CVD is currently the leading cause of illness and death in developed countries, and over the past decade, it has become apparent that chronic inflammation plays a major role in its development( Reference Munro and Cotran 1 , Reference Alexander 2 ). C-reactive protein (CRP) is a commonly measured marker of cardiovascular risk, and elevated concentrations are associated with CVD, in both cross-sectional( Reference Mendall, Patel and Ballam 3 ) and many longitudinal studies( Reference Ridker, Cushman and Stampfer 4 – Reference Kuller, Tracy and Shaten 6 ). However, a direct causal link between CRP and the development of CVD has not been identified. On the other hand, serum amyloid A (SAA), another marker of inflammation, is produced acutely by the liver and chronically by hypertrophic adipocytes and its expression is regulated by inflammation-associated cytokines and activated monocytes and macrophages( Reference Akira, Hiranom and Tagam 7 – Reference Zhao, He and Shi 10 ), and it may have a causal role in the development of CVD. This concept is based on the fact that one of the major proatherogenic properties of SAA is its rapid association with HDL, especially with HDL3, in the circulation, which results in the production of dysfunctional HDL( Reference Jahangiri, de Beer and Noffsinger 11 ). Normally, the major antiatherogenic role of HDL is its involvement in reverse cholesterol transport. However, when HDL is associated with SAA, this and other antiatherogenic properties may be attenuated or lost( Reference Clifton, Mackinnon and Barter 12 , Reference Van Lenten, Hama and de Beer 13 ). Dysfunctional HDL binds to proteoglycans on the vascular wall, favouring their retention and subsequent modification by the vascular matrix, which has an important role in the formation of macrophage foam cells. The presence of SAA within HDL fractions also decreases the efflux of cholesterol from lipid-laden macrophages( Reference Artl, Marsche and Lestavel 14 ). In addition, HDL-associated enzymes may be influenced by the presence of SAA. For example, the activity of cholesteryl ester transfer protein (CETP) may be increased( Reference Park, Shin and Kim 15 ), which leads to altered lipoprotein remodelling( Reference Zeller, Masson and Farnier 16 ) and may contribute to an atherogenic phenotype. In support of this, we have found that increased lycopene intake leads to a reduction in the concentrations of SAA and a concomitant decrease in the activity of CETP( Reference McEneny, Wade and Young 17 ).
Therefore, considering the above-mentioned findings, it is likely that factors that reduce systemic concentrations of SAA and, therefore, its association with HDL may reduce the burden of CVD risk. One such factor that may mediate this response is increased fruit and vegetable (F&V) intake. Meta-analyses of prospective cohort studies have suggested an association between increased F&V intake and reduced CVD risk( Reference Dauchet, Amouyel and Hercberg 18 ) and stroke( Reference He, Nowson and MacGregor 19 ). However, although these observational studies have demonstrated a significant inverse relationship between high F&V intake and CVD risk, no biological marker that responds to increased F&V intake has been identified. For example, intervention studies examining the inflammatory molecule CRP have reported conflicting results( Reference Freese, Vaarala and Turpeinen 20 – Reference Fisk, Middaugh and Rhee 22 ), while observational studies have rarely shown an association between increased F&V intake and reduced CRP concentrations after adjustment for possible confounding factors( Reference Gao, Bermudez and Tucker 23 – Reference Esmaillzadeh, Kimiagar and Mehrabi 25 ). In fact, both the FAVRIT (Fruit and Vegetable Randomised Intervention Trial) and ADIT (Ageing and Dietary Intervention Trial) studies have found that CRP does not respond to increased F&V intake( Reference McCall, McGartland and McKinley 26 – Reference Gibson, Edgar and Neville 28 ). However, we suggest that SAA may respond to inflammatory changes brought about by increased F&V intake, based on the concept that SAA has been documented as a more sensitive marker of inflammatory changes than CRP( Reference Yamada 29 , Reference Bozinovski, Hutchinson and Thompson 30 ) and on the fact that we have found that SAA responds to increased intake of foods rich in lycopene, while CRP is unresponsive( Reference McEneny, Wade and Young 17 ).
Therefore, the present study was carried out to investigate whether (1) SAA responded to increased F&V intake, while other inflammatory markers were unresponsive, and (2) increased F&V intake lowered HDL-associated inflammation and thereby influenced the antiatherogenic properties of HDL. This was achieved by utilising samples from two previous F&V intervention studies( Reference McCall, McGartland and McKinley 26 – Reference Gibson, Edgar and Neville 28 ) and was not part of the original analyses.
