Emerging research suggests that dietary patterns can have a significant impact on vascular function and subsequent effects on health and disease( Reference Vanhoutte 1 ). Vascular endothelial function, most often measured non-invasively by assessing flow-mediated dilation (FMD), has been studied as a surrogate marker for atherosclerosis and an indicator of vascular health and reactivity( Reference Corretti, Anderson and Benjamin 2 ). It has been hypothesised that endothelial function may serve as an integrating index of risk factor burden and genetic susceptibility and that endothelial dysfunction will prove to be a preclinical marker for CVD. However, it remains to be determined whether an improvement in endothelial function directly translates into improved clinical outcomes.
A multitude of factors can affect endothelial function. These include a single energy-dense meal( Reference Vogel, Corretti and Plotnick 3 ), psychological stress( Reference Gu, Tang and Yang 4 ), circulating concentrations of oestrogen and progesterone( Reference Knowlton and Lee 5 , Reference Simoncini 6 ), smoking( Reference Celermajer, Sorensen and Bull 7 – Reference Messner and Bernhard 9 ), acute changes in glucose concentrations( Reference Ceriello, Quagliaro and Piconi 10 , Reference O'Keefe, Gheewala and O'Keefe 11 ), shifts in electrolyte concentrations( Reference Liu, Peng and Lu 12 ) and pharmaceuticals( Reference Bryan 13 ). A variety of dietary ingredients (DI) that are pharmacologically active are known to affect endothelial function including caffeine( Reference Shechter, Shalmon and Scheinowitz 14 ), flavanols found in cocoa( Reference Heiss, Finis and Kleinbongard 15 – Reference Grassi, Mulder and Draijer 17 ), tea( Reference Duffy, Keaney and Holbrook 18 , Reference Vita 19 ) and soya isoflavones( Reference Teragawa, Higashi and Kihara 20 ). An array of DI have been clinically tested and reported to alter endothelial function. DI may alter endothelial function through multiple pathways such as by mediating the release of NO altering cell signalling pathways( Reference Steffen, Jung and Klotz 21 – Reference Shapiro, Lev and Cohen 24 ), decreasing the production of pro-inflammatory cytokine concentrations( Reference Spector, Kaduce and Figard 25 – Reference Massaro, Habib and Lubrano 27 ), down-regulating the expression of cellular adhesion factors( Reference Saravanan, Davidson and Schmidt 28 – Reference De Caterina, Madonna and Massaro 31 ), and increasing or modulating the concentrations of antioxidant enzymes( Reference Guo, Rimbach and Moini 32 – Reference Forstermann and Munzel 34 ) or indirectly by lowering the blood concentrations of homocysteine( Reference Wald, Wald and Morris 35 ).
Systematic reviews and meta-analyses are dependent on the validity and overall strength of the primary studies that are included in the review. It is equally important for researchers to provide an adequate description of the methodology employed in their studies to allow for reproducibility within the field. A number of standardised procedures have been developed to support a credible systematic review involving dietary constituents( Reference Chung, Balk and Ip 36 – Reference Stroup, Berlin and Morton 38 ). We utilised the patient, intervention, comparator and outcome (or PICO) method to formulate our research questions( Reference Lichtenstein, Yetley and Lau 39 ).
Thus, the purpose of this review is to provide a quality assessment of the primary studies reported in the literature carried out using as interventions select DI purported to affect vascular endothelial function. These DI were folic acid, n-3 fatty acids, cocoa and isoflavones. Specifically, this review was designed to answer the following questions:
-
(1) How complete are the descriptions of DI interventions documented in randomised controlled trials measuring vascular function in targeted populations?
-
(2) Do the quality and completeness of reporting vary by the type of journal?
