The protein and amino acid (AA) demands of animals for normal physiological states (e.g. growth, pregnancy and lactation) are now well characterised, especially for pigs and poultry( Reference Wu 1 ) and with progress made in ruminants( Reference Patton, Hristov and Lapierre 2 ). These requirements are altered, however, in response to injury, infection and parasitic challenge. In many cases, impact of these challenges can be ameliorated and the rate of recovery improved by provision of additional protein in the diet( Reference Ishibashi, Plank and Sando 3 – Reference Kidane, Houdijk and Athanasiadou 6 ). Often, however, the amount of protein required is not known, with consequences for both animal performance and farm economics.
More importantly, protein per se may not be required, but instead the metabolic responses to the challenge may increase the demand for specific AA. For example, practical and theoretical arguments have been presented for additional arginine in cardiovascular and pulmonary disorders( Reference Yeh, Yeh and Lin 7 – Reference Zhou, Wu and Yin 10 ), for cysteine( Reference Lyons, Rauh-Pfeiffer and Ming-Yu 11 ), leucine( Reference Bruzzone, Siegel and Chiarla 12 ) and threonine( Reference Faure, Chone and Mettraux 13 ) in sepsis, for glutamine in endotoxaemia( Reference Zhou, Wu and Yin 10 ), and for phenylalanine to support acute-phase protein synthesis( Reference Reeds, Fjeld and Jahoor 14 ). In other situations, combinations of AA have been proposed( Reference Zhou, Wu and Yin 10 , Reference Breuille, Bechereau and Buffiere 15 , Reference Laurichesse, Tauveron and Gourdon 16 ). Obviously different stages of injury, infection or recovery may require differences in the combinations and absolute quantities of AA.
The current study addresses two objectives. First, to identify which AA have increased demand and, second, how much is required during acute endotoxaemia, based on intravenous infusion of lipopolysaccharide (LPS). The first objective can be addressed from the changes in plasma AA concentration compared with the healthy state and how these respond to supplemental AA, as has been used for AIDS patients( Reference Laurichesse, Tauveron and Gourdon 16 ). For the second objective, in terms of quantifying requirements a factorial approach based on dose–responses is an effective, albeit laborious, technique( Reference Bruzzone, Siegel and Chiarla 12 , Reference Breuille, Bechereau and Buffiere 15 ). The current study, in twin sheep, introduces measurement of dynamic rates of whole-body disposal of individual AA and how these change in response to the endotoxin challenge and supplementation with additional AA. In theory this should both identify and quantify the critical AA needed to restore the challenged state to healthy homoeostasis.
Methods
Animals and husbandry
For the pilot study, healthy lambs (10–16 months old, body weight (BW) 35–55 kg) were selected from the Rowett flock of Scottish Blackface×Greyface. For the main study, six pairs of twin lambs (12 months old, BW 37–57 kg) were selected from the same Rowett flock. There were four pairs of male twins and two pairs of female twins. Twins within each pair were closely matched for BW and condition score. The sheep were then housed indoors in individual pens for 3 months to acclimatise to experimental conditions, during which time they were offered daily 2×500 g of a mixed concentrate-forage diet (CF, g/kg as fed: barley 300, hay 500, molasses 100, fishmeal 90, salts and vitamins 10; 830 g DM/kg; 21·5 g N/kg DM, estimated metabolisable energy 11·0 MJ/kg DM( Reference Lobley, Bremner and Zuur 17 )). This fixed intake provided 1·0–1·3 of estimated maintenance metabolisable energy requirement depending on metabolic BW (kg0·75) of individual sheep. For the experimental measurements, the sheep were held in metabolic cages for 7 d with 1 kg/d CF provided as 24 equal portions through automated feeders. On day 6, polyvinyl catheters (Portex NT2, Smiths Medical) were placed into each external jugular vein, one inserted 25 cm for infusions and the other inserted at 15 cm for blood sampling. All procedures were approved by the Ethical Review Committee of the Rowett Institute of Nutrition and Health and conformed to UK legislation under the Animals (Scientific Procedures) Act 1986.
