Sarcopenia is a progressive and generalised skeletal muscle disorder that is associated with increased likelihood of adverse outcomes including falls, fractures, physical disability and mortality(Reference Cruz-Jentoft, Bahat and Bauer1–Reference Zhang, Wang and Dou3). The diagnosis of sarcopenia is based on two or three of the following components: (a) low muscle mass, (b) low muscle strength and (c) low physical performance(Reference Polyzos and Margioris4). Each of these components can be evaluated by several methods and classified by different cut points(Reference Dam, Peters and Fragala5–Reference Prado, Purcell and Alish8).
The European Working Group on Sarcopenia in Older People published an original definition for sarcopenia in 2010 (EWGSOP)(Reference Cruz-Jentoft, Baeyens and Bauer6) and a revised version in 2018 (EWGSOP2)(Reference Cruz-Jentoft, Bahat and Bauer1). The Foundation of the National Institutes of Health (FNIH)(Reference Studenski, Peters and Alley9), the Asian Working Group on Sarcopenia (AWGS)(Reference Chen, Liu and Woo10) and the International Working Group on Sarcopenia(Reference Fielding, Vellas and Evans11) also published guidelines for sarcopenia diagnosis. Sarcopenia definition varies based on these guidelines and an unique definition is not yet available(Reference Dennison, Sayer and Cooper12,Reference Kim, Jang and Lim13) . Therefore, sarcopenia prevalence varies widely depending on the criteria used. Additionally, body composition variables including muscle mass, and muscle function parameters used for sarcopenia diagnosis, may vary according to ethnic diversity(Reference Woo, Arai and Ng14,Reference Du, Goates and Arensberg15,Reference Heo, Faith and Pietrobelli16) .
Primary sarcopenia is caused by ageing itself, whereas secondary sarcopenia is caused by disuse, systemic diseases including chronic kidney disease (CKD) and inadequate nutrition(Reference Cruz-Jentoft, Bahat and Bauer1,Reference Choi17,Reference Scherbakov and Doehner18) . The risk of skeletal muscle loss and dysfunction should also be considered in people with obesity, especially in individuals whose age is greater than 65 years or with concomitant metabolic complications, chronic diseases, acute complications or on long-term glucocorticoid treatment(Reference Barazzoni, Bischoff and Boirie7).
While individuals with obesity have increased body adiposity, they may also have increased muscle mass. However, significant changes in muscle metabolism, impairment of muscle strength and endurance may result from the high body adiposity(Reference Barazzoni, Bischoff and Boirie7,Reference Cava, Yeat and Mittendorfer19) in this population. The reduced physical activity inherent in sarcopenia contributes to the development of obesity. While the excessive adiposity, especially in the form of visceral fat, is associated with inflammation, which is an important risk factor for sarcopenia. Obesity and sarcopenia share common pathophysiological mechanisms, such as increased pro-inflammatory cytokines, oxidative stress, insulin resistance and hormonal alterations(Reference Choi17). The joint occurrence of obesity and sarcopenia may potentiate such conditions, and the European Society for Clinical Nutrition and Metabolism and the European Association for the Study of Obesity recognise the scientific and clinical importance of the simultaneous occurrence of obesity and sarcopenia(Reference Polyzos and Margioris4,Reference Barazzoni, Bischoff and Boirie7,Reference Choi17,Reference Marty, Liu and Samuel20) .
Kidney transplantation is the treatment of choice for end-stage renal disease(Reference Magee and Pascual21). Renal transplant recipients (RTR) may present an increased risk of sarcopenia due to factors related to the pretransplant period such as dialysis therapy, metabolic acidosis and inflammation(Reference Fahal22). Obesity is a common condition after kidney transplantation and occurs in up to 50 % of patients. Obesity may precipitate the development of sarcopenia(Reference Chan, Bosch and Jones23) as well as the use of immunosuppressive drugs mainly corticosteroids(Reference Dennison, Sayer and Cooper12,Reference Hasselgren, Alamdari and Aversa24,Reference Dasarathy25) . Although there is evidence of a decrease in lean body mass(Reference Netto, Alves-Filho and Mazzali26,Reference Harada, Nakamura and Hotta27) , the prevalence of sarcopenia and its relationship with body adiposity in RTR is still not completely understood.
To our knowledge only four studies evaluated the presence of sarcopenia in RTR(Reference Ozkayar, Altun and Halil28–Reference Małgorzewicz, Wołoszyk and Chamienia31). Three of these studies were conducted in Asia(Reference Ozkayar, Altun and Halil28–Reference Yanishi, Tsukaguchi and Kimura30) and one in Europe(Reference Małgorzewicz, Wołoszyk and Chamienia31). None used the EWGSOP, EWGSOP2 or FNIH criteria. The study with the largest number of participants (n 166), used only muscle strength to diagnose sarcopenia(Reference Ozkayar, Altun and Halil28). Two studies used the AWGS criteria and included less than sixty participants(Reference Yanishi, Kimura and Tsukaguchi29,Reference Yanishi, Tsukaguchi and Kimura30) . The most recent study evaluated only overweight individuals (BMI ≥ 25 kg/m2) (n 70)(Reference Małgorzewicz, Wołoszyk and Chamienia31). Therefore, the aim of the present study was to evaluate the prevalence of sarcopenia and its components according to EWGSOP, EWGSOP2 and FNIH criteria in adult RTR not classified as underweight according to BMI. As a secondary objective, we investigated the relationship of sarcopenia and its components with total and abdominal body adiposity.
