While the glucose facilitation of cognition effect is fairly well established, a number of experiments have failed to replicate these beneficial effects. This observation suggests the existence of moderating factors that need to be identified in order to understand the variability observed in human studies.
Previous research has already identified a number of factors responsible for inter- and intra-individual differences in response to glucose administration. These include task difficulty(Reference Owens and Benton1–Reference Meikle, Riby and Stollery6), task domain(Reference Foster, Lidder and Sünram7–Reference Riby, Meikle and Glover9), age(Reference Meikle, Riby and Stollery6, Reference Riby, Meikle and Glover9), glucoregulatory control(Reference Messier, Desrochers and Gagnon10–Reference Sünram-Lea, Owen and Finnegan12) and BMI(Reference Sünram-Lea, Owen and Finnegan12). More specifically, in terms of task and stimuli characteristics that can moderate the cognitive benefits of glucose administration, it has been argued that glucose facilitation in healthy young participants tends to be demonstrated on tasks that require greater ‘cognitive effort’(Reference Kennedy and Scholey4) and/or that the glucose enhancement effect is most robust in tasks that pertain to hippocampal memory systems(Reference Foster, Lidder and Sünram7, Reference Messier8). Individual differences in somatic and behavioural state or trait also appear to moderate the glucose facilitation effect. For example, it has been suggested that glucose administration preferentially improves memory in human subjects that have poor glucose regulation and that the effects are therefore less likely to be observed in good glucose regulators(Reference Messier8, Reference Awad, Gagnon and Desrochers11). Moreover, in our own laboratory, we have recently observed both direct effects of glucose regulation (irrespective of drink) on mood and cognition, and moderation of the dose–response profile by inter-individual differences in glucose response(Reference Sünram-Lea, Owen and Finnegan12). In general, our data suggested that individuals with better glycaemic control also demonstrate performance improvements following higher glucose dosages. Insulin resistance and poor glucose tolerance become a greater issue in middle age; however, our previous research has demonstrated that even young healthy individuals may show performance decrements due to poor glucoregulation, which may be ameliorated by supplementation. Therefore, as a direct extension of Sünram-Lea et al. (Reference Sünram-Lea, Owen and Finnegan12), which also used healthy young adults (aged 18–25 years), we intended to further examine individual differences in a young sample. Furthermore, while many studies into the glucose enhancement effect are restricted to singe doses (typically 25 g), it was felt that restricting the study to one dose may have resulted in missing some glucose effects observed in our previously work; therefore, in the present study, both the lower dose of 25 g and a higher dosage of 60 g were used to examine individual differences in this sample.
It is important to note that none of the above-mentioned studies has used a standardised oral glucose tolerance test (OGTT) for the evaluation of glucose tolerance. The OGTT involves administration of a 75 g glucose load after a minimum 8 h fast and is the ‘gold standard’ test for the diagnosis of diabetes mellitus (WHO, 1999). The importance of using a standard OGTT to evaluate glucose tolerance is highlighted by the observation that in our previous study, we observed that participants, classified as high responders following a 60 g load, did not show significant differences in glycaemic response following the administration of 25 g glucose(Reference Sünram-Lea, Owen and Finnegan12). Consequently, administration of less than 75 g glucose might not be sufficient to expose differences in glucose regulation and tolerance in healthy young adults. Even though the present findings support the notion that glucoregulation is a moderating factor, future research using a standard glucose tolerance test for classification purposes is necessary. Moreover, there is currently no consensus as to which glucoregulatory index is best suited to predict cognitive performance in response to glucose administration in normoglycaemic samples. Previously used estimates of glucoregulation included fasting levels, peak glucose levels, recovery and evoked glucose to baseline levels and AUC(Reference Donohoe and Benton2, Reference Donohoe and Benton3, Reference Messier, Desrochers and Gagnon10, Reference Craft, Murphy and Wernstrom13–Reference Vanhanen and Soininen18). Implementation of a proper OGTT for classification purposes might also help establish which glucoregulatory index is the better predictor of glucose effects on cognition.