Pilot studies
The amounts of both LPS (from Escherichia coli; Sigma) and supplemental AA required for the main study were estimated on the basis of a pilot study that involved six sheep that were supplied with the CF diet at hourly intervals by means of automated feeders. Two of each sheep were continuously infused via a jugular vein with either 2, 3 or 4 ng LPS/kg BW per min dissolved in sterile saline for 24 h. Rectal temperatures were recorded hourly, as was food intake. Blood samples were also taken hourly for measurement of clinical parameters and plasma AA concentrations. These preliminary data showed a longer period of inappetence as the dose of LPS increased (<4 h, 8–12 h and 16–20 h for 2, 3 and 4 ng LPS/kg BW per min, respectively) and also higher mean rectal temperatures (+1·0, +1·9 and +2·6°C above background, respectively). Therefore, infusion of 2 ng LPS/kg BW per min was selected, and a further three sheep were infused with this dose of endotoxin for 24 h, and measurement of plasma AA concentrations was made at hourly intervals.
Main study
The protocol for the main study is shown in Fig. 1. The LPS was dissolved in sterile physiological saline (150 µg/500 ml) and infused at a rate of 2 ng/kg BW per min for 20 h in one twin. The other twin of each pair was infused on the same day with a similar volume of sterile saline. From 12 h onwards a mixture of stable isotope-labelled AA was infused into all animals as described previously( Reference Savary-Auzeloux, Hoskin and Lobley 18 ). Briefly, 1 g [U-13C]whole algal hydrolysate prepared from Celtone-C (Martek Biosciences Corporation), plus 100 mg l-[5-13C]methyl methionine 100 mg, 120 mg l-[1-13C]cysteine and 150 mg l-[2-15N]glutamine (all sourced from Cambridge Isotope Laboratories), was dissolved in 100 ml of buffered physiological saline (pH 7·4) containing 50 000 IU of heparin and infused at 10 g/h. Methionine, cysteine and glutamine were added, as these AA are lost during the acid hydrolysis preparation of the whole algal powder. All of the [U-13C]AA from the algal hydrolysate were as the l-enantiomer (>99·8 %), except for lysine, which contained 23·1 % of the d-isomer. Measurement of the stable isotope enrichments allowed quantification of the irreversible loss rates (ILR) through plasma of the individual AA. From 16 h onwards the supplemental AA were infused (via a Y-piece) into all sheep, at rates calculated to restore plasma concentrations to pre-LPS values for those animals that received the LPS and based on the data from the pilot studies. The control (saline-infused) sheep of each pair received the same amount of AA infusion as the LPS-infused twin. The supplemental l-AA were of European Pharmacopeia grade (Ajinomoto Foods Europe S.A.S.) and infused at 42 g/h at the following concentrations (mmol/kg infusate): alanine 32·3, arginine 34·4, aspartate 9·0, cysteine 28·3, glutamate 31·0, glutamine 8·1, glycine 137·2, histidine 15·9, isoleucine 16·7, leucine 10·4, lysine·HCl 27·5, methionine 11·6, phenylalanine 5·2, proline 18·7, serine 36·0, threonine 40·2, tryptophan 2·5, tyrosine 1·3 and valine 16·1; pH adjusted to 7·4. The amounts infused were calculated as the product of the irreversible loss rate for each AA, determined previously for similar sheep( Reference Savary-Auzeloux, Hoskin and Lobley 18 , Reference Lobley, Connell and Revell 19 ), multiplied by the fractional decrease in plasma AA concentrations observed during the pilot studies (see examples in Fig. 2). Blood samples (n 3) were drawn continuously( Reference Hoskin, Savary and Zuur 20 ) over 40 min periods between 14 and 16 h and at hourly periods between 17 and 20 h (n 4), covering the last 2 h of the pre-AA infusion period and the last 3 h of the AA infusion. Plasma was immediately prepared at 4°C and stored at −80°C for subsequent analysis. Throughout the infusion periods, rectal temperatures were recorded hourly, as were any orts.

Fig. 1 Experimental protocol for the main study. Six pairs of twin sheep with one twin infused intravenously with saline and the other twin with lipopolysaccharide (LPS) from Escherichia coli (2 ng/kg body weight per min), both for 20 h. At 12 h, an 8 h intravenous infusion of stable isotope-labelled amino acids (AA) was started. At 16 h, a 4 h intravenous infusion of unlabelled AA was started. Continuous blood samples were taken between 14 and 20 h, as indicated. Sheep were euthanised at 20 h and samples of liver and kidney were taken.