Methods
This cross-sectional study was conducted in RTR under regular treatment at the renal transplant outpatient clinic at Pedro Ernesto University Hospital (Rio de Janeiro State University – Rio de Janeiro, Brazil). The present study followed the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Committee on Ethics and Research of Pedro Ernesto University Hospital (CAAE: 50747615.4.0000.5259). Written informed consent was obtained from all participants.
The following population was recruited for the study: men and women aged between 18 and 65 years, who had received a kidney transplant at least 6 months before inclusion in the study and who were regularly using corticosteroids as part of their immunosuppressive regimen. The exclusion criteria were diagnosis of AIDS, cancer, autoimmune diseases, acute illness, amputation, liver failure and mental disorders; pregnant or lactating women; RTR undergoing dialysis; BMI < 18·5 kg/m2; and inability to walk 6 m.
Individuals who met eligibility criteria and agreed to participate in the study were submitted to clinical, nutritional and laboratory evaluations. Anthropometric measurements and blood collection were performed from 07.00 to 09.00 hours after a 12-h fasting period. Data collected from patient chart included date of transplantation, type of graft donor, co-morbidities and current use of drugs. During an interview, participants were asked about the renal replacement therapy prior to transplantation and lifestyle habits. Participants who reported smoking at least one cigarette daily or those who stopped smoking within the previous 6 months were classified as smokers. Participants who reported consumption of alcoholic beverages one or more times/week were considered alcohol consumers. Habitual physical activity was evaluated using the Baecke questionnaire. This validated questionnaire assesses physical activity at three subscales, namely, at work, sports during leisure time and other physical activities during leisure time(Reference Baecke, Burema and Frijters32,Reference Florindo and Latorre33) . The Brazilian Institute of Geography and Statistics endorses categories according to skin colour white, brown, black, yellow and indigenous, given the high population miscegenation(Reference Petrucelli and Saboia34).
Anthropometric assessment
The anthropometric measurements were performed by two experienced renal dietitians. Height was measured using a stadiometer accurate to ±0·5 cm, and weight was obtained with a digital scale accurate to ±0·1 kg (Filizola S.A.), after participants wearing light clothing, with no shoes, attempted to empty their bladder. BMI was calculated using the standard equation (kg/m2)(35).
Waist circumference (WC) was measured in the standing position midway between the lowest rib and the iliac crest at mid-exhalation(36). Anthropometric measurements were taken twice, and the mean values were used. Waist:height ratio (WHtR) was obtained by dividing WC (cm) by height (cm).
Participants with BMI ≥ 30 kg/m2 were classified as obese(35). Abdominal obesity was defined according to the following criteria: (1) WC > 90 cm in men and >80 cm in women(Reference Alberti, Eckel and Grundy37) and (2) WHtR > 0·52 in men and >0·53 in women(Reference Pitanga and Llessa38).
Dual-energy X-ray absorptiometry
Dual-energy X-ray absorptiometry (DXA) procedure was performed by a trained technician using a GE Medical Systems Lunar® with the participant in the supine position. The DXA system performs rectilinear scans over the length of the body. The scan begins at the top of the participant’s head and moves downward toward the feet. The program allows scanning up to 205 lines. During the scan, the source shutter opens to emit an X-ray beam. The software calculates fat mass, lean tissue and bone mineral mass. Fat-free mass is calculated as the sum of lean tissue plus bone mineral mass. Body composition was evaluated in whole body and different sites such as trunk. Obesity according to the percentage of total body fat (% BF) was defined using the cutoffs proposed by Heo et al. (Reference Heo, Faith and Pietrobelli16).
Laboratory parameters
Blood samples were analysed to measure creatinine and albumin. These analyses were performed at the University Hospital’s central laboratory. Serum creatinine was determined by kinetic method (creatinine calibrated to IDMS: COBAS 6000 (Roche/Hitachi)). Serum concentration of albumin was determined by colorimetric method. The glomerular filtration rate was estimated (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration equation(39).