Another potential source for variability appears to be neuroendocrine and, more specifically, cortisol status at the time of testing. For example, in our own laboratory, we have observed that administration of a glucose drink (25 g) can significantly blunt the cortisol response to a brief naturalistic stressor that has both a psychological and a physical component, especially firefighting training(Reference Robinson, Leach and Sünram-Lea19). More specifically, the data revealed that firefighters who ingested a glucose drink had significantly lower cortisol levels compared with those who received a placebo drink, suggesting that cortisol levels remain low in a stressful situation when glucose is administered. In addition, it was observed that individuals with a greater cortisol response were more susceptible to the glucose facilitation of cognition. Since it has been suggested that performing a cognitive task itself could act as a psychosocial stressor(Reference Gibson and Green20), it is possible that glucose administration might lower the cortisol response in healthy young participants, and individual differences in cortisol response may in turn moderate cognition and the effects of glucose administration on cognition.
A further potential moderating variable of the glucose facilitation effect appears to be weight. Our own data suggest that participants with a BMI of 25 kg/m2 and below benefit from the administration of higher glucose loads (65 g), whereas those with a higher BMI (>25 kg/m2) show decrements when given a high glucose load(Reference Sünram-Lea, Owen and Finnegan12). Body weight adjusted for stature is often used as an alternative to the measurement of adipose tissue mass in the evaluation of individuals or populations for obesity(Reference Keys, Fidanza and Karvonen21). BMI strongly correlates with body fat and other measures related to adiposity, and thus is a reasonable index of adiposity(Reference Keys, Fidanza and Karvonen21, Reference Strain and Zumoff22). Consequently, the moderating effects of body composition (BC) merit further investigation, and the present study aimed to recruit a sample of participants with a range of BMI scores, as a proxy for adipose tissue mass. However, while BMI is a useful index, this measure provides no information regarding actual BC such as body fat and muscle weight. Therefore, a number of other body measurements including body fat were measured in the present study in order to acquire a more comprehensive index of BC for the analysis.
Consequently, in order to further our understanding of the response variability observed in human studies, the aim of the present study was to investigate some of the factors that have been observed to exert a moderating role on the behavioural response to glucose facilitation, including glucose regulation, BC and hypothalamic–pituitary–adrenal axis response, were evaluated and their moderating effects on glucose facilitation effects investigated.
Methods
Study population
A total of twenty-four healthy young individuals took part in the present study. The age range was 18–30 years (mean age 20 years), with a mean BMI of 24 kg/m2. Participants were recruited via an opportunity sample from the University of Lancaster. Only those between the age range of 18–30 years were recruited (mean age 20 (sd 1·693) years). Of the participants, ten were overweight or obese ranging from 25 to 32·2 kg/m2 (mean BMI 27·61 (sd 2·927) kg/m2) and fourteen participants were of low or normal body mass ranging from 16·10 to 24·7 kg/m2 (mean BMI 20·79 (sd 2·927) kg/m2). BMI classifications were carried out in accordance with the WHO guidelines. All procedures were carried out with a signed consent of the participants, and they were tested according to the national and local ethics guidelines in accordance with the Declaration of Helsinki, and ethical approval was gained from the Lancaster University Board of Ethics. Participants were excluded from the study on the basis of several criteria. Information regarding these criteria was gathered using a modified version of the Blood Services screening questionnaire (National Blood Service, 2002). Exclusion criteria included current active infections, jaundice within the last year, hepatitis, haemophilia, HIV antibody positive, diabetes mellitus, awakening times of earlier than 06.30 hours or later than 08.00 hours, medications which have been shown to affect cortisol, such as antidepressants or the oral contraceptive pill, and intolerance or allergic reaction to substances that contain phenylalanine. Participants received £30 sterling for taking part in the experiment.
Treatment and design
The study followed a mixed, placebo-controlled design, with a counterbalanced within-participant design for drink administration and a between-participant design for BC and glucose regulation. Treatment order was randomly assigned using a Latin square design and drink administration was double-blind (all drinks were matched for sweetness and colour). Drinks were manufactured by GlaxoSmithKline and contained 330 ml of non-carbonated solutions with additions of glucose (0, 25 and 60 g glucose) as required, and sweetness and flavour was matched using artificial sweeteners and pharmacologically inactive flavourings.