Fig. 2 Pilot study. Effect of a 24 h continuous intravenous infusion of lipopolysaccharide (2 mg/kg body weight per min) on the proportional changes in plasma amino acid (AA) concentrations (pre-infusion values set at 100 %); ○, serine; ●, glutamine; △, threonine; ▲, phenylalanine; □, methionine; ■, cysteine, ×branch chain AA (leucine+isoleucine+valine). Values are means, with standard errors, based on data from five sheep, except for glutamine and cysteine, for which values are from four sheep.
At the end of the study, the sheep were immediately euthanised with Na pentobarbital given intravenously, and samples of liver and kidney were quickly excised, frozen in liquid N2 and stored at −80°C until later analysis for enzyme activity.
Analyses
Commercial kits were used for plasma measurement of total protein, albumin, urea, glucose (kits 981–387, 981–767, 981–818 and 981–304, respectively; Thermo Scientific) and lactate (kit 735–10; Trinity Biochemicals) on a clinical analyser (Kone Limited). The activity of serine-threonine dehydratase (SDH; EC 4.2.1.16) was assayed on extracts from frozen liver and kidney, based on the method of Ogawa et al. ( Reference Ogawa, Pitot and Fujioka 21 ) modified as described( Reference Rees, Hay and Antipatis 22 ). SDH was selected because, in the pilot study, threonine showed the greatest proportional decrease in plasma concentration, and other studies have shown this enzyme to be sensitive to threonine supply and status( Reference Rees, Hay and Antipatis 22 , Reference Su, Kanamoto and Miller 23 ). Enrichments of the various AA for ILR determination were determined by GC MS as described previously( Reference Savary-Auzeloux, Hoskin and Lobley 18 , Reference Lobley, Holtrop and Horgan 24 ) and included use of a chiral column to quantify the amount of l-lysine( Reference Lobley, Connell and Revell 19 ). AA concentrations were determined by gravimetric procedures based on isotope dilution( Reference Lobley, Holtrop and Horgan 24 , Reference Calder, Garden and Anderson 25 ) with appropriate correction for enrichments in the plasma during the ILR procedure.
Statistics
All statistical analyses were conducted with GenStat 13th edition (version 13.2; VSN International). Preliminary ANOVA indicated that for several variables there was a strong twin effect and this was included in the main analysis as a random effect. Background (pre-infusion) samples were analysed by ANOVA with twin pair as a random effect, but effects of twin were only observed for plasma isoleucine with greater values for those allocated to the LPS group (80·7 v. 70·4 µmol/kg; P=0·026).
For the main analysis, data consisted of six twin pairs (twelve sheep) where one sheep of each twin pair received LPS and the other sheep received saline (LPS status). For each sheep, the mean of measurements made before infusion of AA was compared with the mean of measurements taken during AA infusion (AA status). Data were analysed by ANOVA where twin pair and sheep, AA status plus their interaction nested within twin pair were set as random effects while AA status, LPS status and their interaction were set as fixed effects. For clinical parameters and plasma AA concentrations, the background sample, taken before any treatments (saline or LPS infusion) were applied, was used as the covariate. This ANOVA gave four residual df for the effect of LPS and five residual df for the effect of AA infusion. The ANOVA for ILR was similar to that for clinical parameters and plasma AA concentrations, but without a covariate correction (with five residual df for the effect of LPS).
For comparison of the difference between pre- and during AA infusion, twin pair was set as the random effect and LPS status as the fixed effect. Only terminal samples were available for kidney and liver from each sheep, and therefore data on SDH activity were analysed within tissue with twin as a random effect and LPS status as the fixed effect. For comparison between kidney and liver, then twin and sheep, tissue and their interaction within twin were treated as random effects and tissue, LPS status and their interaction as fixed effects.
For all statistical comparisons, significance was assessed at P<0·05 and a tendency at P<0·10. When significant effects were observed (P<0·05) the difference between treatments was analysed by means of the post-hoc t test based on the relevant standard error of the difference between means and corresponding residual df based on the ANOVA output. Data in the tables and figure are presented as the arithmetic average of the original values and not as the covariate-adjusted predicted means from the ANOVA. Unless otherwise indicated, all data were collected and reported (i.e. no missing values). Visual inspection of the residual plots from the ANOVA output indicated that the assumptions of normality and constant variance had been met.