Methods
Process of article selection
The PICO questions guided the systematic search conducted using PubMed, EMBASE and the Cochrane Library collection from January 2000 to August 2012. Keywords in the search string included select vascular endothelium measures, vitamins, trace elements, fatty acids and botanicals (see online supplementary materials for the complete search string). Articles were included in the review if they reported studies carried out in healthy subjects, subjects with risk factors for CVD or clinically stable populations with chronic disease; studies with a randomised controlled trial design; studies in which the DI was consumed orally, in a defined supplement or ingredient form; and studies in which standard vascular assessment measures were reported. In addition, articles were limited to studies using DI that had been included in a published meta-analysis on endothelial dysfunction and considered a topic of public health importance. Studies were excluded if they were not randomised, the intervention was a whole or unrefined food, and the DI was used in combination with a drug therapy or another DI; if there was no dependent endothelial dysfunction measure; or if they were carried out in clinically unstable subjects. Studies reporting inflammatory markers without vascular measures were not included in this review. Abstracts were reviewed manually by two of the authors. Full-text articles were retrieved for manual review if inclusion by abstract was questionable. A total of fifty-two full-text articles were reviewed and excluded as they did not meet the inclusion criteria. Additional studies were also identified manually from published systematic reviews and meta-analyses on the same topic.
Quality scoring parameters
A variety of clinical trial quality tools appropriate for botanical and non-botanical DI intervention studies were employed. All studies were evaluated using the scoring tool of Jadad et al. ( Reference Jadad, Moore and Carroll 40 ), designed to measure bias in clinical trial design (Table 1). For evaluating key methodological questions that should be addressed in DI intervention studies, guidelines for reporting micronutrient and botanical interventions( Reference Chung, Balk and Ip 36 , Reference Gagnier, Boon and Rochon 41 ) were modified to arrive at quantitative scores. For determining a DI quality score, ten key methodological guidelines were selected to use as the basis for the scoring tool. These guidelines were derived either from the Consolidated Standards of Reporting Trials (CONSORT) herbal guidelines for botanicals( Reference Gagnier, Boon and Rochon 41 ) or from the nutritional quality guidelines for the non-botanical DI( Reference Chung, Balk and Ip 36 ). A numerical score of ‘1’ was assigned if the criterion was met and a score of ‘0’ if the criterion was not met (Table 2). A quality score sheet or Corretti score( Reference Corretti, Anderson and Benjamin 2 ) was developed for reporting guidelines for intervention trials utilising the FMD ultrasound technique to measure endothelial function. Furthermore, twelve key methodological guidelines for the ultrasound assessment of the brachial artery proposed by Corretti were selected to use as the basis for the scoring tool (Table 3). A five-point placebo quality score was similarly developed based on the review criteria as reported by Golomb et al. ( Reference Golomb, Erickson and Koperski 42 ) (see online supplementary table). Journal types were tabulated using the National Library of Medicine designation for core clinical journals to assess whether reporting characteristics varied by journal categories and by description of study design and DI intervention. Journal types were sorted by core clinical, nutrition specialty, cardiovascular, not included in core clinical, and medical, not included in core clinical (see online supplementary table). Demographic and background data were extracted by two authors. Jadad, DI and placebo quality scoring was independently performed and scores were recorded by two authors in a blinded fashion. Corretti scores were independently recorded by three authors in a blinded fashion. Discrepancies in scoring were discussed and a consensus was reached.
Table 1 Jadad score for dietary ingredients: number of papers that responded to each of the questions

Table 2 Dietary ingredient (DI) score: number of papers that responded to each of the questions

IUPAC, International Union of Pure and Applied Chemistry.
Table 3 Corretti flow-mediated dilation (FMD) score for dietary ingredients: number of papers that responded to each of the questions

2D, two-dimensional; ECG, electrocardiography.
