Dietary isoflavones, a subclass of flavonoids, have been the focus of much of the recent interest in the nutritional benefits of soya foods(Reference Horn-Ross, Barnes and Lee1). The exact role that isoflavones play in the health-related effects of soya foods and their potential for adverse effects have been controversial and difficult to discern(Reference Cassidy, Albertazzi and Lise Nielsen2, Reference Rüfer, Kulling, Dunkelberg, Gebel and Hartwig3). This may be in part due to the lack of basic knowledge regarding absorption, distribution, metabolism and excretion.
In a recently published study, we investigated the difference in the bioavailability of the isoflavone daidzein (DAI) in the aglycone and glucoside form when administered as pure compounds(Reference Rüfer, Bub and Möseneder4). In most subjects the plasma appearance and disappearance curves revealed a biphasic pattern with an early peak before the peak plasma concentration which has also been reported by other authors(Reference Setchell, Brown and Desai5, Reference Cassidy, Brown and Hawdon6). Possible reasons for this behaviour discussed so far are enterohepatic recycling and some small initial absorption in the upper gastrointestinal tract along with predominant absorption in the large intestine.
A third explanation might be the absorption of isoflavones via a chylomicron-mediated mechanism and distribution of isoflavones bound to lipoproteins throughout the body. Several observations provide evidence for this hypothesis: It is known that water-insoluble compounds, for instance, some lipid-soluble vitamins, can be transported as compounds of lipoprotein complexes(Reference Wasan and Cassidy7). In addition, it has been suggested that the lipoprotein pathway plays a role in the transport of the flavonolignan silibinin in rats(Reference Svagera, Skottová and Vána8). Furthermore, a higher fat content resulting in a greater chylomicron formation has been shown to increase the bioavailability of the flavonole quercetin in pigs(Reference Lesser, Cermak and Wolffram9). In vitro an incorporation of isoflavones in HDL and LDL as well as of 17β-oestradiol in HDL has been demonstrated(Reference Meng, Lewis and Wahala10–Reference Abplanalp, Scheiber and Moon12). Despite these facts, there is a lack of basic knowledge regarding the association of isoflavones with lipoproteins in vivo. However, it is essential to study this distribution in order to understand the increased LDL oxidation resistance after isoflavone administration(Reference Tikkanen, Wahala and Ojala13–Reference Wiseman, O'Reilly and Adlercreutz15). Oxidative modification of LDL particles is considered to be a prerequisite for the uptake of LDL by macrophages in the artery wall, an initial step in the formation of atheroma(Reference Tikkanen, Wahala and Ojala13).
Therefore, the objective of the present study was to investigate the distribution of the isoflavone DAI in plasma lipoproteins after consumption of DAI in both the aglycone and glucoside form.
Materials and methods
Chemicals
Both forms of DAI, aglycone and 7-O-β-d-glucoside, were isolated by high-speed countercurrent chromatography of a commercially available DAI preparation (TCI Tokyo Casei, Tokyo, Japan) or of soya flour, respectively, as described by Rüfer et al. (Reference Rüfer, Bub and Möseneder4). [3,4,8-13C]DAI was obtained from Nigel Botting, University of St Andrews (St Andrews, UK). Enzymes and all other chemicals used were obtained from Sigma-Aldrich (Taufkirchen, Germany).
Human intervention study
The intervention study was performed as a randomised, double-blind, cross-over study. For information on subjects and study design (run-in phase and intervention period), see Rüfer et al. (Reference Rüfer, Bub and Möseneder4). Data of only five subjects were included in the present study since the plasma amounts available from two subjects were not sufficient to perform all the required analyses. The study protocol was approved by the Ethics Committee of Landesärztekammer Baden-Württemberg. The dose of DAI and daidzein-7-O-β-d-glucoside (DG) (1 mg isoflavone calculated as aglycone equivalents/kg body weight) was given in a hard gelatin capsule with the first breakfast after 12 h overnight fasting. Isoenergetic and standardised meals (breakfast, lunch and dinner) as well as snacks were provided after administration of the isoflavone preparation as well as after the blood samples taken at 2, 4·5, 8, 12, 24 and 48 h.