Results
During the LPS infusion, rectal temperature increased from 2 h onwards (P=0·009) and was maintained, on average, 1·0°C above that of saline-treated sheep (P=0·004) for the remainder of the infusion times. Rectal temperature was not changed by the AA infusion. During the first 4 h of infusion the sheep receiving LPS ate each hourly portion more slowly than did their saline-infused counterparts and on occasion did not consume the portion completely. The orts were not removed, but after 4 h these had been consumed, and by 8 h onwards the rate of consumption of each portion was similar between the twin pairs. This return to the normal rate of food consumption was established several hours before the metabolic measurements were started.
There were no differences (P>0·05) in biochemical parameters for background (pre-infusion) samples between sheep from each twin pair subsequently allocated to either saline or LPS treatment (Table 1). Animals subsequently infused with LPS showed decreased plasma glucose (−32 %; P=0·014) and increased lactate (+43 %; P=0·027), whereas urea showed a marginal increase (+9 %; P=0·055). Infusion of AA tended to increase urea (+5 %; P=0·088), whereas the effect on lactate tended to be greater for the saline-infused animals (P=0·077). Neither LPS nor AA infusion had an effect on either plasma total protein or albumin.
Table 1 Effects on concentrations of plasma metabolites in six pairs of twin sheep, with one of each twin infused intravenously for 20 h with either saline (control) or lipopolysaccharide (LPS) infusion (2 ng/kg body weight per min). From 16 to 20 h all sheep were also infused intravenously with an amino acid (AA) mix (+AA) (Mean values and standard error of the difference between means)

a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05); NS (P>0·10).
* There were no significant differences (P>0·10) in background values between twin sheep subsequently allocated to either saline or LPS infusion.
† Average of three samples drawn continuously over 40 min between 14 and 16 h (before AA infusion).
‡ Average of four samples drawn continuously every hour between 16 and 20 h (during AA infusion).
§ By ANOVA with twin pair, plus sheep, AA status and their interaction nested within twin pair, as random effects and AA status, LPS status and their interaction as fixed effects, and with background values (prior to any infusions) used as covariate, four residual df for LPS and five for AA. Standard error of the difference between mean values are for the LPS status×AA status interaction.
Between 14 and 16 h, when animals received either saline or LPS (but not the AA infusion) the presence of endotoxin caused a decrease (P<0·05) in plasma concentrations (range 19–67 %) for most AA (see Table 2). The exceptions were leucine (downward tendency, −30 %; P=0·063), tyrosine (no change) and phenylalanine (20 % increase; P=0·047).
Table 2 Plasma amino acid (AA) concentrations (µmol/kg) either before (14–16 h) or during (16–20 h) infusion of an AA mixture during a 20 h infusion of either saline (control) or lipopolysaccharide (LPS) (2 ng/kg body weight per min) into one of each of six pairs of twin sheep (Mean values and standard error of the difference between means)

a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05); NS (P>0·10).
* By ANOVA with twin pair, plus sheep, AA status and their interaction nested within twin pair, as random effects and AA status, LPS status and their interaction as fixed effects, and with background values (prior to any infusion) treated as covariate, four residual df for LPS status and five for AA status. Standard error of the difference between mean values are for AA status×LPS status interaction.
† There were no significant differences (P>0·10) in background values between twin sheep subsequently allocated to either saline or LPS infusion, except for isoleucine, which was greater for those allocated to the LPS group (80·7 v. 70·4 µmol/kg; P=0·026).
‡ Average of three samples drawn continuously over 40 min between 14 and 16 h (before AA infusion).
§ Average of four samples drawn continuously every hour between 16 and 20 h (during AA infusion).
Each pair of twins received the same amount of infused AA, and the consequent increase in plasma concentration was similar for alanine, glutamine, histidine, isoleucine, leucine, tryptophan, tyrosine and valine whether infused with saline or with LPS (this is shown by the non-significant LPS×AA interactions shown in Table 2). For the other AA, phenylalanine showed a greater incremental change (P=0·041) with LPS than with saline, whereas the remainder all showed lower responses (P<0·05) with the endotoxin challenge, although this was only a tendency (P=0·066) for lysine.