Statistical analyses
The statistical analyses were performed using SAS 9.2 (SAS Institute, Inc.). Descriptive statistics, means and standard errors were obtained for all the continuous variables and percentages were obtained for all the categorical variables for the following seven groups: (1) folic acid; (2) n-3 fatty acids; (3) nutrient group (folic acid+n-3 fatty acids); (4) cocoa; (5) isoflavones; (6) botanical group (cocoa+isoflavones); (7) nutrient group and botanical group combined. Significance was set at P< 0·05. The SAS general linear model procedure (PROC GLM; SAS Institute, Inc.) was used to conduct ANOVA. Regression analysis was used to compare the folic acid, n-3 fatty acid, cocoa and isoflavone intervention studies, with the folic acid intervention studies being used as the reference group. Outcome data (FMD measures) from individual studies were not pooled for the statistical analyses.
Results
Search results
Four DI that had been the subject of meta-analyses were evaluated: folic acid, n-3 fatty acids, cocoa and isoflavones. These DI met the inclusion criteria (Fig. 1). The DI that were excluded because they did not meet the inclusion criteria included antioxidants, B vitamins, amino acids, vitamin C, vitamin E, α-lipoic acid and those using combination ingredients. This resulted in a dataset of seventy randomised controlled trials: fifteen folic acid( Reference Doshi, McDowell and Moat 43 – Reference Woodman, Celermajer and Thompson 57 ); twenty n-3 fatty acids( Reference Woodman, Mori and Burke 58 – Reference Wong, Yiu and Li 77 ); fifteen cocoa( Reference Balzer, Rassaf and Heiss 78 – Reference Wang-Polagruto, Villablanca and Polagruto 92 ); twenty isoflavones( Reference Chan, Lau and Yiu 93 – Reference Villa, Costantini and Suriano 112 ). These articles provided a description of DI and standardised vascular-dependent measures of endothelial function (see online supplementary materials for the complete data design tables).

Fig. 1 Results of the PubMed search strategy with the resulting seventy studies included in the review. Studies were excluded if the dietary ingredients (DI) evaluated had not been the subject of a meta-analysis and were not randomised, the intervention was a whole or unrefined food, and the DI was used in combination with a drug therapy or another DI; if there was no dependent endothelial dysfunction measure; or if the study sample comprised clinically unstable subjects. RCT, randomised controlled trials.
Description of the dataset
A total of 3959 randomised subjects, mean age 51 (se 0·21) years (range 9–79 years), were represented in the dataset. Of the studies included, 66 % had healthy subjects enrolled, with the majority including both men and women. Approximately one-third (36 %) of the subjects continued using concomitant medications during the study period. Cross-over design studies accounted for 47 % of those included in the dataset. The most common measure used to evaluate vascular endothelial function was FMD at 81·4 %; other measures (e.g. pulse wave velocity, forearm arterial blood flow, augmentation index, ankle-brachial pressure index, cardio-ankle vascular index, fingertip pulse wave amplitude and hyperaemic response) were seldom used in the studies (Table 4).
Table 4 Vascular-dependent measures of endothelial function
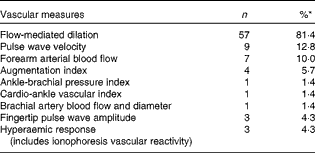
* Will not add to 100 %, as several studies used multiple measures.
Quality scoring parameters
The overall mean Jadad score was 3·50 (se 0·13) (maximum score of 5). The mean Jadad scores were 3·53 (se 0·21), 3·40 (se 0·25), 3·30 (se 0·36) and 3·70 (se 0·20) for the folic acid, n-3 fatty acid, cocoa and isoflavone intervention studies, respectively, and did not differ statistically among the supplement studies (Table 1). Studies achieving a Jadad score ≥ 3 were considered to be free of bias and therefore of higher quality. A Jadad score of 5 was achieved by nine studies.
The overall mean DI score was 5·40 (se 0·25) out of 10. Studies carried out on the four DI evaluated in this review achieved an individual DI quality reporting score. The mean DI quality scores were 4·13 (se 0·34), 5·20 (se 0·47), 6·13 (se 0·41) and 6·00 (se 0·59) for the folic acid, n-3 fatty acid, cocoa and isoflavone intervention studies, respectively (Table 2 and Fig. 2). Of the articles reviewed, 73 % had included the DI source or supplier, 64 % included the intervention dosage and study duration, 57 % included the baseline nutrient biomarker assessment, and only 39 % included the baseline nutrient assessment. A statistically significant higher DI quality score was achieved by botanical supplement studies than by nutrient supplement studies (P= 0·01). Only thirteen studies achieved a DI quality score ≥ 8 and one study achieved a score of 10.

