Gestational diabetes mellitus (GDM) is one of the most common pregnancy complications. The global prevalence of GDM was 14·0 %(Reference Wang, Li and Chivese1). GDM is related to perinatal diseases and is closely related to adverse maternal and children’s health after pregnancy(Reference McIntyre, Catalano and Zhang2). Therefore, early identification of GDM-related risk factors is essential for preventing GDM and improving maternal and children’s health. In addition to some traditional risk factors, increasing evidence indicates that one-carbon metabolism (OCM) related nutrients (folate and vitamin B12) are associated with GDM(Reference Lai, Pang and Cai3–Reference Li, Tian and Wang7).
Multiple prospective cohort studies have shown that excessive folate supplementation and higher blood folate levels are associated with a higher incidence of GDM(Reference Zhu, Ge and Huang8,Reference Li, Zhang and Huang9) . In contrast to folate, maternal vitamin B12 levels were shown to be negatively correlated with GDM risk(Reference Lai, Pang and Cai3–Reference Chen, Zhang and Chen6). Since folate and vitamin B12 are interrelated in the OCM pathway, several studies have shown that higher folate coupled with lower vitamin B12 was associated with a higher risk of GDM(Reference Lai, Pang and Cai3,Reference Li, Hou and Yan4) . Furthermore, homocysteine (Hcy) is a sensitive marker of folate and vitamin B12 insufficiency. However, the association between Hcy and GDM remains controversial(Reference Zheng, Deng and Qiao10).
Folate and vitamin B12 play a crucial role in the OCM pathway, which is driven by the folate and methionine cycles(Reference Clare, Brassington and Kwong11). The folate cycle begins with the conversion of dietary folate into dihydrofolate, which is subsequently reduced to tetrahydrofolate (THF). THF is next converted to 5,10-methylene-THF, which is then reduced to 5-methyl-THF by methylenetetrahydrofolate reductase (MTHFR). As part of the methionine cycle, 5-methyl-THF donates a methyl group to regenerate methionine and THF from Hcy by the vitamin B12-dependent methionine synthase (MTR) and methionine synthase reductase (MTRR). In addition to food and dietary supplements intake, serum folate, vitamin B12 and Hcy levels are influenced by specific genetic variants in the genes encoding these enzymes, particularly two common functional polymorphisms (rs1801131 and rs1801133) within the MTHFR gene(Reference Hiraoka and Kagawa12). Therefore, the polymorphisms of genes related to the OCM pathway may also be associated with GDM. A few recent studies have explored the relationship between MTHFR rs1801133 SNP and GDM. However, they did not find a significant association between them(Reference Khan, Shaik and Kamineni13,Reference Liu, Liu and Ma14) . Our previous study evaluated the association between twelve OCM-related gene polymorphisms and GDM. We found that MTHFR rs1801131 was an independent risk factor for GDM, and pregnant women with the homozygous wild-type MTHFR rs1801131 are more susceptible to OCM-related GDM. However, no significant association was identified between MTHFR rs1801133 SNP and GDM using the traditional statistical methods(Reference Li, Tian and Wang7).
Compared with MTHFR rs1801131, the effect of rs1801133 gene polymorphism on serum folate and Hcy levels was more predominant. Numerous studies have revealed that individuals with the MTHFR rs1801133 CC genotype have significantly higher folate levels and lower Hcy levels in serum than those with the CT and TT genotypes(Reference Shane, Pangilinan and Mills15,Reference Jin, Cheng and Chen16) . Considering folate is well associated with the risk of GDM, we speculate that the relationship between rs1801133 polymorphism and GDM is hidden or suppressed by OCM nutrients. Coincidentally, mediation analysis can contribute to a better understanding of the relationship between an independent and dependent variable through a mediator variable when these variables do not have an apparent direct association(Reference MacKinnon, Fairchild and Fritz17). Therefore, the present study applied the mediation analysis to clarify the relationship between MTHFR rs1801133 polymorphism and GDM through folate, vitamin B12 and Hcy, respectively.