In the period before AA infusion, LPS infusion compared with saline resulted in an increase in ILR (see relevant data in Table 3) for phenylalanine (17 %; P=0·005), but decreases for isoleucine (−14 %; P=0·020), methionine (−47 %; P=0·004), serine (−36 %; P=0·038), threonine (−36 %; P<0·001) and valine (−13 %; P=0·026). These differential responses in ILR to LPS treatment were maintained during the period of AA infusion (see relevant data in Table 3), especially for methionine (−38 %; P=0·004) and phenylalanine (+27 %; P<0·001). In addition, histidine ILR also increased more to infused AA when LPS was present (+24 %; P=0·036), whereas glutamate decreased (−10 %; P=0·035). These responses were reflected in main effects for LPS (P<0·05) for isoleucine, methionine, phenylalanine and threonine plus tendencies (P<0·10) for glutamine and serine (Table 3).
Table 3 Irreversible loss rate (mmol/h) either before (12–16 h) or during (16–20 h) an amino acid (AA) mixture was infused during a 20 h infusion of either saline (control) or lipopolysaccharide (LPS) (2 ng/kg body weight per min) into six pairs of twin sheep with one of each pair infused (Mean values and standard error of the difference between means)

a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05); NS (P>0·10).
* Average of three samples drawn continuously over 40 min between 14 and 16 h (before AA infusion).
† Average of four samples drawn continuously every hour between 16 and 20 h (during AA infusion).
‡ By ANOVA with twin, plus sheep, AA status and their interaction nested within twin, as random effects and AA status, LPS status and their interaction as fixed effects, five residual df for LPS status and five for AA status. Standard error of the difference between mean values are for AA status×LPS status interaction.
Infusion of AA increased ILR for most AA, regardless of whether saline or LPS was infused (Table 3), with glutamine, leucine, phenylalanine and tyrosine as the exceptions. The magnitude of ILR response to AA infusion was greater for LPS-infused sheep for histidine, serine and threonine as shown by the AA status×LPS status interaction (Table 3) and with a similar tendency for valine. In contrast, the response for glycine tended to be less (P=0·071) when the sheep received LPS compared with saline. The increase in ILR matched the amount infused for alanine, aspartate, glutamate, glutamine, isoleucine, lysine and methionine (Table 4) plus serine in the saline-infused sheep. For the other AA, the relative increases were variable, and there were differences between saline and LPS-treated animals. For example, for both treatments cysteine ILR increased by more than the amount infused (P<0·01), whereas glycine (P<0·001) and proline (P<0·05) showed a smaller response than expected (Table 4). For arginine, histidine, leucine, phenylalanine, threonine, tyrosine and valine the increase in ILR was by less than the amount infused for the saline-treated sheep, but was greater for serine in animals that received LPS (Table 4).
Table 4 Comparison of the rate of infusion of a mixture of amino acid (AA, mmol/h) during 16–20 h of the experiment with the difference in irreversible loss rate (ILR) (mmol/h) of individual AA between pre- and during the intravenous infusion of the AA mixture in six pairs of twin sheep when one of each twin was infused with either saline (control) or lipopolysaccharide (LPS) (2 ng/kg body weight per min) for 20 h (Mean values and standard error of the difference between means)

* These values are the differences between pre- and during AA infusion shown in Table 3. The statistical outcome is the same as for the AA status×LPS status interaction term in Table 3.
† The rate of AA infused is subtracted from the difference between ILR measured for the pre- and during AA infusion and the net values analysed based on twin pair as a random effect and LPS status as a fixed effect. The mean values were then tested for statistical difference from zero based on the appropriate standard error and df. This analysis indicates whether the AA infusion causes a smaller, larger or equal effect on plasma ILR and whether the response differs between the saline- and LPS-infused sheep.
The maximal activity of SDH was increased in both kidney (83 %; P=0·008) and liver (90 %; P=0·019) for those animals that received the endotoxin (Table 5). On the basis of log-transformed data (the variance for liver was greater than for kidney), the specific activity of SDH was 4-fold greater in the liver than in the kidney (P=0·016), but this difference was not affected by LPS status.
Table 5 Kidney and liver serine-threonine dehydratase (SDH) activities (nmol/min per mg tissue protein) in six pairs of twin lambs with one of each twin infused intravenously for 20 h with either saline (control) or lipopolysaccharide (LPS) (2 ng/kg body weight per min) and with an AA mixture infused for the last 4 h (Mean values and standard error of the difference between means)
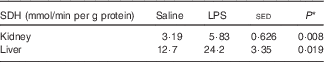
* By one-way ANOVA with twin pair and sheep within twin pair as random effects and LPS status as fixed effect (5 residual df).