Fig. 2 Mean dietary ingredient (DI) quality scores for the folic acid, n-3 fatty acid, cocoa and isoflavone intervention studies. * DI score was significantly different from that of the cocoa intervention studies (P= 0·04). † DI score was significantly different from that of the isoflavone intervention studies (P= 0·03).
Studies utilising folic acid as the intervention most consistently used 5 mg/d (range 400 μg–1600 mg); one study used folic acid in the form of 5-methyltetrahydrofolate. None of the articles reporting folic acid intervention studies provided the specific chemical formula for the DI used (e.g. sodium folate or calcium folate). Placebo or comparator ingredients were poorly characterised. The duration of studies ranged from 1 to 52 weeks, and blood folic acid concentrations were determined before or after the intervention period in nine of the fifteen studies.
The n-3 fatty acid intervention studies included trials utilising fish-oil mixtures containing both DHA and EPA, capsules containing DHA alone, or capsules containing EPA alone. Of the articles reporting n-3 fatty acid intervention studies, 60 % provided the specific chemical form for the DI used (e.g. ester or TAG). The doses of fish-oil mixtures ranged from 1·0 to 4·0 g/d; DHA from 290 mg to 1·5 g/d, and EPA from 300 to 1800 mg/d. The duration of studies ranged from 2 to 152 weeks. Olive oil was the predominant comparator oil utilised.
A wide range of interventions containing cocoa (Theobroma cacao) flavanols were utilised including beverages, bars and powder. The chemical composition of the DI was clearly defined in 87 % of the articles. The doses of flavanols ranged from 88 to 963 mg/d and studies lasted from 2 d to 12 weeks. Acute sampling or sampling at multiple time points within 1 d of study was performed in five trials included the dataset. The comparators used in the cocoa intervention studies were typically low-flavonoid preparations.
Unlike in the cocoa intervention studies, the chemical compositions of the DI used in the soya isoflavone (Glycine max) intervention studies were poorly described. Intervention DI included a range of isoflavones as purified capsules, powder, spread, tablets and fortified soya germ pasta. Comparator formulations were varied and included matching placebo, components not specified, caseinate protein powder, milk protein powder, cereal bars or conventional pasta. Doses ranged from 33 to 200 mg/d and studies lasted from 2 to 56 weeks. Only four (20 %) of the studies provided information on the amounts of the glycoside form (as genistin, daidzin or glycitin) as well as the aglycone form (as genistein, daidzein or glycitein) of the isoflavone present in the intervention.
A positive vascular outcome was reported as determined by the study investigators. FMD, assessed by brachial artery ultrasound, was used as the primary outcome measure in fifty-seven (81 %) of the seventy trials. A positive FMD outcome was reported in 61 % of the articles (93 % for cocoa, 62 % for n 3-fatty acids, 48 % for isoflavones and 46 % for folic acid). Differences were found between cocoa and folic acid intervention studies (P= 0·02) and cocoa and isoflavone intervention studies (P= 0·02) (Fig. 3). The overall mean Corretti score for the fifty-seven studies utilising the FMD methodology was 7·01 (se 0·32) out of 12. The mean Corretti scores were 7·27 (se 0·56), 7·46 (se 0·79), 6·29 (se 0·61) and 7·11 (se 0·56) for the folic acid, n-3 fatty acid, cocoa and isoflavone intervention studies, respectively. The nutrient DI (folic acid and n-3 fatty acid) studies had slightly higher Corretti scores (7·34 (se 0·49)) than the botanical DI (cocoa and isoflavone) studies (6·76 (se 0·41)). There were no statistically significant differences among the supplement studies (Table 3). Over 90 % of the studies failed to adequately describe the equipment used to measure FMD and more than half of the studies failed to provide an adequate description of the procedures used for vascular image acquisition and measurement. Lastly, one-third of the studies did not have adequate sample size for determination of the primary outcome measure of FMD by the Corretti guidelines.