Methods
Study population
Details of the study design have been described in our previous study(Reference Li, Tian and Wang7). Briefly, pregnant women participating in this research were recruited in the Gene-Environment-Nutrient–Epigenetics interaction on Maternal and Children health study (GENEMaC) from October 2017 to September 2018 in Tianjin, China. Of the 1505 participants, 1254 pregnant women were involved in the final analysis according to the study flowchart (online Supplementary Fig. 1). The Ethics Committee of Metabolic Diseases Hospital and Institute of Endocrinology, Tianjin Medical University and Tianjin Xiqing Hospital approved the research. All procedures involving human participants were performed under the ethical standards of the Declaration of Helsinki Ethical Principles. All participants provided signed informed consent before participating in this study.
Sample collection and covariates assessment
Fasting blood samples were obtained from each pregnant woman at 24–28 gestational weeks when they underwent a routine screening for GDM. Aliquots of blood samples and serum were frozen and stored at –80 °C until further use. Information on age, education, smoking and drinking habits, parity and family history of diabetes were collected by a structured questionnaire at study enrolment. Participants’ height, current weight and prepregnancy weight were also obtained through questionnaires and verified in the medical system.
Diagnosis of gestational diabetes mellitus
The primary outcome of interest for this study was the prevalence of GDM. A 75-g oral glucose tolerance test (OGTT) was used to identify GDM. The cut points of glucose values of 75-g OGTT: fasting plasma glucose (FPG) 5·1 mmol/l, 1-h plasma glucose (1-h PG) 10·0 mmol/l and 2-h plasma glucose (2-h PG) 8·5 mmol/l. GDM can be diagnosed when any one value is equalled or exceeded.
Determination of one-carbon metabolism-related nutrients
The method for determining folate, vitamin B12 and Hcy in maternal serum has been described in detail in our previous studies(Reference Li, Tian and Wang7,Reference Zhang, Zhang and Li18) . Briefly, folate and vitamin B12 were measured using the Architect-i2000SR automated chemiluminescence immunoassay system and its supporting kit (Abbott Diagnostics). A circulating enzymatic method was adopted for the determination of serum Hcy by the automatic biochemical analyzer (Dirui CS-T300) and Hcy kit (MedicalSystem Biotechnology).
Genotyping of MTHFR rs1801131 and rs1801133
The two SNP in MTHFR (rs1801311 and rs1801133) were genotyped at Biowing biotechnology using the assay conditions described previously(Reference Li, Tian and Wang7,Reference Chen, Zhou and Li19) . In brief, human genomic DNA were extracted from blood samples with RelaxGene Blood DNA System (Tiangen Biotech) following the manufacturer’s instructions. After three-round multiplex PCR amplification, the products were subjected to next generation sequencing platform to genotype SNP precisely.
Statistical analysis
The Chi-square test or Fisher’s exact test was applied for categorical variables to examine the differences between different rs1801133 genotypes. For continuous variables with skewed distribution, the Kruskal–Wallis H test was employed to determine the differences among rs1801133 CC, CT and TT genotypes. Spearman’s correlation was conducted to estimate monotonic relationships among serum folate, vitamin B12 and Hcy levels. Linear regression was applied to examine associations of rs1801133 genotypes and serum OCM nutrients with plasma glucose levels. Logistic regression was used to calculate OR and 95 % CI for rs1801133 genotypes and serum OCM nutrients on GDM. Potential maternal confounders, including age, prepregnancy BMI, education, smoking, drinking, family history of diabetes, parity and rs1801131 genotypes, were adjusted in all models. In addition, serum OCM nutrients (folate, vitamin B12 and Hcy) were mutually adjusted to evaluate the association of OCM nutrients with blood glucose levels and GDM. When estimating the association of rs1801133 genotypes with blood glucose levels and GDM, maternal confounders and serum OCM nutrients were adjusted.
The best inheritance model for MTHFR rs1801133 on GDM was the dominant model based on the smallest value of the Akaike Information Criterion and Bayesian Information Criterion (see the Results section). Therefore, the mediating effects of OCM nutrients on the relationship between rs1801133 genotypes and blood glucose levels (FPG, 1-h PG and 2-h PG) and GDM were examined based on the dominant model (Fig. 1). According to the recommendation by Baron and Kenny(Reference Baron and Kenny20), the mediation model included the following steps: (1) the linear or logistic regression model estimated the association of rs1801133 genotypes with blood glucose levels and GDM without adjusting for OCM nutrients; (2) the relationship between rs1801133 genotypes and OCM nutrients was evaluated by a linear regression model (a path); (3) the association between OCM nutrients and GDM (b path) was analysed using a logistic regression model; (4) the OCM mediator (folate, vitamin B12 and Hcy) was added to the c path. If the path between rs1801133 genotypes and GDM was no longer significant, it could be concluded that the association between rs1801133 genotypes and GDM was fully mediated by OCM nutrients (c’ path). Otherwise, the association might be only partially mediated by OCM nutrients.