Muscle mass
Muscle mass was evaluated using the appendicular skeletal muscle mass (ASM) obtained with DXA and estimated as the sum of muscle mass of the four limbs. The skeletal muscle mass index (SMI) was determined as ASM divided by height (m2) (SMI/ht2) as recommended by the EWGSOP(Reference Cruz-Jentoft, Baeyens and Bauer6) and EWGSOP2(Reference Cruz-Jentoft, Bahat and Bauer1), and as ASM divided by BMI (SMI/BMI) as recommended by FNIH(Reference Studenski, Peters and Alley9). Low muscle mass was defined according to (1) EWGSOP as SMI/ht2 < 7·26 kg/m2 in men and <5·5 kg/m2 in women(Reference Cruz-Jentoft, Baeyens and Bauer6); (2) EWGSOP2 as SMI/ht2 < 7·26 kg/m2 in men and <5·5 kg/m2 in women(Reference Cruz-Jentoft, Bahat and Bauer1); and (3) FNIH as SMI/BMI < 0·789 in men and <0·512 in women(Reference Studenski, Peters and Alley9).
Muscle strength
Muscle strength was assessed by hand grip strength (HGS) using a handheld dynamometer (Baseline® Smedley Spring Dynamometer; Fabrication Enterprises Inc.), according to the protocol recommended by the American Association of Hand Therapists(Reference Roberts, Denison and Martin40). Participants were first familiarised with the device and were then evaluated seated, shoulders adducted and neutrally rotated, elbow flexed at 90°, forearm in neutral and wrist between 0 and 30° of dorsiflexion. Participants were instructed to grip the dynamometer with the maximum strength in response to a voice command. Measurements were repeated at 1 min intervals and obtained three times for each hand in a rotational way. The highest value of three measurements in each hand was considered for the study. Low muscle strength was diagnosed according to (1) EWGSOP as HGS <30 kg in men and <20 kg in women(Reference Cruz-Jentoft, Baeyens and Bauer6), (2) EWGSOP2 as HGS <27 kg in men and <16 kg in women(Reference Cruz-Jentoft, Bahat and Bauer1) and (3) FNIH as HGS <26 kg in men and <16 kg in women(Reference Studenski, Peters and Alley9).
Physical performance
Physical performance was evaluated by usual gait speed (m/s). Participants were asked to stand stationary with their feet behind a starting line marked with tape, then, following the examiner’s command of ‘Go’, to walk at their usual pace over a 6-m course and to stop just past the finish line. Timing was started with the first foot fall and stopped when participant’s first foot completely crossed the 6-m end line. The faster of two trials (in m/s) was used for the present analyses(Reference Cesari, Kritchevsky and Newman41). Low physical performance was defined as usual gait speed <0·8 m/s according to EWGSOP(Reference Cruz-Jentoft, Baeyens and Bauer6)and FNIH(Reference Studenski, Peters and Alley9)and as gait speed ≤ 0·8 m/s according to EWGSOP2(Reference Cruz-Jentoft, Bahat and Bauer1).
Sarcopenia diagnosis
The diagnosis of sarcopenia was made according to the three-guideline criteria of low muscle mass, low muscle strength and low physical performance based on the respective cutoff values mentioned above.
• EWGSOP criteria: low muscle mass + low muscle strength and/or low physical performance(Reference Cruz-Jentoft, Baeyens and Bauer6).
• EWGSOP2 criteria: low muscle strength + low muscle mass(Reference Cruz-Jentoft, Bahat and Bauer1).
• FNIH criteria: low muscle strength + low muscle mass(Reference Studenski, Peters and Alley9).
Participants were stratified into two groups according to the presence of sarcopenia using these three-guideline recommendations (EWGSOP, EWGSOP2 and FNIH): sarcopenia group (with sarcopenia) and control group (without sarcopenia).
Statistical methods
The total base cohort population of RTR, followed in Pedro Ernesto University Hospital, is approximately 450. Considering 20·5 % frequency of sarcopenia observed on the study conducted by Ozkayar et al. (Reference Ozkayar, Altun and Halil28), an α error = 0·05 and β error = 0·20, the minimum sample size should be 160 participants (within 95 % CI).
Categorical variables were expressed as percentages and compared by the χ 2 test. Normality was tested by the Shapiro–Wilk normality test, and skewed data were log transformed to improve normality. Mean values and standard errors were used to summarise continuous variables with normal distribution, while medians and interquartile intervals were used to summarise variables with non-normal distribution. The differences between groups were analysed using either Student’s t test or the Mann–Whitney test, as appropriate. Multiple logistic regressions were performed to assess the association of sarcopenia with the presence of excess body adiposity.
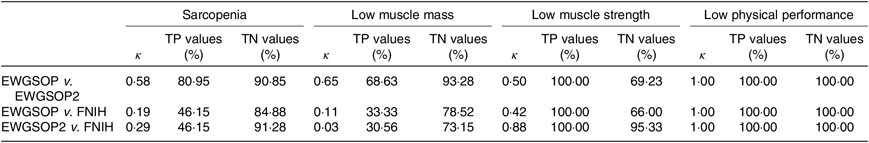
The κ test was used to evaluate the agreement between the diagnosis of sarcopenia, low muscle mass, low muscle strength and low physical performance using the three different criteria (EWGSOP, EWGSOP2 and FNIH). Considering the κ values, the degree of agreement was classified as none (0), slight (0–0·2), fair (0·2–0·4), moderate (0·4–0·6), substantial (0·6–0·8) or almost perfect (0·8–1·0)(Reference McGinn, Wyer and Newman42). The true positive values (sensitivity) and the true negative values (specificity) were assessed by the receiver–operator curve analysis.