Blood was obtained via an indwelling cannula for samples up to 12 h, and thereafter by venepuncture into pre-chilled tubes containing EDTA (1·6 g/l; Monovette-Sarstedt, Nümbrecht, Germany). Plasma was collected after centrifugation at 1500 g for 10 min at 4°C. Sucrose (75 %) and 3,5-di-tert-4-butylhydroxytoluene (BHT) (0·5 g/l in 50 % methanol) solutions were added to give final concentrations of 1·5 g sucrose/l plasma and 5 mg BHT/l plasma, respectively. Samples were stored at − 80°C until lipoprotein separation.
Analytical methods
Lipoprotein separation
Plasma (2 ml) was used for the isolation of lipoproteins. VLDL, LDL and HDL were separated by sequential flotation ultracentrifugation (Optima K-100, rotor 50.4 Ti; Beckmann, Munich, Germany) in a KBr density gradient according to the method described by Clevidence & Bieri(Reference Clevidence, Bieri and Packer16). After HDL separation the remaining bottom fraction was defined as the non-lipoprotein plasma (ρ >1·21 g/ml). Purity of each lipoprotein fraction was determined by agarose gel electrophoresis (Hydragel; Sebia, Norcross, GA, USA) and subsequent Sudan Black staining. Chylomicrons were prepared by overlaying 2 ml plasma with 2 ml 0·9 % NaCl solution after ultracentrifugation at 10 000 g and 10°C for 40 min.
Clean-up of lipoprotein samples
The concentrations of DAI in the different fractions obtained after lipoprotein separation of 2 ml plasma were measured by GC/MS using a stable isotopically labelled internal standard [3,4,8-13C]DAI. Total DAI was determined after extraction and enzymic hydrolysis with a mix of β-glucuronidase and sulfatase. Results are expressed as amount of DAI per ml plasma.
The separated lipoprotein fractions were equilibrated with 100 pmol [3,4,8-13C]DAI for 30 min at room temperature and diluted with five volumes of 0·5 m-triethylammonium sulfate (pH 5). Further details on sample clean-up, enzymic hydrolysis and derivatisation for GC/MS analysis are described by Rüfer et al. (Reference Rüfer, Bub and Möseneder4).
Gas chromatography/mass spectrometry conditions
Isoflavone trimethylsilylether derivatives were quantified by GC/MS analysis. GC/MS conditions can be found in Rüfer et al. (Reference Rüfer, Bub and Möseneder4). The selected ion monitoring was applied to monitor two ions for each analyte, where the first was used for quantification and the second was used as confirmation of the presence of the analyte: m/z 398 and 383 for DAI as well as m/z 401 and 386 for [3,4,8-13C]DAI. DAI was quantified using ten calibration levels between 0 and 10 pmol. The limit of detection was 8 fmol. The recovery averaged 94 to 106 % obtained after spiking lipoprotein fractions with DAI and subtracting the basal values from the blank fractions. The intra- and inter-assay coefficients were always less than 5 %.
Pharmacokinetics and statistical analysis
Maximal concentrations (Cmax) and the time required to attain Cmax (tmax) were obtained directly by visual inspection of each subject's concentration–time profile. The area under the concentration–time profiles from 0 h to infinity (AUCinf) as well as the half-life of elimination (t1/2) were calculated using PK SOLUTIONS™ 2.0 (Summit Research Services, Montrose, CO, USA) computer software as described by Rüfer et al. (Reference Rüfer, Bub and Möseneder4).
All statistical calculations were performed using the STATVIEW program (version 5.0; SAS Institute, Cary, NC, USA). Results are reported as mean values and standard deviations. Error variance was tested and found to be homogeneous. The differences between the mean values of Cmax, tmax, t1/2 and AUCinf after administration of the two isoflavone preparations were statistically analysed using the paired Student's t test. Changes between the baseline (0 h) and the following time points among the two treatment groups were tested for significance by repeated-measures ANOVA. Differences were considered significant at P < 0·05.