Fig. 1. Conceptual diagram of mediation analysis. a, regression coefficient of X v. Mi; b, regression coefficient of Mi v. Yi; a * b, indirect effect, regression coefficient of the mediation effect; c, total effect, regression coefficient when X v. Yi (no mediator variable Mi in the model); c’, direct effect, regression coefficient when X v. Yi (mediator variable Mi in the model). All models were adjusted for age, prepregnancy BMI, education, smoking, drinking, family history of diabetes, parity and rs1801131 genotypes. Additionally, serum vitamin B12 and Hcy were adjusted when folate worked as mediator; folate and Hcy were adjusted when vitamin B12 worked as mediator; and folate and vitamin B12 were adjusted when Hcy worked as mediator.
All statistical analyses were performed using R (version 4.1.2; R Project for Statistical Computing). Mediation analysis was implemented with the R package ‘bruceR’. A P-value < 0·05 was considered to be statistically significant.
Results
General information on the study population
The genotype frequencies for the MTHFR rs1801133 were in Hardy–Weinberg equilibrium. Genotype distribution of the rs1801133 SNP was 19·14 %, 48·64 % and 32·22 % for the CC, CT and TT genotypes, respectively (online Supplementary Table 1). There were no significant differences in demographic characteristics among different MTHFR genotypes. However, there were significant differences in folate (CC: 10·75 ng/ml, CT: 8·90 ng/ml and TT: 9·40 ng/ml) and Hcy (CC: 4·84 μmol/l, CT: 4·93 μmol/l and TT: 5·20 μmol/l) levels among the three groups. In addition, pregnant women with the rs1801133 CT and TT genotypes were more likely to have the rs1801131 TT genotype. The three groups observed no significant differences in blood glucose levels and GDM prevalence (Table 1). The correlations of serum folate and vitamin B12 concentrations with Hcy levels are displayed in Supplementary Fig. 2. Serum folate was positively correlated with vitamin B12, and serum Hcy levels were negatively correlated with folate and vitamin B12 concentrations.
Table 1. Characteristics of the study population according to MTHFR rs1801133 genotypes (n 1254)

OCM, one-carbon metabolism; FPG, fasting plasma glucose; GDM, gestational diabetes mellitus.
Values are n (%) or median (25th and 75th percentiles). P-values were obtained by Krushkal–Wallis H test for continuous variables and Chi-square test or Fisher’s exact test for categorical variables.
Associations of one-carbon metabolism indicators with blood glucose and gestational diabetes mellitus
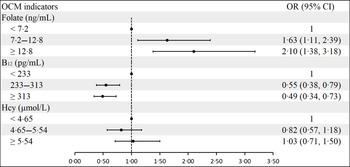
Folate concentrations were positively associated with blood glucose concentrations in both crude and adjusted models, especially for 1-h PG and 2-h PG. However, serum vitamin B12 levels were negatively correlated with FPG concentrations in crude and adjusted models. There was no significant association between serum vitamin B12 and 1-h PG and 2-h PG. In contrast to folate, serum Hcy levels had a negative correlation with 2-h PG concentrations in crude (β: −0·08; 95 % CI: −0·13, −0·04) and adjusted (β: −0·06; 95 % CI: −0·10, −0·01) models (Table 2). The association between OCM indicators and GDM can be found in Fig. 2. Serum folate was positively related to GDM. However, vitamin B12 was negatively related to GDM. There was no significant association between Hcy and GDM risk.
Table 2. Association of maternal serum folate, vitamin B12 and Hcy levels with FPG, 1-h PG and 2-h PG (n 1254)

Hcy, homocysteine; FPG, fasting plasma glucose; 1-h PG, 1-h plasma glucose; 2-h PG, 2-h plasma glucose; OCM, one-carbon metabolism.
* Adjusted for age, prepregnancy BMI, education, smoking, drinking, family history of diabetes, parity and rs1801131 genotypes. Additionally, serum vitamin B12 and Hcy were adjusted in the folate group; folate and Hcy were adjusted in the vitamin B12 group; and folate and vitamin B12 were adjusted in the Hcy group.