Blood glucose measurement
Blood glucose measurements were obtained using an over-the-counter finger-prick glucose monitor. The ExacTech® blood glucose monitoring equipment (supplied by MediSense Britain Limited) was used following the recommended procedure. The high accuracy and consistency of MediSense blood glucose sensors has been established previously.
Body composition measurement
BC measures were taken using a Tanita body composition monitor which uses bioelectrical impedance analysis. Impedance analysis is the use of safe, low-level electrical signals that are passed through the body via the footpads on the monitor platform. It is easy for the signal to flow through fluids in the muscle and other body tissues but meets resistance as it passes through body fat, as it contains little fluid. Impedance readings are then entered into medically researched mathematical formulas to calculate an indirect measurement of BC; for example, free fat mass, muscle mass and basal metabolic age are determined using this measurement (for further information see http://www.tanita.eu/; Tanita UK Limited).
Cortisol measurement
Saliva samples were collected using a salivette saliva sampling device (Sarstedt Limited), and participants were instructed to give saliva samples by placing a salivette under their tongue for a timed 2 min period. The samples were stored at − 80°C until analysis. Saliva was recovered from the salivettes by centrifugation and salivary volume determined by weighing. This allowed for calculation of the saliva flow rate. Cortisol concentration (nmol/l) in saliva was determined by the high-sensitivity salivary cortisol enzyme-linked immunoassay kit (Salimetrics) according to the manufacturer's instructions.
Procedure
Before the start of the study, participants were phone-screened in order to ensure their suitability for the study; if suitable, treatment order was then randomly allocated with treatments being counterbalanced. Each participant was required to attend the laboratory on four separate occasions. All participants were instructed to fast 12 h before each test session (standard fasting period for the OGTT) and to refrain from smoking or ingesting any other stimulants for 6 h before testing. Participants were tested between 09.00 and 12.00 hours with a 1-week washout period between the test sessions.
The OGTT was carried out at the first visit between 09.30 and 12.30 hours for all participants. Upon entering the laboratory, participants' blood glucose was measured (baseline levels). Participants were then instructed to drink a solution containing 75 g glucose over a timed period of 5 min. Further blood glucose measures were taken at half-hourly intervals for a total of 3 h. Measurements of height, weight, the percentage of body fat, basal metabolic age (Tanita UK Limited) and the waist:hip ratio were also measured. Following the OGTT, participants were then familiarised with the cognitive test battery by completing all of the cognitive tests to be assessed at later visits; this was done in order to reduce error and practice effects. Completion of the cognitive tests was intended to familiarise participants with the test battery and procedure, and data from this training session were not included in the statistical analysis. For active study days, each visit was at the same time of day (between 09.00 and 12.00 hours) with a 1-week washout period between the study days. Following baseline blood glucose and cortisol measurements, participants received either a glucose-containing drink (containing 25 or 60 g glucose) or a placebo drink, depending on the treatment they were assigned to. At 15 min after consumption of the drink, a further blood glucose measurement was taken followed by cognitive and mood testing. Once the assessment of cognition and mood was completed final blood glucose and cortisol samples were taken. At the end of the fourth visit, participants were debriefed and thanked for taking part.
Cognitive tests and mood assessment
Computerised assessment was used to evaluate cognitive performance. A selection of computer-controlled tasks was administered with parallel forms of the tests being presented at each test session. The order in which parallel task versions were administered was fully counterbalanced across the participants and conditions. Task presentation was via computer screen, and with the exception of written word recall tests, all responses were recorded via button responses. In each testing session, the following assessments were administered.
Word presentation
A list of twenty words matched for frequency (M= 34·37), concreteness (M= 4·99) and imagery (M= 4·97)(Reference Clark and Paivio23) was presented on the monitor at the rate of 1 every 2 s for participants to remember. While the participants were presented with the twenty-item word list, they were also required to perform two types of complex hand motor sequences, which were practised with each participant before the first presentation of the word list. Participants were instructed to share their attention equally between the two tasks, and were told that they should perform to the best of their ability on each of the two tasks. See Sünram-Lea et al. (Reference Sünram-Lea, Owen and Finnegan12), for full details of the motor task. The ability of the participants to perform the hand movement task was not assessed and incorrect hand movements were not recorded.
Immediate word recall
This test session assessed immediate free recall memory performance from a supraspan word list. Participants were given 60 s to write down as many of the words as possible. Recall was scored as the number of correct words and the number of errors.