All statistical analyses were performed using STATA12.0 software (StataCorp LP). A P value <0·05 was considered statistically significant.
Results
Out of 337 interviewed volunteers, 187 met the eligibility criteria and agreed to participate, 185 completed all the study protocol and thus were included in the statistical analysis. The participants’ median age was 50·0 (range 18–65) years and 57 % (n 106) were males. The mean eGFR was 55·80 (se 1·52) ml/min per 1·73 m2 and the time from transplantation was 117·0 (range 6–493) months.
The prevalence of sarcopenia, low muscle mass, low muscle strength and low physical performance according to the three guidelines are presented in Fig. 1. The prevalence of sarcopenia according to the three criteria was 7 % (FNIH), 11 % (EWGSOP2) and 17 % (EWGSOP). The frequency of low muscle mass ranged from 19 to 28 %, according the three criteria used, while the prevalence of low muscle strength showed a wider range from 18 to 46 %. The low physical performance prevalence was similar among the three criteria (10 %).

Fig. 1. Prevalence of sarcopenia, low muscle mass, low muscle strength and low physical performance according to European Working Group on Sarcopenia in Older People (EWGSOP; ![]() ), European Working Group on Sarcopenia in Older People revised version in 2018 (EWGSOP2;
), European Working Group on Sarcopenia in Older People revised version in 2018 (EWGSOP2; ![]() ) and Foundation of the National Institutes of Health (FNIH;
) and Foundation of the National Institutes of Health (FNIH; ![]() ) criteria in renal transplant recipients.
) criteria in renal transplant recipients.
The agreement, according to κ values, in the diagnosis of sarcopenia, low muscle mass and low muscle strength between (1) EWGSOP and EWGSOP2 criteria was moderate, substantial and moderate, respectively; (2) EWGSOP and FNIH criteria was slight, slight and moderate, respectively; and (3) EWGSOP2 and FNIH criteria was fair, slight and almost perfect, respectively. The highest true positive and true negative values for the diagnosis of sarcopenia and low muscle mass were observed for EWGSOP v. EWGSOP2 and for the diagnosis of low muscle strength were observed for EWGSOP2 v. FNIH. The agreement in the diagnosis of low physical performance was almost perfect among the criteria used, as all participants were included in the same classification using the three criteria and the true positive and negative values were 100 % (Table 1).
Table 1. κ, True positive (TP) and true negative (TN) values for agreement in the diagnosis of sarcopenia, low muscle strength, low muscle mass and low physical performance, according to the European Working Group on Sarcopenia in Older People (EWGSOP), European Working Group on Sarcopenia in Older People revised version in 2018 (EWGSOP2) and Foundation of the National Institutes of Health (FNIH) criteria (κ Values and percentages)