Fig. 2. Association of maternal serum folate, vitamin B12 and Hcy levels with GDM. a Adjusted for age, prepregnancy BMI, education, smoking, drinking, family history of diabetes, parity and rs1801131 genotypes. Additionally, serum vitamin B12 and Hcy were adjusted in the folate group; folate and Hcy were adjusted in the vitamin B12 group; and folate and vitamin B12 were adjusted in the Hcy group. GDM, gestational diabetes mellitus.
Association of MTHFR rs1801133 polymorphism with blood glucose and gestational diabetes mellitus
There were no substantial differences regarding the blood glucose levels of the increase of the T allele in rs1801133 reflected by the beta values (online Supplementary Table 2). Compared with pregnant women with the CC genotype, participants with CT (OR: 1·34; 95 % CI: 0·86, 2·07) and TT (OR: 0·99; 95 % CI: 0·60, 1·62) genotypes did not have a significantly higher risk of GDM (Table 3). Similarly, there were no significant associations between MTHFR rs1801133 SNP and GDM in the dominant, recessive, over-dominant and additive models (Table 3).
Table 3. Odds ratios of MTHFR rs1801133 genotype for GDM (n 1254)

GDM, gestational diabetes mellitus; AIC, Akaike Information Criterion; BIC, Bayesian Information Criterion; Hcy, homocysteine.
* Crude model.
† Adjusted for age, prepregnancy BMI, education, smoking, drinking, family history of diabetes, parity, serum folate, vitamin B12, Hcy and rs1801131 genotypes.
Mediation of one-carbon metabolism indicators on the association of MTHFR rs1801133 genotype with blood glucose and gestational diabetes mellitus
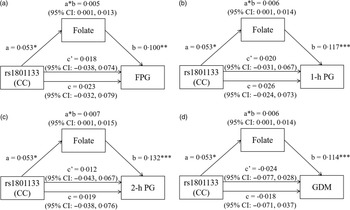
The mediation effects of serum folate on the association of MTHFR rs1801133 genotype with FPG, 1-h PG, 2-h PG and GDM are shown in Fig. 3. Using the CT/TT genotypes as the reference, the total effect model predicting FPG revealed a non-significant effect of MTHFR rs1801133 CC genotype after adjustment for maternal characteristics, rs1801131 genotypes and serum vitamin B12 and Hcy levels. Once folate was added to the model, the direct effect of the MTHFR rs1801133 CC genotype on FPG was also insignificant (Fig. 3(a)). However, there was a significant indirect effect of the MTHFR rs1801133 CC genotype on FPG. The regression coefficient of the mediation effect was 0·005 (95 % CI: 0·001, 0·013), indicating that folate plays a mediating role in the effect of MTHFR rs1801133 on FPG (Fig. 3(a)). Similarly, the indirect effects of the MTHFR rs1801133 CC genotype on 1-h PG (β: 0·006; 95 % CI: 0·001, 0·014), 2-h PG (β: 0·007; 95 % CI: 0·001, 0·015) and GDM (β: 0·006; 95 % CI: 0·001, 0·014) were significant (Fig. 3(b), (c) and (d)). The regression coefficients of the path ‘a’ (rs1801133 CC genotype v. folate) and path ‘b’ (folate v. blood glucose levels and GDM) indicate that MTHFR rs1801133 CC genotype indirectly predicted higher blood glucose levels or higher GDM risk by increasing serum folate concentrations. No significant indirect effect was found between the MTHFR rs1801133 CC genotype and FPG, 1-h PG, 2-h PG and GDM when vitamin B12 worked as mediators (online Supplementary Fig. 3). However, we did observe a significant indirect effect of wild-type MTHFR rs1801133 (β: 0·004; 95 % CI: 0·001, 0·009), such that the CC genotype predicted higher 2-h PG indirectly through decreasing serum Hcy levels (online Supplementary Fig. 4C).