Materials and methods
Study groups
The present study was carried out using samples from two previous Food Standards Agency-funded studies. The first study examined the effect of increased F&V intake in a group of subjects with mild hypertension (the FAVRIT study). The second study examined the effect of increased F&V intake in an older population (the ADIT study).
Fruit and Vegetable Randomised Intervention Trial study design
Details of the FAVRIT study have been published previously( Reference McCall, McGartland and McKinley 26 , Reference McCall, McGartland and McKinley 27 ). In brief, 112 participants aged between 40 and 65 years, with brachial blood pressure in the range of 140–190 mmHg (systolic) and 90–110 mmHg (diastolic), were recruited from medical outpatient clinics and through local press release. Exclusion criteria were diabetes mellitus, an acute coronary ischaemic attack within the past 3 months, dietary requirements, food sensitivities or vegetarian/vegan diet by choice, oral anticoagulation therapy, BMI >35 kg/m2, excessive alcohol consumption (defined as >221 g/week in men and 166 g/week in women), fasting TAG concentration >4 mmol/l, or pregnancy/lactation. Suitable participants gave written informed consent and were put on a 4-week washout phase, during which F&V consumption was limited to 1 portion/d. After the washout phase, the participants were randomised to one of three groups, consuming 1, 3 or 6 portions of F&V daily for 8 weeks. They were asked to maintain other aspects of their lifestyle. Fasting blood samples were collected before and after the intervention period and were separated appropriately for the proposed assays and stored at − 75°C until analysis. This study was approved by the Research Ethics Committee of Queen's University Belfast.
Ageing and Dietary Intervention Trial study design
Details of the ADIT study have been published elsewhere( Reference Gibson, Edgar and Neville 28 ). In brief, eighty-two free-living, healthy older participants (aged 65–85 years) with low F&V intake ( ≤ 2 portions/d) were recruited. Exclusion criteria were consumption of special diets, use of nutritional supplements or medications known to affect the variables being assessed, excessive alcohol consumption (>221 g/week in men or >166 g/week in women), BMI >35 kg/m2, history of diabetes or dementia, inability to provide informed consent, any other problems that would prevent adherence to a high-F&V diet, or a recent infection ( < 3 weeks since the completion of any antibiotic course or symptoms of viral illness). Following acquisition of written informed consent, the participants were randomised to one of two arms – either to increase F&V consumption to at least 5 portions/d or to follow their normal diet (therefore consuming ≤ 2 portions/d) for 16 weeks. They were asked to maintain other aspects of their lifestyle. Fasting blood samples were collected at baseline and week 16, separated appropriately for the proposed assays and stored at − 75°C until analysis. A total of eighty subjects were included in the final analysis: thirty-nine in the ≤ 2 portions/d group and forty-one in the 5 portions/d group.
The study was approved by the Office for Research Ethics Committees Northern Ireland (ORECNI) and was registered in ClinicalTrials.gov (no. NCT00858728).
Specific dietary advice was given to all participants of the FAVRIT and ADIT studies helping ensure a similar energy and macronutrient intake from their normal diet, while weekly contact encouraged compliance. Compliance was monitored using diet history, interview and laboratory assessment of micronutrient status (data not shown).
Serum analysis
Measurement of serum carotenoid concentrations
The serum concentrations of carotenoids were measured by HPLC, as described by Craft( Reference Craft 31 ).
Measurement of high-sensitive C-reactive protein, IL-6 and E-selectin concentrations
The concentrations of high-sensitive C-reactive protein (hsCRP) were measured in the primary analyses( Reference McCall, McGartland and McKinley 27 , Reference Gibson, Edgar and Neville 28 ) by an immunoturbidimetric assay (Randox), using an ILab-600 biochemical analyser (Instrumentation Laboratories), and their values are reported in comparison with SAA values. The serum concentrations of IL-6 and E-selectin were measured using ELISA procedures, as per the manufacturer's instructions (product no.: HS600B and DSLE00; Randox).