The participants’ demographic and clinical characteristics according to the presence of sarcopenia are summarised in Table 2. Considering the EWGSOP and the EWGSOP2 criteria, lower physical activity (total score) was observed in participants presenting sarcopenia compared with those without sarcopenia. According to the FNIH criteria, participants with sarcopenia were significantly older (Table 2).
Table 2. Demographic and clinical characteristics according to the diagnosis of sarcopenia (European Working Group on Sarcopenia in Older People (EWGSOP), European Working Group on Sarcopenia in Older People revised version in 2018 (EWGSOP2) and Foundation of the National Institutes of Health (FNIH) criteria) in renal transplant recipients
(Mean values with their standard errors for normal distributions; medians and interquartile ranges for non-normal distributions; absolute values and percentages)
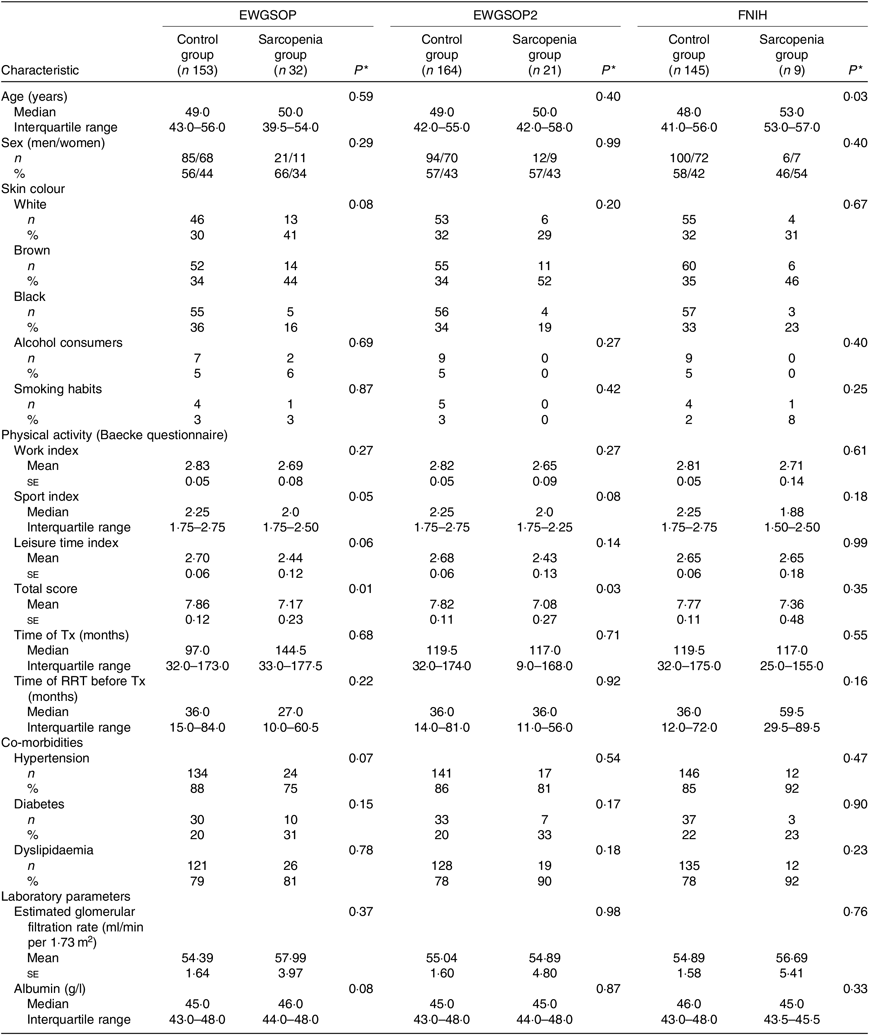
Tx, transplantation; RRT, renal replacement therapy.
* P values refer to control group v. sarcopenia group.
Body adiposity parameters evaluated by anthropometry (BMI, WC and WHtR) and DXA (% BF and percentage trunk fat) were significantly lower in the sarcopenia group compared with the control group, according to the EWGSOP and the EWGSOP2 criteria (including both sexes in the analyses) (Table 3). Conversely, according to the FNIH criteria, sarcopenia group compared with control group (including both sexes in the analyses) presented significantly higher values for WHtR (Table 3).
Table 3. Parameters of body adiposity according to the diagnosis of sarcopenia (European Working Group on Sarcopenia in Older People (EWGSOP), European Working Group on Sarcopenia in Older People revised version in 2018 (EWGSOP2) and Foundation of the National Institutes of Health (FNIH) criteria) in renal transplant recipients
(Mean values with their standard errors for normal distributions; medians and interquartile ranges for non-normal distributions)
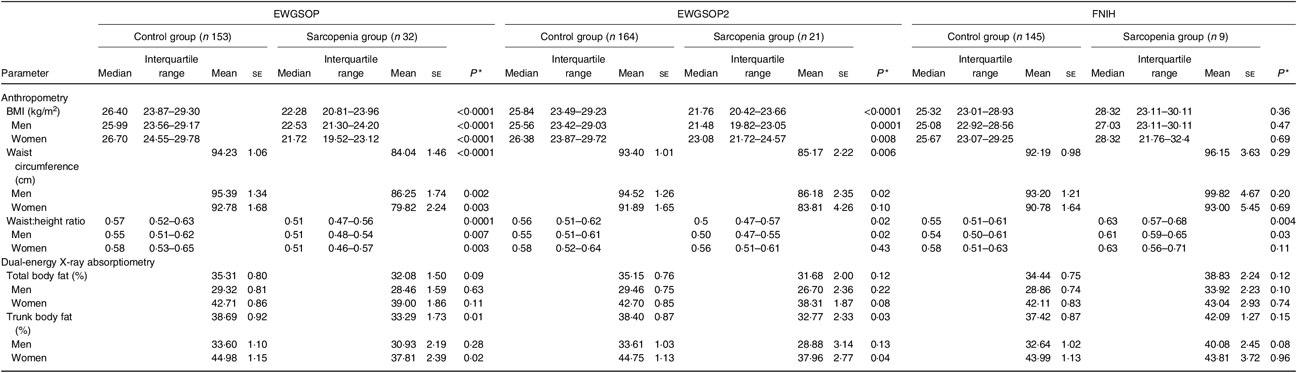
* P values refer to control group v. sarcopenia group.
The frequency of sarcopenia, low muscle mass, low muscle strength and low physical performance according to the presence of excessive body adiposity is shown in Table 4. The frequency of sarcopenia defined by the EWGSOP and the EWGSOP2 criteria was significantly lower in individuals classified as presenting excessive total body adiposity (overweight and obesity) evaluated by BMI. The frequency of sarcopenia defined by EWGSOP was lower in individuals presenting abdominal obesity evaluated by WC and WHtR. Considering the recommendations of the FNIH criteria, the frequency of sarcopenia was significantly higher in subjects classified as presenting abdominal obesity according to WHtR (Table 4).