Fig. 3. The mediation effect of folate on the association of MTHFR rs1801133 with (a) FPG, (b) 1-h PG, (c) 2-h PG and (d) GDM. All models were adjusted for age, prepregnancy BMI, education, smoking, drinking, family history of diabetes, parity, rs1801131 genotypes, serum vitamin B12 and Hcy. a, regression coefficient of rs1801133 v. folate; b, regression coefficient of folate v. FPG, 1-h PG, 2-h PG and GDM; a * b, indirect effect, regression coefficient of the mediation effect; c, total effect, regression coefficient when rs1801133 v. FPG, 1-h PG, 2-h PG and GDM (no mediating variable folate in the model); c’, direct effect, regression coefficient when rs1801133 v. FPG, 1-h PG, 2-h PG and GDM (mediating variable folate in the model). * P < 0·05, ** P < 0·01, *** P < 0·001. FPG, fasting plasma glucose; 1-h PG, 1-h plasma glucose; 2-h PG, 2-h plasma glucose; GDM, gestational diabetes mellitus.
Discussion
In this study, we evaluated the mediation effect of serum OCM nutrients on the association between MTHFR rs1801133 polymorphism and GDM in Chinese Han pregnant women for the first time. Although there was no significant association between MTHFR rs1801133 genotypes and GDM risk, we found a novel and significant indirect effect of MTHFR rs1801133 polymorphism on blood glucose levels and GDM through modifying serum folate levels.
Folate and vitamin B12 are essential water-soluble vitamins. In addition to food and dietary supplementation, genetic polymorphisms in MTHFR, MTR and MTRR are closely related to serum folate, vitamin B12 and Hcy levels(Reference Hiraoka and Kagawa12,Reference Thuesen, Husemoen and Ovesen21) . Among these genes, rs1801133 in MTHFR is the most well-known genetic factor influencing OCM status. It was reported that carriers of the rs1801133 T allele have lower enzyme activity, which leads to decreased folate and increased Hcy concentrations(Reference Tsang, Devine and Cordero22). In contrast, the rs1801133 CC genotype is associated with higher folate and lower Hcy. In the present study, the prevalence of the rs1801133 CC, CT and TT genotypes were 19·14 %, 48·64 % and 32·22 %, respectively. This was in line with previously reported CC, CT and TT frequencies in Tianjin, China (21·4 %, 48·3 % and 30·3 %, respectively)(Reference Yang, Liu and Li23). We found that pregnant women with the rs1801133 CC genotype have higher folate and lower Hcy concentrations. These results are consistent with previous findings that the wide-type (CC) of MTHFR rs1801133 was associated with increased folate but decreased Hcy levels(Reference Hiraoka and Kagawa12). Furthermore, there was no significant difference in vitamin B12 levels among rs1801133 CC, CT and TT genotypes. Our findings were in line with most previous studies that genetic polymorphisms in MTHFR (rs1801131 and rs1801133) have no association with vitamin B12 concentrations(Reference Surendran, Adaikalakoteswari and Saravanan24). However, a study from Jordan indicated that the rs1801133 T allele was associated with vitamin B12 deficiency(Reference Al-Batayneh, Zoubi and Shehab25). In addition to ethnic differences, it could be postulated that the rs1801133 polymorphism might be related to decreased intestinal absorption of vitamin B12(Reference Shiran, Remer and Asmer26).
Although the relationship between high folate and low vitamin B12 and GDM is well studied, their association with blood glucose is limited. A prospective cohort study from the UK revealed that folate was positively associated with 2-h PG, and vitamin B12 was negatively associated with FPG and 2-h PG. However, they did not provide the data of 1-h PG(Reference Saravanan, Sukumar and Adaikalakoteswari5). Our study indicated that serum folate was positively associated with blood glucose levels. However, this association was more evident in 1-h PG and 2-h PG. For vitamin B12, a significantly positive correlation was found only for FPG. Although the association between Hcy and GDM was insignificant, it was negatively correlated with 2-h PG. This contrasts sharply with the relationship between folate and blood glucose levels since Hcy is a surrogate marker of folate deficiency. Our findings were in line with recently published results, which indicated that Hcy is inversely associated with blood glucose levels(Reference Lai, Pang and Cai3). However, other studies revealed that the relationship between Hcy and GDM remains controversial(Reference Zheng, Deng and Qiao10,Reference Radzicka, Ziolkowska and Zaborowski27) . Therefore, a comprehensive evaluation of the factors related to Hcy metabolism may help clarify the association between Hcy and GDM.