Isolation of HDL2 and HDL3 from serum
HDL2 and HDL3 were isolated from serum by rapid ultracentrifugation, according to the method of McPherson et al. ( Reference McPherson, Young and McKibben 32 ). This method is a three-step procedure: crude HDL was isolated by 2 h rapid ultracentrifugation, which allows crude HDL to sediment at the bottom of the ultracentrifuge tube. This crude HDL was then subfractionated into HDL2 and HDL3 by two 2 h sequential rapid flotation ultracentrifugation procedures, with total isolation time being 6 h. HDL2 and HDL3 were stored at − 75°C until the analyses described below were carried out. HDL subfractions are stable when frozen at − 75°C for up to 1 year following their isolation from serum (data not shown).
Serum, HDL2 and HDL3 analyses
Determination of total protein concentration
The concentration of protein in HDL2 and HDL3 was determined spectrophotometrically, as described by McEneny et al. ( Reference McEneny, McMaster and Trimble 33 ).
Determination of serum amyloid A concentrations
The concentrations of SAA in serum, HDL2 and HDL3 were determined using an ELISA procedure (KHA0011; Invitrogen Life Technologies), as per the manufacturer's instructions. This commercially available ELISA recognises the SAA isoforms 1 and 2. The concentrations of serum-SAA, HDL2-SAA and HDL3-SAA are expressed as μg/l.
Measurement of cholesteryl ester transfer protein activity
The activity of CETP in serum, HDL2 and HDL3 was measured using a commercially available fluorometric assay, as per the manufacturer's instructions (RB-CETP; Roar Biomedical, Inc.). The activity of CETP was compared with that of a known concentration of CETP; therefore, it is expressed as μmol/l in serum and as μmol/mg protein in HDL2 and HDL3. The values of CETP were standardised to total protein concentration in HDL2 and HDL3 to obtain an estimation of the activity of this enzyme within an individual HDL particle.
Statistical analyses
Normally distributed continuous variables are summarised as means and standard deviations. Skewed variables were logarithmically transformed for parametric analysis, and these are summarised as geometric means and interquartile ranges.
Between-group comparisons of change in each outcome variable were made using one-way ANOVA for the FAVRIT study. Because the intervention involved increasing numbers of portions of F&V, a test for linear trend across the groups was used. Between-group comparisons of change in each outcome variable were made using independent-samples t tests for the ADIT study. Within-group analyses were conducted using paired-samples t tests for both the FAVRIT and ADIT studies. Associations between outcome variables were tested using Pearson's correlation coefficients. All tests were two-tailed, and a P value < 0·05 was considered statistically significant. The analyses were carried out using the software SPSS (version 17.0.1; SPSS, Inc.).
Results
Subject characteristics
The baseline characteristics of the FAVRIT cohort have been described previously( Reference McCall, McGartland and McKinley 26 , Reference McCall, McGartland and McKinley 27 ); however, in brief, the following baseline characteristics were similar among the groups randomised to receive a 1-, 3- or 6-portion F&V/d intervention: age (52·4 (sd 7·9) v. 56·1 (sd 8·4) v. 53·7 (sd 7·1) years); BMI (29·7 (sd 4·4) v. 28·2 (sd 3·2) v. 28·8 (sd 3·3) kg/m2); blood pressure (systolic: 139·4 (sd 15·0) v. 144·6 (sd 18·1) v. 145·3 (sd 15·7) mmHg; diastolic: 82·0 (sd 11·9) v. 81·1 (sd 11·1) v. 86·3 (sd 11·0) mmHg); antihypertensive medication use; lipid-lowering therapy (P>0·05 for all comparisons). Following intervention and assessment by dietary recall, F&V intake was found to increase across the three groups (pre v. post: 0·9–1·1, 1·1–3·2 and 1·1–5·6 portions/d in the 1-, 3- and 6-portion groups, respectively; P< 0·001 for linear trend), which was accompanied by an increase in serum lutein (P< 0·05) and β-cryptoxanthin (P< 0·001) concentrations, while the increase in zeaxanthin and vitamin C concentrations approached significance (P= 0·09 and 0·06, respectively). In addition, BMI remained unaltered following the 1-, 3- and 6-portion F&V/d interventions (P>0·05 for all comparisons). All the above results have been reported in detail in McCall et al. ( Reference McCall, McGartland and McKinley 26 , Reference McCall, McGartland and McKinley 27 ).