Fig. 3 Percentage of articles reporting a positive flow-mediated dilation outcome as determined by the investigator. * Score was significantly different from that of the folic acid intervention studies (P= 0·02). † Score was significantly different from that of the isoflavone intervention studies (P= 0·02).
Overall, placebo controls and/or comparators were poorly described; only 41 % of the included articles had detailed descriptions of the placebo treatment and only 31 % of the articles had adequate descriptions of all its constituents. The rationale for the type of control or placebo used was also lacking, as well as its physical appearance and likeness to the intervention under study (see online supplementary materials for the placebo scoring sheet).
Journal characteristics
Journal types were sorted by core clinical (29 %), nutrition related (20 %), other cardiovascular (29 %) that were not included in core clinical and other medical (23 %) that were also not included in core clinical. Core clinical journal type was defined using the National Library of Medicine definition( 113 ). The distribution of DI articles across the journal categories was somewhat uniform (see online supplementary materials for journal listing). No association was evident between journal type and Jadad or Corretti score, but a significant association was evident between journal type and DI quality score. The DI quality score for studies reported in core clinical journals was 5·30 (se 0·77). Studies reported in nutrition-related journals achieved the highest DI quality score (mean 7·00 (se 0·84)), and the scores of studies reported in these journals were significantly different from those of studies reported in other cardiovascular journals (mean 4·85 (se 0·57); P= 0·02) and other medical journals (mean 4·81 (se 0·56); P= 0·02). Of the thirty-five journals, eleven (28 %) included endorsement of the CONSORT guidelines.
Subgroup analyses
Subgroup analyses by clinical trial design features revealed non-cross-over FMD studies, independent of the DI, to have significantly higher Jadad scores than cross-over studies (3·76 (se 0·14) v. 3·21 (se 0·20); P= 0·03). The analysis of trial design by DI quality score or Corretti score revealed no differences between non-cross-over and cross-over studies. No association was evident between the population group (primary v. secondary) and the Jadad, DI quality or Corretti score.
Discussion
The DI selected in this quality review have been shown to alter endothelial function and have been studied extensively as well as included in meta-analyses. To our knowledge, this is the first review to evaluate the quality reporting of a select group of DI interventions and corresponding comparators for endothelial dysfunction. In addition, a scoring system was developed to evaluate the reporting of the methodology used to assess FMD and endothelial function, which is not typically done in systematic reviews and meta-analyses. Other reviews and meta-analyses have addressed issues of bias, dose–response effects and sensitivity analysis in concert with a pooled meta-analysis of the data on cocoa( Reference Hooper, Kay and Abdelhamid 114 , Reference Shrime, Bauer and McDonald 115 ), n-3 fatty acids( Reference Pase, Grima and Sarris 116 , Reference Wang, Liang and Wang 117 ), soya isoflavones( Reference Beavers, Beavers and Miller 118 ) and folic acid( Reference McRae 119 , Reference de Bree, van Mierlo and Draijer 120 ), and most of them have concluded that supplementation improved the parameters of endothelial function. We have shown that the peer-reviewed literature is significantly deficient in providing an adequate description of the background and intervention diets as evaluated previously in a critical appraisal of the reporting of systematic reviews of micronutrients and health( Reference Chung, Balk and Ip 36 ). Although the use of quality measures or scoring tools does not necessarily correlate with improved outcomes or the strength of treatment effects across studies, these measures may be appropriate in specific well-defined areas of study and offer some benefits( Reference O'Keefe, Gheewala and O'Keefe 11 , Reference Moher, Cook and Eastwood 37 , Reference Balk, Bonis and Moskowitz 121 , Reference Moher, Pham and Jones 122 ).