Table 4. Frequency of sarcopenia, low muscle mass, low muscle strength and low physical performance (European Working Group on Sarcopenia in Older People (EWGSOP), European Working Group on Sarcopenia in Older People revised version in 2018 (EWGSOP2) and Foundation of the National Institutes of Health (FNIH) criteria) according to the presence of excessive total or abdominal body adiposity in renal transplant recipients
(Absolute values and percentages)
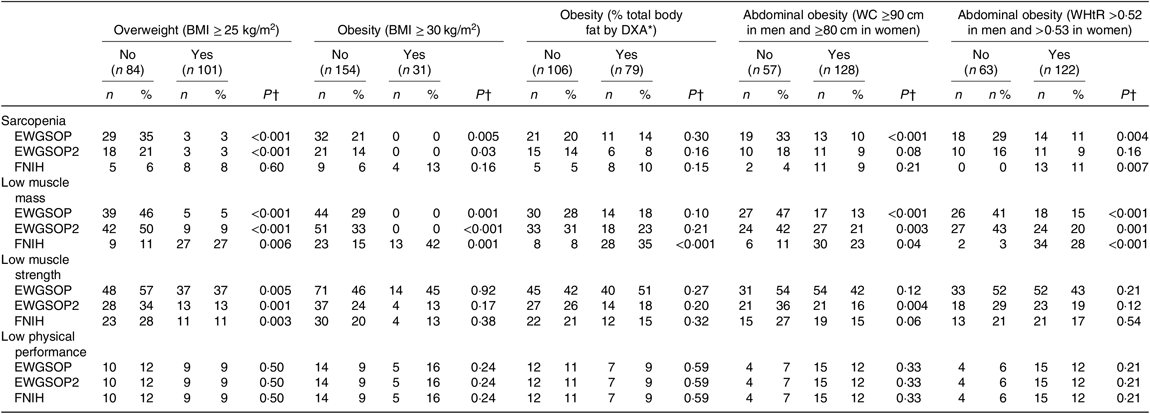
DXA, dual-energy X-ray absorptiometry; WC, waist circumference; WHtR, waist:height ratio.
* According to sex, race ethnicity and age(Reference Heo, Faith and Pietrobelli16).
† P values refer to individuals with excessive total or abdominal adiposity v. individuals without excessive total or abdominal body adiposity.
Individuals presenting excessive total and abdominal body adiposity based on anthropometric measures presented a lower frequency of low muscle mass defined by EWGSOP and EWGSP2 criteria. Whereas the frequency of low muscle mass defined by FNIH criteria was significantly higher in individuals with excessive total and abdominal body adiposity, according to anthropometric measures and to % BF evaluated by DXA. The frequency of low muscle strength defined according to the three criteria (EWGSOP, EWGSOP2 and FNIH) was significantly lower in participants with overweight (according to BMI) and according to EWGSOP2 in individual presenting with abdominal obesity (according to WC) (Table 4). The presence of low physical performance was not associated with the presence of excessive body adiposity (Table 4).
The association between sarcopenia and its components with excessive body adiposity was also evaluated using the OR analyses. These analyses are shown in Table 5 and were performed according to the subgroups of high body adiposity assessed by BMI (overweight), % BF (obesity) and WC (abdominal obesity), considering adequate sample size (as presented in Table 4). After adjustment for age, sex, eGFR and time from transplantation, the OR for (1) sarcopenia, low muscle mass and low muscle strength defined by EWGSOP and EWGSOP2 criteria were significantly lower in overweight individuals (BMI), obese individuals (% BF) and individuals with abdominal obesity (WC); (2) low muscle mass defined by FNIH was significantly higher in overweight RTR (BMI) and obese RTR (% BF); and (3) low muscle strength defined by FNIH was lower for overweight (BMI) and abdominal obesity (WC) (Table 5).
Table 5. Risk for sarcopenia, low muscle mass, low muscle strength and low physical performance (European Working Group on Sarcopenia in Older People (EWGSOP), European Working Group on Sarcopenia in Older People revised version in 2018 (EWGSOP2) and Foundation of the National Institutes of Health (FNIH) criteria) according to the presence of excessive total or abdominal body adiposity in renal transplant recipients
(Odds ratios and 95 % confidence intervals)
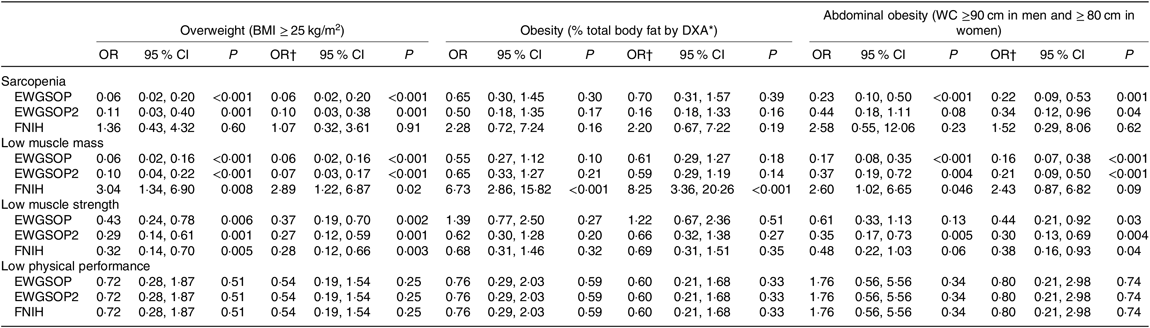
DXA, dual-energy X-ray absorptiometry; WC, waist circumference.