Computerised serial sevens task
This task evaluated working memory performance(Reference Scholey, Harper and Kennedy24). Participants were required to compute a running subtraction of 7, starting from a randomly generated number. Participants were given 120 s to complete this task. This task was administered twice consecutively totalling 240 s. The task was scored as number of correct subtractions.
Computerised Corsi block-tapping task
This task assessed the visual memory span(Reference Milner25). Illuminated buttons appeared on the screen. The buttons flashed after each other in a tempo of one per s. Then, the participants pointed to the buttons in the same order as they appeared on the screen. The task was scored as the number of correct responses.
Computerised serial threes
This task evaluated working memory performance(Reference Scholey, Harper and Kennedy24). Participants were required to compute a running subtraction of 3, starting from a randomly generated number. Participants were given 120 s to complete this task. This task was administered twice consecutively totalling 240 s. The task was scored as the number of correct subtractions.
Delayed word recall
This task assessed delayed free recall memory performance from a supraspan word list. Participants were given 60 s to write down as many of the words as possible. The task was scored as the number of correct recalls.
Bond and Lader visual analogue scales
A total of sixteen visual analogue scales(Reference Bond and Lader26) were presented on the monitor. Subjective responses were measured via a mouse-click. Participants were instructed to ‘use the mouse to position the arrow at the point on the scale that represents how you feel at the present time’. The responses to the sixteen individual scales were combined as recommended by the authors to form three mood factors: ‘alertness’; ‘calmness’; ‘contentedness’.
Statistical analysis
All data were analysed using SPSS statistical software (IBM SPSS). Before the analysis of moderating factors, drink effects on blood glucose values and cortisol values were examined using a two-way repeated-measures ANOVA, the factors being dose (three levels; 0, 25 and 60 g glucose) and time (blood glucose, three time points; cortisol, two time points) with repeated measures on both factors. Where significant statistical effects were identified by ANOVA, Bonferroni post hoc testing was subsequently conducted. Drink effects on mood and cognition were analysed using Dunnett's test (two-sided) for planned (a priori) comparisons. As mood was assessed before and after drink administration, scores were transformed into change from baseline scores (post–pre-dose score). Cognitive performance was only assessed after drink administration; therefore, absolute measures were used for the analysis.
Analysis of moderating factors; post hoc division of sample based on glucose regulatory indices
AUC
The moderating effects of glucose regulation were assessed by the calculation of the AUC of evoked glucose levels. Glucose regulation has been routinely indexed using the AUC(Reference Moore, Cherrington and Mann27–Reference Convit, Wolf and Tarshish30). However, differences in calculations have been noted, and we therefore elected to examine two formulae for computation of the AUC: (1) AUC with respect to increase (AUCI) and (2) AUC with respect to ground (AUCG)(Reference Pruessner, Kirschbaum and Meinlschmid31). Both computations were used since it has been suggested that both formulae reveal different information(Reference Pruessner, Kirschbaum and Meinlschmid31), with AUCG being related to total peripheral circulating glucose (i.e. taking into account basal glucose levels) and AUCI being related to the sensitivity of the system (i.e. how efficiently the glucose–insulin system responds to a glucose load, not taking into account basal circulating glucose). This was followed by the post hoc division of all participants into two equal groups depending on the AUC (AUCI: small ( ≤ 11·33), large (>11·33); AUCG: small ( ≤ 45·13), large (>45·13)). This division was carried out for the AUCG and AUCI observed during the standard OGTT (75 g).
Fasting glucose and glucose concentration 2 h after ingestion
In addition, we determined glucose regulation by a median split of (1) fasting glucose levels (low ( ≤ 5·25) and high (>5·26)) and (2) levels observed 2 h after ingestion (low ( ≤ 7·10) and high (>7·11)) observed during the standard OGTT.
Post hoc division of samples based on hypothalamic–pituitary–adrenal axis activation
In order to asses the moderating effects of cortisol response to cognitive testing (a potential stressor(Reference Gibson and Green20)) and controlling for potential drink effects on cortisol levels, change from baseline levels following placebo administration was calculated. Given that cortisol levels fell throughout testing, participants were banded into two groups using a median split: those participants who experienced the greatest fall throughout testing (difference from baseline ≤ − 0·12 μg/dl) and those who experienced a more modest fall in cortisol response throughout the testing session (difference from baseline ≥ − 0·11 μg/dl).