Discussion
The decreased plasma concentrations observed for most essential and non-essential AA in response to LPS are similar, but not identical, to changes observed previously in steers( Reference Waggoner, Loest and Mathis 26 ) and foals( Reference Zicker, Spensley and Rogers 27 ) as well as in pigs with respiratory disease( Reference Le Floc’h, Deblanc and Cariolet 28 ). Under the conditions of endotoxaemia within the current ovine model, although the majority of AA (except leucine and tyrosine) showed altered plasma concentrations when LPS was infused, nine of these did not show a differential response compared with saline during the AA infusion (which can be taken as a surrogate for a protein source of similar AA composition). Therefore, simple changes in plasma concentrations do not necessarily reflect challenge-specific metabolic needs.
The concentration of AA in plasma depends on the balance between inflow (absorption, endogenous protein breakdown plus, in the case of non-essential AA, synthesis de novo) and outflow (removal for protein synthesis and AA oxidation). If one, or more, AA are utilised in response to a challenge then this will create an imbalance relative to the others that will be in corresponding ‘excess’ and need to be oxidised to maintain homeostasis. Thus, it is possible for all plasma AA to decrease even if the metabolic demands have altered only for a single AA.
Inappetence accompanies some disease states( Reference Breuille, Bechereau and Buffiere 15 , Reference Stipkovits, Ripley and Tenk 29 )and will therefore reduce inflow from digestion and alter plasma AA concentrations( Reference Le Floc’h, Deblanc and Cariolet 28 ), but this complication was avoided within the current study by the low dose of endotoxin infused. Although inflow from endogenous protein degradation can increase during infection( Reference Luiking, Poeze and Ramsay 9 ), plasma AA concentrations often decrease, indicating that outflows to protein synthesis and/or pathways of AA oxidation must increase by proportionally more( Reference Le Floc’h, Deblanc and Cariolet 28 ). Furthermore, the variety and complex nature of diseases, infections and injuries means that each will have different requirements for specific AA, while the severity and duration of recovery will also impact nutrient demand. Indeed, the basal diet itself can alter the magnitude of response, as shown by studies in which low- and high-protein diets were tested during challenge situations( Reference Houdijk, Kyriazakis and Jackson 4 ) or where nutrient demand was altered( Reference Houdijk, Jackson and Coop 30 ). In the present study a good-quality mixed ration was offered with adequate N to support limited growth.
In the experimental conditions employed, the endotoxaemia was assessed as mild (based on small rise in body temperature, the limited period of inappetence and the time taken for plasma AA concentrations to return to control values in the pilot study) and only the plasma concentration of phenylalanine increased. On the basis of the AA composition of acute-phase proteins and their increased synthesis by the liver during infection it has been hypothesised that demand for phenylalanine would increase( Reference Reeds, Fjeld and Jahoor 14 ). Such increased demands may be counteracted by less production of serum albumin, as occurs in response to reduced intake( Reference Connell, Calder and Anderson 31 ) or certain infections( Reference Zhou, Ren and Han 32 ), although this can vary with stage of infection( Reference Ruot, Bechereau and Bayle 33 ). In the current study, it would appear that any additional needs for phenylalanine have been met either from increased mobilisation of tissue protein and/or from reduced hepatic catabolism.
Glutamine is essential for lymphocyte metabolism( Reference Lobley, Hoskin and McNeil 34 ) and therefore support of the immune system. Substantial amounts of free glutamine are present in muscle( Reference Borgenvik, Nordin and Mikael 35 , Reference Sales, Pacheco and Blair 36 ), and when protein from this tissue is mobilised in response to infection there may be an associated release of sarcoplasmic free glutamine, but this appears to be insufficient to meet the metabolic demand by other tissues because the plasma concentrations are not maintained. The effect of the endotoxin challenge on plasma tryptophan was marginal, and this is perhaps surprising when the high abundance of this AA in positive acute-phase proteins( Reference Reeds, Fjeld and Jahoor 14 ) plus the stimulation of the kynurenine pathway during immune responses( Reference Wirthgen, Tuchscherer and Otten 37 ) is considered. This may be partly due to the low dose of LPS used compared with other studies( Reference Wirthgen, Tuchscherer and Otten 37 ).
The reasons for the decreases in plasma concentrations observed for the other AA may involve either a requirement to support specific metabolic processes related to the endotoxaemia and/or because they are in excess from breakdown of tissue protein to specific AA when they will be catabolised to establish a new homeorhetic state. Which of these is the reason cannot be ascertained by the response to LPS alone, but needs to be assessed from the combined effect of LPS and AA.