Since the relationship between folate, vitamin B12 and GDM is significant, and genetic variants in MTHFR can significantly affect the OCM nutrient status, we speculate that SNPs in this gene may be closely related to blood glucose levels and GDM risk. However, we did not observe a significant association between MTHFR rs1801133 SNP and GDM under different genetic models. Similarly, we did not find a significant association between mutant T and blood glucose levels at different time points of OGTT (online Supplementary Table 2). The research on the relationship between MTHFR rs1801133 SNP and GDM is still limited. Studies from India, Australia and South Africa showed that the maternal MTHFR rs1801133 genotype was not associated with GDM risk(Reference Khan, Shaik and Kamineni13,Reference Dias, Adam and Rheeder28,Reference Jankovic-Karasoulos, Furness and Leemaqz29) . Similar results were also found in the Chinese population(Reference Liu, Liu and Ma14). Our findings and others indicated that the relationship between MTHFR rs1801133 and GDM may not be direct. This also urged us to consider new and more sensitive methods to reveal the relationship between MTHFR rs1801133 and GDM.
The MTHFR rs1801133 polymorphism affects serum OCM nutrient levels, and folate, vitamin B12 and Hcy affect blood glucose levels and GDM occurrence. It is reasonable to raise the question of whether a genetic variant in MTHFR rs1801133 will affect blood glucose levels and GDM occurrence by influencing OCM nutrient levels. A question like that suggests a chain of relations where an independent variable (X) affects a mediating variable (M), which then affects a dependent variable (Y). Therefore, the mediation analysis is the best way to determine the effect of M transmits the effect of X on Y(Reference Preacher30,Reference Lee, Cashin and Lamb31) . Consistent with our hypothesis, serum folate mediated the effect of the MTHFR rs1801133 on FPG, 1-h PG, 2-h PG and GDM. The regression coefficients of the MTHFR rs1801133 polymorphism with folate and folate with blood glucose levels and GDM indicated that the MTHFR rs1801133 CC genotype predicts higher blood glucose levels or evaluated GDM risk indirectly through increasing serum folate concentrations. However, the mediating role of vitamin B12 in transmitting the effect of the MTHFR rs1801133 polymorphism on blood glucose levels and GDM was insignificant. This may be attributed to the fact that the effect of the MTHFR rs1801133 polymorphism on serum vitamin B12 levels is not apparent. Although no significant indirect effect was found between MTHFR rs1801133 polymorphism and GDM when Hcy served as a mediating variable, the mediation effect of Hcy in transmitting the effect between MTHFR rs1801133 polymorphism on 2-h PG was significant (online Supplementary Fig. 4(c)). Although this is the first study on the relationship between MTHFR and GDM by mediation analysis, a few studies have focussed on the association between genetic polymorphisms and metabolic disease through this analysis approach(Reference Xu, Lv and Xie32,Reference Guerra-García, Moreno-Macías and Ochoa-Guzmán33) . It highlights that mediation analysis is a powerful method to test a hypothetical causal chain where one variable affects a second variable and, in turn, that variable affects the third variable.
The mechanisms linking high folate and low vitamin B12 levels and increased GDM risk remain unclear. However, serum folate mediated the effect of MTHFR rs1801133 SNP on GDM, which provided a biological pathway for understanding how the MTHFR polymorphism affects GDM during pregnancy. Our previous study revealed that the wild-type (TT) genotype of MTHFR rs1801131 was significantly associated with an increased risk of GDM. In addition, pregnant women with homozygous T alleles are more prone to have OCM-related GDM(Reference Li, Tian and Wang7). However, the present study showed an indirect effect of MTHFR rs1801133 SNP on GDM by modifying serum folate levels using the mediation approach. The two studies all pointed out that the wild-type genotypes of MTHFR rs1801131 and rs1801133 with higher folate can increase the risk of GDM but through different ways, as rs1801131 acting directly and rs1801133 indirectly. In the OCM pathway, Hcy can be remethylated to methionine via vitamin B12-dependent MTR and MTRR utilising the methyl group from 5-methyl-THF. Without vitamin B12, 5-methyl-THF becomes metabolically trapped in this form since producing 5-methyl-THF from 5, 10-methylene-THF by MTHFR is irreversible(Reference Maher and Sobczyńska-Malefora34). In addition, the wild-type genotypes of MTHFR rs1801133 and rs1801131 facilitate reducing 5, 10-methylene-THF to 5-methyl-THF. The above evidence indicates that folate/vitaminB12 imbalance coupled with pregnant women’s MTHFR rs1801133 CC and rs1801131 TT genotype is more likely to accumulate 5-methyl-THF in the body. Although the role of 5-methyl-THF in the pathogenesis of GDM is unclear, recent animal studies suggested that consuming 5-methyl-THF diets will increase the weight of the dams and their female offspring(Reference Pannia, Hammoud and Simonian35,Reference Pannia, Hammoud and Kubant36) . Therefore, 5-methyl-THF may play a role in GDM occurrence, and further studies are needed to clarify the underlying mechanism.