The baseline characteristics of the ADIT cohort have also been described previously( Reference Gibson, Edgar and Neville 28 ); however, in brief, the following baseline characteristics were similar between the 2- and 5-portion groups : age (71·1 (sd 5·0) v. 70·9 (sd 5·0) years); BMI (28·1 (sd 4·5) v. 28·5 (sd 10·9) kg/m2); blood pressure (systolic: 150·5 (sd 24·4) v. 152·9 (sd 20·9) mmHg; diastolic: 84·1 (sd 10·9) v. 87·0 (sd 10·9) mmHg); antihypertensive medication use; lipid-lowering therapy (P>0·05 for all comparisons). Following intervention and assessment by dietary recall, F&V intake was found to increase across the two groups (from 1·4 to 1·8 and 1·4 to 6·0 portions/d in the 2- and 5-portion groups, respectively; P< 0·001), which was accompanied by an increase in serum lutein (P< 0·05), β-cryptoxanthin (P< 0·01), lycopene (P< 0·05), zeaxanthin (P< 0·001) and vitamin C (P< 0·001) concentrations. In addition, BMI remained unaltered following the 2- and 5-portion F&V/d interventions (P>0·05 for both comparisons). All the above results have been reported in detail in Gibson et al. ( Reference Gibson, Edgar and Neville 28 ).
Serum analysis
High-sensitive C-reactive protein, IL-6 and E-selectin concentrations
The concentrations of hsCRP in the FAVRIT and ADIT cohorts have been reported previously( Reference McCall, McGartland and McKinley 27 , Reference Gibson, Edgar and Neville 28 ); however, the concentrations of hsCRP, IL-6 and E-selectin were unaffected by increasing F&V intake in both studies (P>0·05 for all comparisons; data not shown).
Serum, HDL2 and HDL3 analyses
Serum amyloid A concentrations in the Fruit and Vegetable Randomised Intervention Trial
Between-group analyses showed that although the concentrations of serum-SAA and HDL2-SAA decreased as F&V intake increased, the decrease was not significant (P= 0·070 and 0·130, respectively, for linear trend), while those of HDL3-SAA decreased significantly as F&V intake increased (P< 0·05 for linear trend) (Table 1). Within-group analyses showed that the concentrations of serum-SAA, HDL2-SAA and HDL3-SAA were unaffected by the 1-portion F&V/d intervention (P>0·05 for all comparisons), and a similar trend was observed for the concentrations of serum-SAA and HDL2-SAA following the 3-portion F&V/d intervention (P>0·05 for both comparisons), although those of HDL3-SAA appeared to decrease, which was unfortunately not significant (P= 0·068). However, following the 6-portion F&V/d intervention, the concentrations of SAA in serum, HDL2 and HDL3 decreased; although this decrease was not significant in serum, it was significant in HDL2 and HDL3 (P= 0·088, 0·038 and 0·041, respectively).
Table 1 Pre- and post-fruit and vegetable (F&V) intervention serum amyloid A (SAA) concentrations in the FAVRIT (Fruit and Vegetable Randomised Intervention Trial) study (Geometric mean values and interquartile ranges (IQR))

* P value within the groups pre- v. post-intervention.
† P value for trend across the groups.
Serum amyloid A concentrations in the Ageing and Dietary Intervention Trial
Between-group analyses of the 2- v. 5-portion groups showed that as F&V intake increased, the concentrations of SAA in serum were unaffected (P>0·050), while those in HDL2 and HDL3 decreased (P= 0·035 and 0·032, respectively) (Table 2). In addition, within-group analyses showed that the concentrations of serum-SAA, HDL2-SAA and HDL3-SAA were unaffected by the 2-portion F&V/d intervention (P>0·05 for all comparisons). However, following the 5-portion F&V/d intervention, the concentrations of SAA in serum tended to decrease (P= 0·05), but decreased significantly in HDL2 and HDL3 (P= 0·001 and 0·040, respectively).
Table 2 Pre- and post-fruit and vegetable (F&V) intervention serum amyloid A (SAA) concentrations in the ADIT (Ageing and Dietary Intervention Trial) study (Geometric mean values and interquartile ranges (IQR))

* P value within the groups pre- v. post-intervention.
† P value for difference between the 2- and 5-portion groups.
Cholesteryl ester transfer protein activity in the Fruit and Vegetable Randomised Intervention Trial
Between-group analyses showed that the activity of CETP in HDL3 decreased as F&V intake increased (P< 0·050 for linear trend) (Table 3). In addition, within-group analyses showed that following the 1- and 3-portion F&V/d interventions, the activity of CETP in serum, HDL2 and HDL3 remained unaltered. However, following the 6-portion F&V/d intervention, although the activity of CETP was unaffected in serum (P>0·050), it decreased in HDL2 and HDL3; although this was not significant in HDL2 (P= 0·052), it was significant in HDL3 (P< 0·050).