Nutrient-specific guidance has been proposed for designing, implementing and reporting clinical studies specifically for soya interventions. It is recommended that the investigator know and report the product source and supplies; analyse, describe and report all the potential bioactive constituents relevant to the study; conduct independent analyses of test and placebo agents; express isoflavone values as aglycone equivalents or present isoflavone values with sufficient information so that they can be readily converted to aglycone equivalents for cross-study comparisons; and refer to the CONSORT guidelines for reporting herbal interventions( Reference Klein, Nahin and Messina 123 ). Similar information for other DI intervention studies is essential to improve the accuracy and enhance the quality of the studies.
The Jadad scores were comparable across the DI studies, with the majority of the studies being of good quality. Higher DI quality scores were achieved by studies using the botanical ingredients cocoa and isoflavones than by those using the nutrient ingredients folic acid and n-3 fatty acids. For cocoa, this may be reflected by repeated use of the same highly characterised product under study. DI quality scores, particularly for folic acid interventions, were significantly affected by the lack of validation of the test materials and lack of documentation of the baseline diet. Many of these studies used pharmacological doses of folic acid for indications of lowering plasma homocysteine concentrations. As expected, the descriptions of DI interventions were more complete in studies reported in nutrition journals. Given the small numbers of studies conducted for each DI, comparison across individual journals was not possible.
Although systematic reviews are prevalent in the literature, there are unique challenges to applying this approach to the field of nutrition science. Nutrition-related considerations include baseline nutrient exposure, nutrient status, bioequivalence of bioactive compounds, bioavailability, multiple and interrelated biological functions, undefined nature of some interventions and uncertainties in dietary intake assessment. In addition, the methodological quality of the primary literature upon which the systematic reviews are based is often poor or inadequately reported, as the individual studies often do not report information that is critical to interpret their findings or to replicate the study. Standards are needed to improve the conduct and reporting of systematic reviews in the field of nutrition science. The quality of clinical trial design can be evaluated and reported in the peer-reviewed literature using various reporting tools. Quality reporting tools for trial design, Jadad scores, and characterisation of botanical and nutrient test materials were used in this review. A nutrient quality score was derived from the proposed Agency for Healthcare Research and Quality guidelines as reported by Chung et al. ( Reference Chung, Balk and Ip 36 ). The 2006 revised CONSORT statement( Reference Gagnier, Boon and Rochon 41 ) guides reporting of randomised control trials of herbal medicine interventions to include the product name, characteristics of herbal product, and qualitative and quantitative testing of the products. Several of these key features were incorporated into the nutrient scoring tool. We relied heavily on the procedure guidelines proposed by Corretti et al. ( Reference Corretti, Anderson and Benjamin 2 ) for evaluating the quality of studies utilising ultrasound measures of FMD, as they were available to most researchers encompassed in this literature review.
Brachial artery FMD provides a non-invasive measure of endothelial function and specific guidelines have been developed to ensure the accuracy and quality of the measurement. The key methodological characteristics of the assessment of endothelial function that were reported in DI intervention studies were documented in this systematic review. The details of the methodology used to assess the outcome measure of FMD were also scarce in the studies reviewed. The procedures used for the measurement of FMD were not uniformly reported in standardised formats or according to the recommended guidelines( Reference Corretti, Anderson and Benjamin 2 ), which adds potential variability and confounding to the measurement of endothelial function. Interestingly, there was under-reporting of specific anatomical imaging sites, CV for test reproducibility and blinding of study personnel to image analysis in the studies. General recommendations from the guidelines for ultrasound assessment of the brachial artery include twenty to thirty subjects for cross-over studies and forty to sixty subjects for parallel-group design studies( Reference Corretti, Anderson and Benjamin 2 ). FMD assessment has become a standard tool and non-invasive method to measure endothelial function( Reference Celermajer, Sorensen and Bull 7 ). FMD correlates with endothelial function of the coronary arteries( Reference Anderson, Uehata and Gerhard 124 ). Brachial artery FMD is reportedly an independent predictor of cardiovascular events( Reference Gokce, Keaney and Hunter 125 – Reference Brevetti, Silvestro and Schiano 127 ). However, this has not been demonstrated in other studies( Reference Fathi, Haluska and Isbel 128 , Reference Frick, Suessenbacher and Alber 129 ). The controversy may be due to the accuracy and variability in the measurement of brachial artery FMD. Factors including expertise of the sonographer, type of ultrasound equipment and technology used, subject preparation, vascular occlusion location, environmental conditions in the room where measurements are taken and method of analyses can influence the measurement of brachial artery FMD. These limitations are revealed by dietary intervention studies measuring brachial artery FMD included in this review.