* According to sex, race ethnicity and age(Reference Heo, Faith and Pietrobelli16).
† Adjusted for age, sex, estimated glomerular filtration rate and time from transplantation.
Discussion
In the present study, the prevalence of sarcopenia in adult RTR, not classified as underweight according to BMI, varied according to the diagnostic criteria: 7 % prevalence (using the FNIH criteria), 11 % prevalence (using the EWGSP2 criteria) and 17 % prevalence (using the EWGSOP criteria). Studies evaluating sarcopenia in RTR using other methods observed similar, 11·8 %(Reference Yanishi, Kimura and Tsukaguchi29) or higher prevalence (20·5 %(Reference Ozkayar, Altun and Halil28), 20·7 %(Reference Yanishi, Tsukaguchi and Kimura30) and 33 %(Reference Małgorzewicz, Wołoszyk and Chamienia31)). This difference in prevalence may be attributed to the different diagnostic criteria and the inclusion of only individuals who were overweight in one study(Reference Małgorzewicz, Wołoszyk and Chamienia31). The risk of sarcopenia prevalence overestimation was carefully prevented by excluding participants older than 65 years and with BMI < 18·5 kg/m2. This approach avoided bias, considering that malnutrition (undernutrition) and older age are associated with increased risk of sarcopenia and are uncommon conditions in RTR(Reference Cruz-Jentoft, Bahat and Bauer1,Reference Cruz-Jentoft, Baeyens and Bauer6,Reference Chan, Bosch and Jones23) .
The frequency of sarcopenia in the present study was not different according to skin colour. It is worth mentioning that in Brazil the classification of ethnicity is a challenge due to the high miscegenation(Reference Petrucelli and Saboia34). Although the parameters used to define sarcopenia may present considerable variation depending on the ethnic groups and different geographic locations(Reference Woo, Arai and Ng14–Reference Heo, Faith and Pietrobelli16), the three consensuses used in the present study to define sarcopenia are applicable in our sample population, considering that the proposed cutoff values were based on studies conducted in different ethnic groups and geographic locations(Reference Cruz-Jentoft, Bahat and Bauer1,Reference Cruz-Jentoft, Baeyens and Bauer6,Reference Studenski, Peters and Alley9) . The existing data regarding body composition, lifestyle and physical activities, factors that may affect the parameters used to define sarcopenia, are scarce among the populations of South America. Thus, a guideline for sarcopenia definition needs to be designed, according to age and gender-specific groups.
Among the three criteria used in the present study, the EWGSOP is the most used in studies conducted in the CKD patients. Thus, considering this criterion solely, we can compare the sarcopenia prevalence in RTR evaluated in the present study with the results of CKD patients who did not undergo renal transplantation. In non-dialysed CKD patients, the prevalence of sarcopenia was 14 % in a study that included CKD stages 3–5(Reference Zhou, Hellberg and Svensson43) and 11·9 % in a study that included CKD stages 2–5(Reference Souza, Oliveira and Barbosa44). Our group observed a similar prevalence (13 %) in a study including participants in CKD stages 3b–4(Reference Fernandes, Barreto Silva and Loivos45). Therefore, according to the EWGSOP criteria, the prevalence of sarcopenia in our RTR was higher than that observed in the above described studies including the non-dialysed CKD patients. Noteworthy is that the studied RTR age range was lower than the above-mentioned CKD patients. Considering dialysis patients, the sarcopenia prevalence was even higher (20 %) in a study conducted with participants aged 18–75 years (mean 53 years)(Reference Isoyama, Qureshi and Avesani46).
Considering the results of κ test, the agreement of sarcopenia diagnosed by EWGSOP v. EWGSOP2 was moderate. However, the agreement of sarcopenia diagnosed by EWGSOP v. FNIH was slight and by EWGSOP2 v. FNIH was fair. This finding may be attributed mainly to the difference in the parameter used to evaluate low muscle mass in the FNIH criteria (SMI/BMI) compared with EWGSOP and EWGSOP2 (SMI/ht2). The agreement between each pair of used criteria for low muscle strength definition was better than the agreement for low muscle mass definition. One possible explanation is the use of the same method (HGS) to evaluate muscle strength. However, as the cutoff points for low muscle strength were different in the three used criteria, the agreement was moderate between EWGSOP v. EWGSOP2 and EWGSOP v. FNIH but was substantial between EWGSOP2 v. FNIH.