Table 3 Pre- and post-fruit and vegetable (F&V) intervention cholesteryl ester transfer protein (CETP) activity in the FAVRIT (Fruit and Vegetable Randomised Intervention Trial) study (Mean values and standard deviations; geometric mean values and interquartile ranges (IQR))
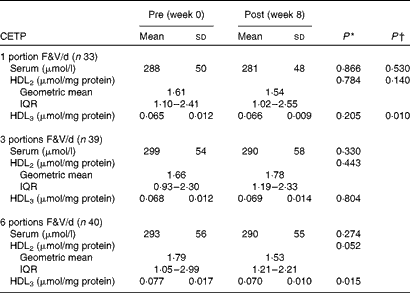
* P value within the groups pre- v. post-intervention.
† P value for trend across the groups.
Cholesteryl ester transfer protein activity in the Ageing and Dietary Intervention Trial
Between-group analyses of the 2- v. 5-portion groups showed that the activity of CETP in serum (P>0·050) and HDL3 (P>0·050) was unaffected (Table 4). However, the activity of CETP in HDL2 decreased (P>0·050). Within-group analyses showed that the activity of CETP in serum, HDL2 and HDL3 was unaffected following the 2-portion F&V/d intervention (P>0·05 for all comparisons). However, following the 5-portion F&V/d intervention, the activity of CETP decreased in HDL2 (P< 0·001), but was unaffected in serum and HDL3 (P>0·050 for both comparisons).
Table 4 Pre- and post-fruit and vegetable (F&V) intervention cholesteryl ester transfer protein (CETP) activity in the ADIT (Ageing and Dietary Intervention Trial) study (Mean values and standard deviations; geometric mean values and interquartile ranges (IQR))
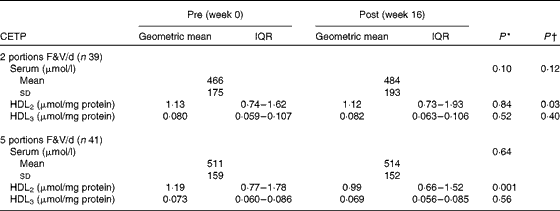
* P value within the groups pre- v. post-intervention.
† P value for difference between the 2- and 5-portion groups.
Correlations between self-reported changes in fruit and vegetable intake, serum antioxidants and serum and HDL2 and HDL3 analyses
Only a few significant correlations were found between the findings from the secondary analysis carried out in the present study and those from the original studies( Reference McCall, McGartland and McKinley 26 – Reference Gibson, Edgar and Neville 28 ) were that:
In the FAVRIT cohort, changes in self-reported F&V intake were negatively correlated with changes in HDL3-CETP activity (r − 0·281, P= 0·016), while changes in serum vitamin C concentrations were negatively correlated with changes in HDL3-SAA concentrations (r − 0·290, P= 0·013).
In the ADIT cohort, changes in self-reported F&V intake were negatively correlated with changes in HDL3-SAA concentrations (r − 0·383, P= 0·001) and serum- and HDL2-CETP activity (r − 0·228, P= 0·047; r − 0·252, P= 0·035, respectively). In addition, changes in serum zeaxanthin and β-cryptoxanthin concentrations were negatively correlated with changes in HDL2-SAA concentrations (r − 0·257, P= 0·037; r − 0·270, P= 0·024, respectively), while changes in serum lycopene concentrations were negatively correlated with changes in HDL3-SAA concentrations (r − 0·320, P= 0·008).
Discussion
The present study was designed based on the knowledge that F&V consumption has been found to be beneficial to CVD health in observational epidemiological studies. However, direct trial evidence of the effect of F&V consumption on a biological marker that is related to changes in CVD health is limited. Although the ability of increased F&V intake to influence CRP concentrations has been widely studied, its usefulness as a marker of inflammatory changes is disputed( Reference Nordestgaard and Zacho 34 ). On the other hand, SAA may have a causal role in the development of CVD( Reference Xie, Ma and Yang 35 ), which we suggest may be due to its association with HDL, as this reduces the antiatherogenic capabilities of this lipoprotein( Reference Jahangiri, de Beer and Noffsinger 11 ).