The theory is that if an AA is required to combat the endotoxaemia directly then the ILR response to the extra infused will differ between the saline- and LPS-treated twin animals. For arginine, aspartate, cysteine, glutamate, lysine (tendency only), glycine, methionine, proline, serine and threonine, there were smaller increments in ILR when the AA were infused into control (saline) animals than their twin treated with LPS. One explanation is that this represents greater net removal in response to the endotoxin challenge. Indeed, a number of studies have implicated greater requirement for arginine in injury and sepsis( Reference Yeh, Yeh and Lin 7 – Reference Zhou, Wu and Yin 10 ), probably due to changes in blood flow with associated need for greater nitric oxide synthesis( Reference Luiking, Ten Have and Wolfe 38 ). Furthermore, arginine supplementation lowered the increased villus height induced by LPS in rats( Reference Zhou, Wu and Yin 10 ), and reduced TNF-α in the ileum. These anti-inflammatory effects were enhanced when glutamine was added to arginine. Similarly, in rats with caecal ligature and puncture, arginine supplementation reduced the production of inflammatory mediators at the site of injury, but had no effect on either nitrogen balance or mortality( Reference Yeh, Yeh and Lin 7 ). Arginine supplementation also improved arterial blood pressure, liver and protein metabolism and intestinal motility in pigs with sepsis( Reference Luiking, Poeze and Ramsay 9 ).
During sepsis in rats, there is increased synthesis of both mucins and gut epithelial proteins, and this increases threonine demand to nearly 3-fold that of normal intake( Reference Faure, Chone and Mettraux 13 ). Although extra requirements to support mucin and gut epithelial protein synthesis may also occur in the current challenge, the increase in SDH activity in both liver and kidney indicated a probable increase in the rate of catabolism of threonine. If synthesis of mucin and other proteins was the key requirement, then some conservation through reduced degradation of threonine might be expected. Instead, the data suggest the opposite, and therefore products of threonine catabolism, such as serine, may be important in combating the consequences of endotoxaemia. This is supported by the alterations also observed for serine metabolism. What mechanisms are involved is unclear, but serine is a known gluconeogenic AA and may be needed to overcome the hypoglycaemia observed in this and other studies( Reference Bruins, Deutz and Soeters 39 ).
In other challenges, either cysteine( Reference Lyons, Rauh-Pfeiffer and Ming-Yu 11 ) or methionine has been identified as a key limiting AA, in combination with threonine in the case of HIV patients( Reference Laurichesse, Tauveron and Gourdon 16 ). Cysteine, from either dietary or endogenous protein sources or from metabolism of methionine, is required for glutathione synthesis. Nonetheless, although cysteine ILR increased by 40 % in children with sepsis, glutathione synthesis decreased by 60 %( Reference Lyons, Rauh-Pfeiffer and Ming-Yu 11 ), which may imply impaired antioxidant protection. In contrast, although appearance of synthesised glutathione in blood was also decreased in septic rats, there was a concomitant increase in synthesis within the liver, lung and spleen( Reference Malmezat, Breuille and Capitan 40 ). This illustrates that caution must be exercised when only plasma kinetics are available, as in the current study. Nonetheless, deficiency of sulphur-AA has been suggested to compromise anti-inflammatory responses( Reference Grimble and Grimble 41 ), supported by earlier observations in sheep treated with endotoxin that showed a marked decrease in plasma methionine( Reference Hofford, Milakofsky and Pell 42 ), whereas in septic rats there was an 80 % increase in the trans-sulphuration pathway that leads to cysteine synthesis( Reference Malmezat, Breuille and Pouyet 43 ). Although the mild endotoxaemia induced in the current sheep did not lead to altered cysteine ILR, the response to supplemental methionine was reduced during the LPS challenge. Catabolism of methionine would not only increase intracellular cysteine for synthesis of glutathione but is linked to synthesis of spermine and spermidine( Reference Ruseva, Lozanov and Markova 44 ), polyamines that are key components during cell proliferation( Reference Landau, Bercovich and Park 45 ), which may be needed to support the increased cell division when villi height increases( Reference Zhou, Wu and Yin 10 ). Nonetheless, in other studies where gut-related responses to LPS have been observed these have usually involved an enteral challenge or use of live bacteria. The current study used parenteral supply of LPS and it is unclear whether this would produce direct effects on the digestive tract. If not, then the severity of the impact and the absolute needs for specific nutrients may be different.