Results
The mean total DAI appearance and disappearance curves for chylomicrons, VLDL, LDL, HDL and the non-lipoprotein fraction are shown in Fig. 1.

Fig. 1 Appearance and disappearance curves for total daidzein in the chylomicrons (–●–), VLDL (–○–), LDL (–▲–), HDL (–△–) and the non-lipoprotein fraction (–■–) in five men after consumption of a single bolus dose of 1 mg/kg body weight daidzein (a) and daidzein-7-O-β-d-glucoside (b) (calculated as aglycone equivalents). Values are means, with standard deviations represented by vertical bars. Daidzein concentrations differed significantly over time between the two treatment groups for all fractions (P < 0·01; repeated-measures ANOVA).
Before consumption of the isoflavone preparations, DAI concentrations were < 20 nmol/l in all fractions. The lipoprotein fraction profiles were similar for all subjects and resembled those obtained in plasma(Reference Rüfer, Bub and Möseneder4). The total DAI appearance and disappearance curves in the VLDL, LDL, HDL, as well as non-lipoprotein fractions, were characterised for all subjects by a rapid increase in the DAI concentration during the first 2–3 h, followed by a slight decline and a second rise that attained the peak concentrations at 6–10 h. A similar two-peak pattern for serum TAG concentrations was observed (data not shown). The curves for total DAI in the chylomicron fraction displayed three peak concentrations which appeared after the volunteers consumed their main meals or snacks. In accordance with the findings for plasma, AUCinf and Cmax in the different lipoprotein fractions as well as in the non-lipoprotein fraction were always statistically significantly higher after the ingestion of DG than of DAI whereas tmax and t1/2 did not differ significantly. The order of the AUCinf, Cmax and the lipoprotein distribution based on AUCinf was irrespective of the administered form as follows: non-lipoprotein fraction > LDL > HDL > VLDL > chylomicrons. The results are summarised in Table 1.
Table 1 Pharmacokinetic variables for total daidzein in the chylomicrons, VLDL, LDL, HDL and the non-lipoprotein fraction in five men following ingestion of a single oral dose of daidzein or daidzein-7-O-β-d-glucoside (1 mg/kg body weight calculated as aglycone equivalents) and lipoprotein distribution based on area under the concentration–time profile from 0 h to infinity (AUCinf)
(Mean values and standard deviations)
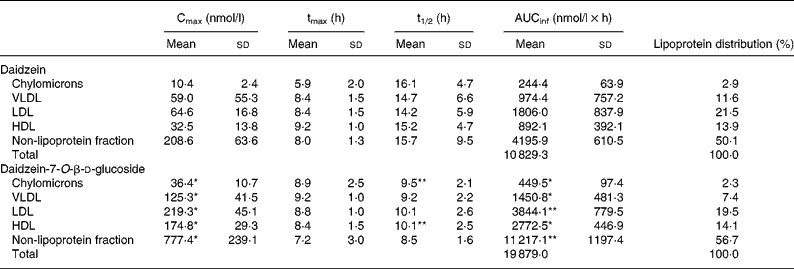
Cmax, maximal concentration; tmax, time required to attain Cmax; t1/2, half-life of elimination.
Mean value was significantly different from that for daidzein (the aglycone form): *P < 0·01, **P < 0·05 (paired Student's t test).