Fruit and vegetable intervention and serum-, HDL2- and HDL3-serum amyloid A concentrations
In the primary analyses of the FAVRIT and ADIT cohorts, subject compliance was confirmed by food diaries and by appropriate changes in serum antioxidants, relative to F&V interventions( Reference McCall, McGartland and McKinley 27 , Reference Gibson, Edgar and Neville 28 ). However, these primary analyses were unable to detect any changes in hsCRP concentrations following increased F&V intake( Reference McCall, McGartland and McKinley 27 , Reference Gibson, Edgar and Neville 28 ). Therefore, the present study investigated whether a change in F&V intake was accompanied by changes in SAA concentrations, especially as SAA may be a more sensitive marker of inflammatory changes than other acute-phase reactants( Reference Yamada 29 , Reference Bozinovski, Hutchinson and Thompson 30 ) and also responds to changes brought about by diet( Reference McEneny, Wade and Young 17 , Reference Esmaillzadeh, Kimiagar and Mehrabi 25 ). However, within the context of a F&V intervention trial, changes in SAA concentrations have not been examined. Subsequently, the results of the present study demonstrate for the first time that SAA does respond to increased F&V intake in hypertensive and elderly populations. This was particularly apparent in the 16-week ADIT study, where both self-reported F&V intake and changes in serum lycopene concentrations were negatively correlated with changes in HDL3-SAA concentrations (r − 0·383, P= 0·001; r − 0·320, P= 0·008, respectively), and the effect observed on lycopene concentrations confirms our previous findings( Reference McEneny, Wade and Young 17 ). Furthermore, the concentrations of both zeaxanthin and β-cryptoxanthin were negatively correlated with HDL2-SAA concentrations (r − 0·257, P= 0·037; r − 0·270, P= 0·024, respectively). In addition, the between-group analyses showed that SAA concentrations in HDL2 and HDL3 decreased as F&V intake increased (P= 0·035 and 0·032, respectively). In the 8-week FAVRIT study, the only significant finding related to SAA was a decrease in HDL3-SAA concentrations observed in the between-group analyses (P= 0·049), which was also negatively correlated with changes in serum vitamin C concentrations. Overall, we suggest that the small disparity between the two studies may be due to (1) study duration, i.e. 8 weeks' duration of the FAVRIT study may be an insufficient time frame to fully detect the effects of an increase in F&V intake, compared with the 16 weeks' duration of the ADIT study; (2) the age difference between the cohorts of both studies, especially as the older subjects in the ADIT study had higher baseline SAA concentrations in serum, HDL2 and HDL3 than their younger counterparts in the FAVRIT study (although this was not examined statistically); this indicates that the older ADIT subjects may have had a greater capacity to respond to increased F&V intake; or (3) an increase in the average intake of F&V to 6 portions/d in the ADIT cohort and of 5·6 portions/d in the FAVRIT cohort, which may also have influenced the results (although this was not examined statistically). However, these suggestions need to be investigated further.
The beneficial effects exerted by increased F&V intake on SAA, but not on the other markers assessed, namely hsCRP, IL-6 and E-selectin, may be explained by the fact that SAA, as well as being expressed by the liver( Reference Uhlar and Whitehead 36 ), is also expressed in and released from hypertrophic adipocytes( Reference Poitou, Viguerie and Cancello 37 , Reference Poitou, Coussieu and Rouault 38 ). This may be particularly relevant, as both cohorts were, on average, overweight, verging on obese( Reference McCall, McGartland and McKinley 26 , Reference Gibson, Edgar and Neville 28 ), and would have higher levels of hypertrophic adipocytes. In addition, these cells, as well as being responsible for the chronic release of SAA, are one of the main storage sites of lipid-soluble antioxidants( Reference Kayden, Hatam and Traber 39 – Reference Perugini, Bagnati and Cau 41 ), which, in the case of tocopherol, lycopene, lutein and β-cryptoxanthin, have been shown to limit the release of pro-inflammatory cytokines and chemokines from these cells( Reference Gouranton, Thabuis and Riollet 42 – Reference Moussa, Gouranton and Gleize 44 ). Therefore, as lutein, lycopene, β-cryptoxanthin and zeaxanthin concentrations increased to varying degrees in the cohorts of both studies( Reference McCall, McGartland and McKinley 26 , Reference Gibson, Edgar and Neville 28 ), we can deduce that this would increase their incorporation into adipocytes, where they may limit the release of SAA, similar to their ability to limit the release of cytokines and chemokines. This concept was further supported by the fact that BMI was unaltered in the cohorts of both studies, indicating that increased F&V intake may have influenced adipocyte function. However, this proposed mechanism needs to be confirmed through further investigations.