Despite claims for the importance of intervention with individual AA, for certain challenges a more effective treatment may require combinations of AA( Reference Zhou, Wu and Yin 10 , Reference Laurichesse, Tauveron and Gourdon 16 ). One example involves sepsis induced by injection of live bacteria into rats and where a factorial approach determined that a combination of cysteine, threonine, serine, aspartate and/or asparagine both reduced the severity of weight loss and hastened the recovery process( Reference Breuille, Bechereau and Buffiere 15 ). Similarly, the approach in the current study would also indicate that several AA are involved in the metabolic response to the endotoxin challenge.
While identification of the key AA needed as part of the animal’s response to endotoxaemia was one aim of the study, the other target was to try and quantify the actual amounts needed. This was estimated on the basis of the simple criterion as to whether the plasma concentrations observed when AA was supplied to the LPS-treated twin restored values to those observed for the control (saline-infused) twin before any intervention. This was achieved for the majority of AA, but with concentrations in excess of control sheep for histidine and phenylalanine but in deficit for glutamine (−15 %), methionine (−22 %), serine (−29 %), leucine (−20 %) and valine (−15 %). The response for phenylalanine fitted with the increase in both concentration and ILR in response to LPS. For histidine, the amount of AA infused exceeded the observed difference in ILR between the saline- and LPS-infused sheep, and this probably accounts for the higher plasma concentration. For most of the remainder of the AA that were not restored to control values in the sheep given both LPS and AA, the amount of AA infused did match the observed difference in ILR between the saline- and LPS-infused twins. Thus for these AA, the simple approach of ILR multiplied by the fractional decrement in plasma AA concentrations did not exactly restore plasma concentrations. This may be due to intracellular use of these AA through mechanisms that are not reflected in plasma fluxes, and this will require further investigation. Indeed, the current ‘difference’ approach does not directly prove how much of each supplemental AA is used to support protein synthesis, or other metabolic mechanisms associated with the inflammatory response, and how much might simply be catabolised as excess to requirements. That would need to be checked by more detailed studies on rates of production of key proteins and intermediary metabolites or direct measures of AA catabolism. The latter is often assessed by rates of 13CO2 oxidation, but this could not be monitored in the current study because of the use of simultaneous infusion of multiple 13C-AA tracers and the presence of other 13C-labelled intermediary metabolites within the whole algal hydrolysate.
The current study combines earlier approaches, based either on changes in plasma AA concentrations or single AA fluxes in response to health challenges, by combining data for all AA fluxes and then predicting the additional AA required on the basis of a priori information. The approach identifies the key AA involved and allowed reasonable assessment of the extra amounts of these required in response to the endotoxaemia. The different proportional responses between individual AA emphasise that targeted supplements rather than just increasing protein intake is the better approach. For example, during the LPS challenge the additional requirements for proline, methionine and threonine would require 42, 73 and 125 g/d, respectively, of a high-quality protein source (beef). Therefore, meeting threonine requirements through additional protein would provide a large excess of other AA, which would be catabolised and represent an inefficient approach in both metabolic and economic terms.
Although there still remain some anomalies, and further study is necessary to elucidate these, the method can be applied to any stress condition and specific phase, so that appropriate adjustments in both the type and amount of supplemental AA can be applied.
Acknowledgements
The expertise of A. Graham Calder and Susan Anderson for the various stable isotope analyses is gratefully recognised. Celine Germaine performed the SDH measurements from the tissue samples as part of a student placement from AgroParisTech, Paris, France.
This study was funded as part of the core grant provided to the Rowett Institute of Nutrition and Health, University of Aberdeen and Biomathematics and Statistics Scotland by Rural and Environment Science and Analytical Services, part of the Scottish Government. Hoskin was a recipient of a FoRST (NZ) award to study abroad.
The authors’ contributions were as follows: S. O. H. and G. E. L. were responsible for study concept and design. S. O. H., G. E. L. and D. M. B. were responsible for data collection and collation. G. E. L. and G. H. were responsible for data analysis and statistical matters. S. O. H., G. E. L. and G. H. were responsible for the first draft and critical revision of the manuscript for important intellectual content.
The authors declare that there are no conflicts of interest.