The divergent association of body adiposity with sarcopenia and low muscle mass defined by the EWGSOP and EWGSOP2 criteria v. the FNIH criteria may be due to the difference in muscle mass index calculation. As mentioned above, the EWGSOP and EWGSOP2 recommend SMI/ht2, while the FNIH criteria recommend SMI/BMI. Many factors determine skeletal muscle mass including the body size (e.g. stature) and adiposity(Reference Heymsfield, Gozalez and Lu47). Obesity is generally accompanied by increased fat and lean mass, but the fat mass increases at a larger scale, resulting in a smaller lean muscle:fat ratio(Reference Yang, Bi and Kuang48). Although body size must be considered in the muscle mass index evaluation(Reference Heymsfield, Gozalez and Lu47), there is no consensus regarding the best index, especially when evaluating sarcopenia in overweight or obese individuals.
Initially, Baumgartner et al.(Reference Baumgartner, Koehler and Gallagher49) suggested the use of SMI/ht2 followed by Janssen et al., who proposed using the weight-adjusted SMI(Reference Janssen, Heymsfield and Ross50). More recently, the FNIH criteria recommended the SMI/BMI after applying a classification and regression tree analytical approach(Reference Studenski, Peters and Alley9,Reference McLean and Kiel51) . Despite the different recommendations, it is recognised that adiposity must be taken into account to obtain a more accurate SMI estimate in overweight and obese individuals(Reference Heymsfield, Gozalez and Lu47,Reference Newman, Kupelian and Visser52) .
Sarcopenia evaluated through SMI/ht2 in older people from the general population showed that this syndrome was associated with lower BMI(Reference Gao, Jiang and Yang53–Reference Lardiés-Sánchez, Sanz-París and Pérez-Nogueras55). Conversely, when muscle mass is evaluated by SMI/BMI, sarcopenia may be associated with higher values of BMI or adiposity. Tyrovolas et al.(Reference Tyrovolas, Koyanagi and Olaya56) studied 18 363 individuals (≥65 years) and showed that the higher % BF was associated with lower SMI/BMI and the presence of sarcopenia.
Impairment of muscle strength is observed in obese individuals due to several mechanisms including decreased physical activity, presence of a pro-inflammatory state and infiltration of fat into the muscle(Reference Marty, Liu and Samuel20). However, in the present study, obesity was not associated with a higher risk of low muscle strength; conversely, we observed a lower OR for low muscle strength in those presenting with overweight and abdominal obesity according to WC. This finding may be related to the length of the time period in which the participants possessed excessive adiposity, given that the majority of our participants gained weight after transplant.
To the best of our knowledge, the present study is the first to evaluate sarcopenia using the EWGSOP, EWGSOP2 and FNIH criteria in RTR. The strengths of the present study include the adequate evaluation of muscle mass (by DXA) and muscle strength using the indices and cutoff points suggested in recent guidelines. The main limitation is its cross-sectional nature, meaning that causality is not likely to be determined, and we could not evaluate the possible association of sarcopenia with outcomes. Therefore, we were not able to evaluate the sarcopenia criteria that can better distinguish patients more prone to worse outcome.
Conclusions
The present study suggests that in adult RTR not classified as underweight according to BMI (1) the prevalence of sarcopenia varied according to the diagnostic criteria: 7 % (FNIH), 11 % (EWGSOP2) and 17 % (EWGSOP); (2) low muscle mass, low muscle function and low physical performance are relatively common conditions, affecting up to 28, 46 and 10 % of the participants, respectively; (3) the association of excessive body adiposity with sarcopenia depends on the index used to evaluate muscle mass and (4) the FNIH criteria was efficient in detecting higher adiposity in individuals with sarcopenia.
Acknowledgements
The authors express their sincere gratitude to Maria de Lourdes Guimarães Rodrigues, Débora Cristina Torres Valença, Sergio Emanuel Kaiser, Bernardo Barreto da Silva Gaspar, Stephanie Giannini, Jessica Veiga Pires, Elisama de Moura Rodrigues Leite and Sabina Clare Valentine.
The present study was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
A. P. M. M. B. contributed to the study conception and design, data collection, assembly, analysis and interpretation, manuscript drafting and the approval of the final version of the manuscript. M. I. B. S. contributed to the study conception and design, data analysis and interpretation, manuscript drafting and the approval of the final version of the manuscript. K. S. d. S. P. contributed to data collection, assembly, analysis and interpretation, manuscript drafting and the approval of the final version of the manuscript. M. S. d. C. contributed to data collection, assembly, analysis and interpretation, manuscript drafting and the approval of the final version of the manuscript. K. T. d. C. R. contributed to data collection, assembly, analysis and interpretation, manuscript drafting and the approval of the final version of the manuscript. E. S. contributed to data collection, manuscript drafting and the approval of the final version of the manuscript. R. B. contributed to the study conception and design, data analysis and interpretation, manuscript drafting and the approval of the final version of the manuscript. M. R. S. T. K. contributed to the study conception and design, data collection, assembly, analysis and interpretation, manuscript drafting and the approval of the final version of the manuscript.
There are no conflicts of interest.