Discussion
It is noteworthy that in human plasma DAI is associated to a remarkable extent with human lipoproteins, namely 47 % after the administration of DAI and DG, respectively. The lipoprotein distribution irrespective of the administered isoflavone form showed a relation to the order of lipoproteins: non-lipoprotein fraction > LDL > HDL > VLDL > chylomicrons. In the non-lipoprotein fraction 53 % of the total DAI concentration based on AUCinf can be detected followed by LDL with 20 %, HDL with 14 %, VLDL with 9·5 % and chylomicrons with 2·5 %. Due to the low amounts found in the chylomicrons, it seems unlikely that the observed isoflavone distribution in VLDL, LDL and HDL results from isoflavone uptake via the chylomicron pathway. Another indication is the rapid increase in the DAI concentration in all fractions after isoflavone administration. The values of tmax in the different fractions do not differ significantly and the increase in the DAI concentration occurs simultaneously. This phenomenon cannot be explained by the fact that DAI is absorbed by the chylomicron pathway because it takes about 4 h before chylomicron remnants are taken up by the liver and DAI is transferred from chylomicrons to lipoproteins of higher density after lipolysis and remodelling(Reference Wasan and Cassidy7). The observed distribution seems to be due to unspecific isoflavone binding to proteins. The protein content in the different lipoprotein fractions decreases from HDL (47 %) to LDL (22 %), VLDL (8 %) and chylomicrons (2 %)(Reference Lesser, Cermak and Wolffram9). Isoflavones exert an extensive unspecific protein binding(Reference Nagel, vom Saal and Welshons17, Reference Csanady, Oberste-Frielinghaus and Semder18). For example, Csanady et al. were able to demonstrate that 88 % of DAI is found bound to plasma proteins(Reference Csanady, Oberste-Frielinghaus and Semder18). Therefore, although the DAI distribution in the different fractions does not resemble that of the protein content of the different fractions in detail one might speculate that the lipoprotein distribution of isoflavones can be explained by unspecific protein binding.
The shapes of all appearance and disappearance curves for total DAI after the ingestion of DAI and DG were similar for all fractions analysed and resembled those obtained for plasma(Reference Rüfer, Bub and Möseneder4). The concentrations found in the different lipoproteins were always greater after the consumption of DG than of DAI. These findings confirm those obtained for plasma DAI concentrations(Reference Rüfer, Bub and Möseneder4). The systemic bioavailability as determined by comparing the plasma AUCinf was found to be 4·5 times and Cmax six times greater after the administration of DG than of DAI(Reference Rüfer, Bub and Möseneder4). In the case of the lipoprotein fractions analysed AUCinf and Cmax are greater after DG than DAI consumption by a factor of 1·5 to 4 and 2 to 5, respectively. Since the degree for the greater concentrations in the lipoprotein fractions and plasma is about the same after the ingestion of DG than of DAI, the intestine rather than transport phenomena in plasma seem to be responsible for the different bioavailabilities of DAI and DG. Several factors might lead to a different absorption of DG: for example, protection of bacterial degradation by the glucoside moiety, increased solubility of the glucoside in the intestine, active transport of the glucoside. These factors are discussed in detail elsewhere(Reference Rüfer, Bub and Möseneder4).
In summary, our findings show a remarkable association between isoflavones and human plasma lipoproteins. These results raise the question about the physiological impact of isoflavones binding to lipoproteins and will help us to understand the observed antioxidative effects of isoflavones in vivo by increasing LDL oxidation resistance after isoflavone intake(Reference Tikkanen, Wahala and Ojala13–Reference Wiseman, O'Reilly and Adlercreutz15). Further studies are needed to investigate the correlation between lipoprotein distribution and oxidation resistance after isoflavone administration.
Acknowledgements
The present study was supported by the Deutsche Forschungsgemeinschaft (grant Ku 1079/6-3).
We thank S. Demirel, S. Herrmann, E. Hoch and U. Stadler-Prayle for their excellent technical assistance and the subjects for taking part in the study.
A. B. and S. E. K. developed the initial idea and designed the study; C. E. R. collected and analysed the data; C. E. R. and A. B. performed the statistical analysis; C. E. R. drafted the manuscript; A. B. and J. M. recruited and checked the volunteers, planned and scheduled meals, oversaw the kitchen personnel and handled, collected and stored all specimens; P. W. provided pure DAI and DG. All co-authors participated in critically revising the manuscript.
None of the authors had any conflict of interest.