Post hoc division of samples based on body composition
In order to assess the moderating effects of BC, a BC score was created using Cronbach's α analysis to assess the inter-item correlation of different body measurements. Cronbach's α analysis was conducted on various (raw data) BC measures. Indices were then transformed to z-scores (Z= (x− mean)/sd) in order to standardise the indices and create a composite score. Indices with a low corrected item-total correlation were removed from the analysis in order to produce a highly correlated composite score. The BC composite score included the following measures: BMI; body fat; muscle mass; basal metabolic age (Chronbach's α = 0·94). The composite score was then calculated by taking the sum of all other variables divided by the number of variables. This was followed by the post hoc division of all participants into two equal groups (low BC ≤ 0·07, high BC ≥ 0·08). A low BC score denotes a low BMI, a low muscle mass, a low basal metabolic age and a low body fat, and vice versa for those with high BC scores. The physical characteristics of the different groups are presented in Table 1.
Table 1 Physiological characteristics of the low- and high-body composition (BC) groups (Mean values and standard deviations)

Separate ANOVA with glycaemic response, change in cortisol levels and BC as the between-subject factor and dose as the within-subject factor were then conducted on cognitive and mood measures as well as on relevant physiological measures (effect of BC on (1) glycaemic response and (2) cortisol response). Where significant statistical effects were identified by ANOVA, Bonferroni comparisons were subsequently conducted. For all post hoc comparisons, only significant effects and interactions of moderating variables or those reaching the significance level are reported.
Results
Glycaemic response
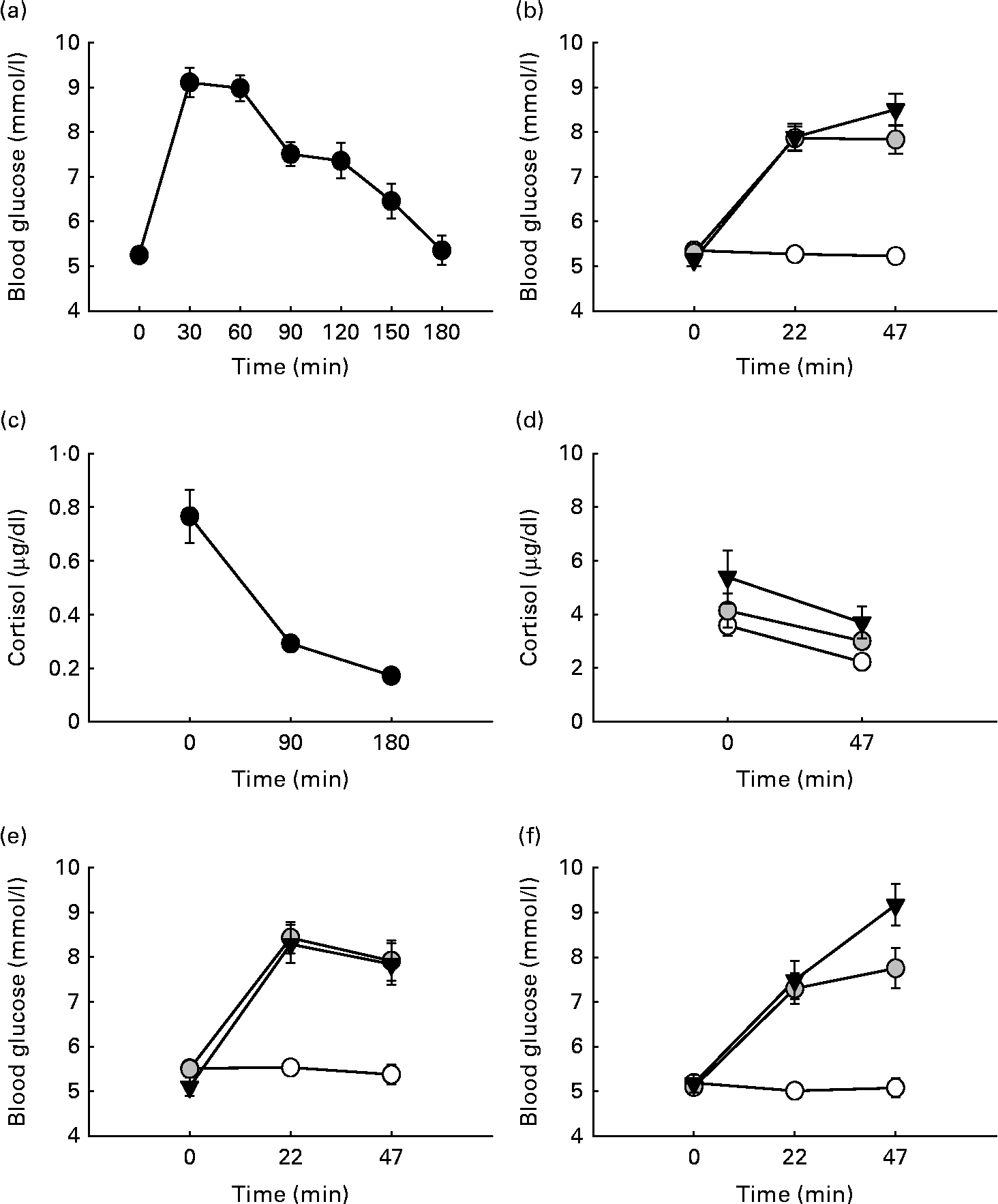
The mean glycaemic response at the OGTT is shown in Fig. 1(a). During cognitive testing, there was a significant main effect of time (F(2,46) = 61·33, P< 0·001) and drink (F(2,46) = 65·32, P< 0·001) on blood glucose values. There was also a significant time × drink interaction (F(4,92) = 30·03, P< 0·001). The post hoc analysis showed that following the administration of the placebo drink, blood glucose levels remained stable, whereas a significant rise in blood glucose levels was observed following the administration of 25 and 60 g glucose at testing times 22 and 47 min compared with baseline (all P< 0·001). Blood glucose levels did not differ significantly between the two glucose dosages (see Fig. 1(b)).