Guidelines for the ultrasound assessment of brachial artery FMD have been developed( Reference Corretti, Anderson and Benjamin 2 , Reference Deanfield, Donald and Ferri 130 , Reference Thijssen, Black and Pyke 131 ). With the advancement of ultrasound technology and widespread clinical applications for testing, these guidelines and recommendations will assist clinicians and researchers in the accurate measurement of endothelial function. This methodology can help track individuals on dietary interventions that have the potential to decrease the risk of CVD. It is broadly applicable to patients because it is non-invasive, stable and reliable( Reference Welsch, Allen and Geaghan 132 ). Ultrasound assessment of brachial artery FMD is also ideal in dietary studies with serial measurements and short-term or long-term durations. However, future studies using this technique need to be standardised using the guidelines for assessment to increase accuracy and minimise all the potential variables.
Limitations of the review
The focus of this review was on the reporting quality of DI purported to affect vascular endothelial function in clinical trials reported in the peer-reviewed literature. Therefore, the degree of reporting bias was not assessed. We were interested in determining whether DI intervention studies included in meta-analyses assessing FMD were adequately and appropriately characterised. We made an attempt to correlate DI quality scores with FMD outcomes. Unfortunately, it was not possible to include a more robust measure of FMD outcome, such as percentage change in FMD, as not all articles uniformly reported FMD outcomes in a similar manner or in similar units. Therefore, the outcome of a ‘positive’ or ‘negative’ FMD study based on the investigator's assessment should be treated with caution. The quality scoring tools used for the determination of a nutrient score, a Corretti score and a placebo score are presented as guidelines for the inclusion of pertinent information for DI intervention studies evaluating FMD; these tools have not been validated yet.
Conclusions
The purpose of this quality review was to highlight the need for accurate and complete reporting by clinical researchers so that their work can be communicated succinctly and replicated by others. The descriptive and statistical treatment of the data suggests weaknesses in the study design and conduct of the study, based on what was reported in the articles. The clinical relevance of this systematic review is that the DI reviewed had favourable effects on endothelial dysfunction as determined by the investigator's assessment of a positive or negative study. Lastly, guideline papers are available for reference to assist investigators in designing DI intervention studies with greater methodological controls and enhanced reporting in peer-reviewed journals.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114514003353
Acknowledgements
The authors thank Joyce Merkel for her technical and editorial contributions and Susan Pilch for conducting the literature searches.
The Office of Dietary Supplements, National Institutes of Health, funded and sponsored this work.
The authors’ contributions are as follows: R. B. C. designed the analysis and performed data extraction and grading of quality scores; C. V. L. contributed to data collection and analysis; L. S., M. B. E. and M. M. E. performed data extraction and grading of quality scores; P. S. contributed to data extraction; C. T. S. performed the statistical analyses. All authors contributed to the drafting of the article and read, reviewed and approved the final manuscript.
The authors report no conflicts of interest. The opinions or assertions reported herein are the those of the author(s) and are not to be construed as official or reflecting the views of the National Institutes of Health, the National Heart, Lung, and Blood Institute, the National Institute of Nursing Research, or the Office of Dietary Supplements.