Folic acid (FA) supplementation in the periconceptional period is recommended to prevent neural tube defects (NTD) worldwide. Some countries, including the USA and Canada, have implemented mandatory fortification of cereal grains with FA to prevent NTD. Although there is no mandatory FA fortification in China, a nationwide FA supplement project has been launched since 2009 with a recommendation of 400 μg/d of folate supplementation from 3 months of preconception until 3 months of pregnancy. A recent study in Tianjin showed that 93·1 % of pregnant women took FA supplements before and/or during pregnancy. However, only 14·4 % of them adhered to the recommendation for FA intake(Reference Yan, Zheng and Cao37). It was reported that 42·3 % of the pregnant women took a FA dose exceeding 400 μg/d, and 69·5 % took FA longer than the recommended duration(Reference Yan, Zheng and Cao37). The indiscriminately FA intake may lead to significantly higher folate levels among pregnant women, especially those with wild-type genotypes of MTHFR, which may account for the high incidence of GDM. However, for pregnant women carrying the mutant allele of MTHFR, higher folate intake may not cause GDM but is beneficial for NTD prevention since the mutant genotypes of MTHFR are associated with lower folate concentrations and are more likely to affect NTD in the offspring. Our findings do not conflict with the current strategy of FA supplementation to prevent NTD, but for MTHFR wild-type genotype carriers, high-dose FA supplementation should be avoided.
The strengths of this study include the availability of individual measurements of serum folate, vitamin B12 and Hcy levels, the ability to estimate the mediation effect of folate on the association between MTHFR rs1801133 polymorphism and GDM, and larger sample sizes that give more reliable results. Despite these advantages, our study was subject to some limitations. First, due to the nature of the cross-sectional design, the temporal link between OCM nutrients and GDM cannot be determined. However, the mediation analysis can estimate how much MTHFR rs1801133 polymorphism may affect GDM through a potential causal mechanism by modifying folate levels. Second, serum folate levels are closely related to folate intake and MTHFR genotype. The lack of data on folate and vitamin B12 from food and dietary supplement limited the application of our findings. Third, folate species, especially 5-methyl-THF, were not distinguished. Further studies are required to verify the potential role of 5-methyl-THF in GDM.
In conclusion, maternal MTHFR rs1801133 polymorphism was not directly associated with GDM risk in Chinese Han pregnant women. However, serum folate can mediate their association indirectly. To our knowledge, this is the first epidemiologic study to use a mediation approach to evaluate the indirect effect of OCM-related gene polymorphisms on GDM through modifying folate, vitamin B12 and Hcy levels. Further mechanistic studies are encouraged to figure out the potential role of OCM nutrients in GDM. At the same time, a more accurate assessment of folate and vitamin B12 supplementation in pregnant women with different genotypes should also be conducted. Our findings provide a feasible GDM prevention strategy via precision nutrition in the future.
Acknowledgements
All the authors would like to thank the participants who took part in this study.
This work was supported by the Fundamental Research Funds for Higher Education of Tianjin Municipal Education Commission (2020ZD14).
Q. Z. and X. Z. conceived and designed the research; Y. W. (Yunguo Wang), Y. S., S. L. (Shuying Li), L. D., C. S. and Y. Y. conducted the research; Y. W. (Yiyun Wang), X. L. and S. L. (Suhui Luo) contributed to laboratory sample testing; Y. W. (Yiyun Wang) and N. Z. performed the statistical analyses; Y. W. (Yunguo Wang) and Q. Z. wrote the manuscript; Q. Z. had primary responsibility for the final content; and all authors contributed to the interpretation of the results and approved the final version of the manuscript.
The authors declare that they have no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114523000314