Fruit and vegetable intervention and serum-, HDL2- and HDL3-cholesteryl ester transfer protein activity
CETP is essential for the normal metabolic functioning of HDL, although it has been suggested that when HDL is associated with SAA, its activity may be altered to a proatherogenic phenotype( Reference Jahangiri, de Beer and Noffsinger 11 , Reference Park, Shin and Kim 15 ). This can reduce the ability of HDL to participate in reverse cholesterol transport( Reference Brites, Bonavita and De Geitere 45 , Reference Palmer, Murphy and Graham 46 ). Therefore, the reduction in the activity of CETP in HDL3 in the FAVRIT cohort and in HDL2 in the ADIT cohort and also the negative correlation of this reduction with self-reported F&V intake demonstrate an anti-atherogenic property of increasing F&V intake and/or decreasing SAA concentrations.
F&V intake may have exerted this effect through one or several mechanisms. First, as CETP is also released by adipocytes( Reference Vassiliou and McPherson 47 ), its expression may have been down-regulated by the increase in lipid-soluble antioxidant concentrations, similar to that suggested for SAA. However, to date, only vitamin E has been investigated in this context, with contradictory findings. In one study, no effect was observed( Reference Napoli, Leccese and Palumbo 48 ), while in another study, vitamin E was found to inhibit the activity of CETP, although the latter study was conducted in hamsters( Reference Shen, Novak and Angel 49 ). Second, the increased F&V intake may have reduced the expression of CETP in the liver, thereby lowering the levels available to associate with HDL. Third, increased F&V intake may, via its ability to lower SAA concentrations within HDL fractions, have altered the conformation of HDL and/or CETP, thus reducing their interaction( Reference Qiu, Mistry and Ammirati 50 ). Unfortunately, as a mass assay was not carried out in the present study, it is difficult to confirm whether the amount of CETP was reduced or remained the same, but its activity was found to be reduced. However, regardless of the mechanism, the present study is the first to show that increased F&V intake leads to reduced CETP activity, which may enhance the antiatherogenic properties of this lipoprotein, although this concept needs to be investigated further.
Conclusions
Overall, by carrying out a further analysis on samples from the FAVRIT and ADIT studies in the present study, we have shown that increased F&V intake ( ≥ 5 portions/d) augments serum, HDL2 and HDL3 antioxidant concentrations and have also shown for the first time that such a dietary pattern lowers the concentrations of the inflammatory marker SAA in HDL2 and HDL3, indicating that this marker may be sensitive to changes in F&V intake in hypertensive and older populations. In addition, we have shown that by decreasing the association of SAA with HDL and by reducing the activity of HDL-associated CETP, increased intake of F&V may enhance the antiatherogenic properties of HDL2 and HDL3. These results highlight a dual antioxidant/anti-inflammatory impact of such a dietary pattern, which would probably affect cardiovascular health.
Overall, the results of the present study provide tangible evidence of the effectiveness of increasing F&V intake, which is an encouraging endorsement of the ‘5-a-day’ public health message and may be of use to health policy makers.
Acknowledgements
The authors thank the Food Standards Agency for funding the original grants, NO2029 (FAVRIT study) and NO5067 (ADIT study), which provided the samples for the present study. They also thank D. O. M. and Dr Claire McGartland for managing NO2029 and Dr Andrew Gibson, C. E. N. and Dr Sarah Gilchrist for managing NO5067.
The present study was funded by the Food Standards Agency and the Department of Health (FSA-DH, UK: NO5087). The FSA-DH had no role in the design and analysis of the study or in the writing of this article. The views expressed in this article are those of the author(s) and not necessarily those of the Department of Health.
The authors' contributions are as follows: J. M. designed the study; N. N. conducted the study; J. V. W. and I. S. Y. designed the FAVRIT and ADIT studies, which generated the samples for the present study; C. E. N. and D. O. M. conducted the FAVRIT and ADIT studies; D. M. and D. E. recruited the FAVRIT and ADIT subjects; J. M., J. V. W. and N. N. wrote the article; J. M. had primary responsibility for the final content. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.