Fig. 1 Participants' glycaemic response to glucose loads over time. (a) Participants' glycaemic response at the oral glucose tolerance test (OGTT). Blood glucose values (mmol/l) are shown following a 75 g glucose load over the course of 180 min. (b) Participants' glycaemic response at testing. Blood glucose values (mmol/l) are shown following 0, 25 and 60 g over the course of the 47 min testing sessions. (c) Participants' cortisol response over time during the OGTT. (d) Participants' cortisol response during testing. (e) Glycaemic profile of participants with low body composition (BC) scores during the test following 0, 25 and 60 g over the course of the 47 min testing sessions. (f) Glycaemic of participants with high BC scores during the test following 0, 25 and 60 g over the course of the 47 min testing sessions. Values are means, with their standard errors represented by vertical bars. ![]() , Placebo;
, Placebo; ![]() , 25 g glucose;
, 25 g glucose; ![]() , 60 g glucose.
, 60 g glucose.
Cortisol response
There was a significant main effect of cortisol over time during the OGTT with cortisol falling over the 3 h period (F(2,38) = 33·562, P< 0·001) being consistent with the usual diurnal fall in cortisol at this time of day (see Fig. 1(c)). There was a significant effect of drink (F(2,24) = 6·162, P= 0.007), with the lowest cortisol levels observed for the placebo group (0·291 (se 0·029)), followed by the 25 g (0·358 (se 0·038)) and 60 g glucose-administered groups (0·454 (se 0·071)). There was also a significant drink × time interaction (F(2,24) = 8·958, P= 0·011). Post hoc comparisons revealed that at the end of the test sessions, cortisol levels were significantly higher following 25 and 60 g glucose compared with placebo (P= 0·024 and P= 0·046, respectively). Cortisol levels fell significantly following placebo (P>0·001), whereas no significant differences compared with baseline were observed following glucose administration (see Fig. 1(d)).
Subjective mood
Planned comparisons showed that compared with placebo, glucose administration did not affect any of the subjective mood measures (see Table 2).
Table 2 Subjective mood measures as a function of dose and time (Mean values and standard deviations)

Cognition
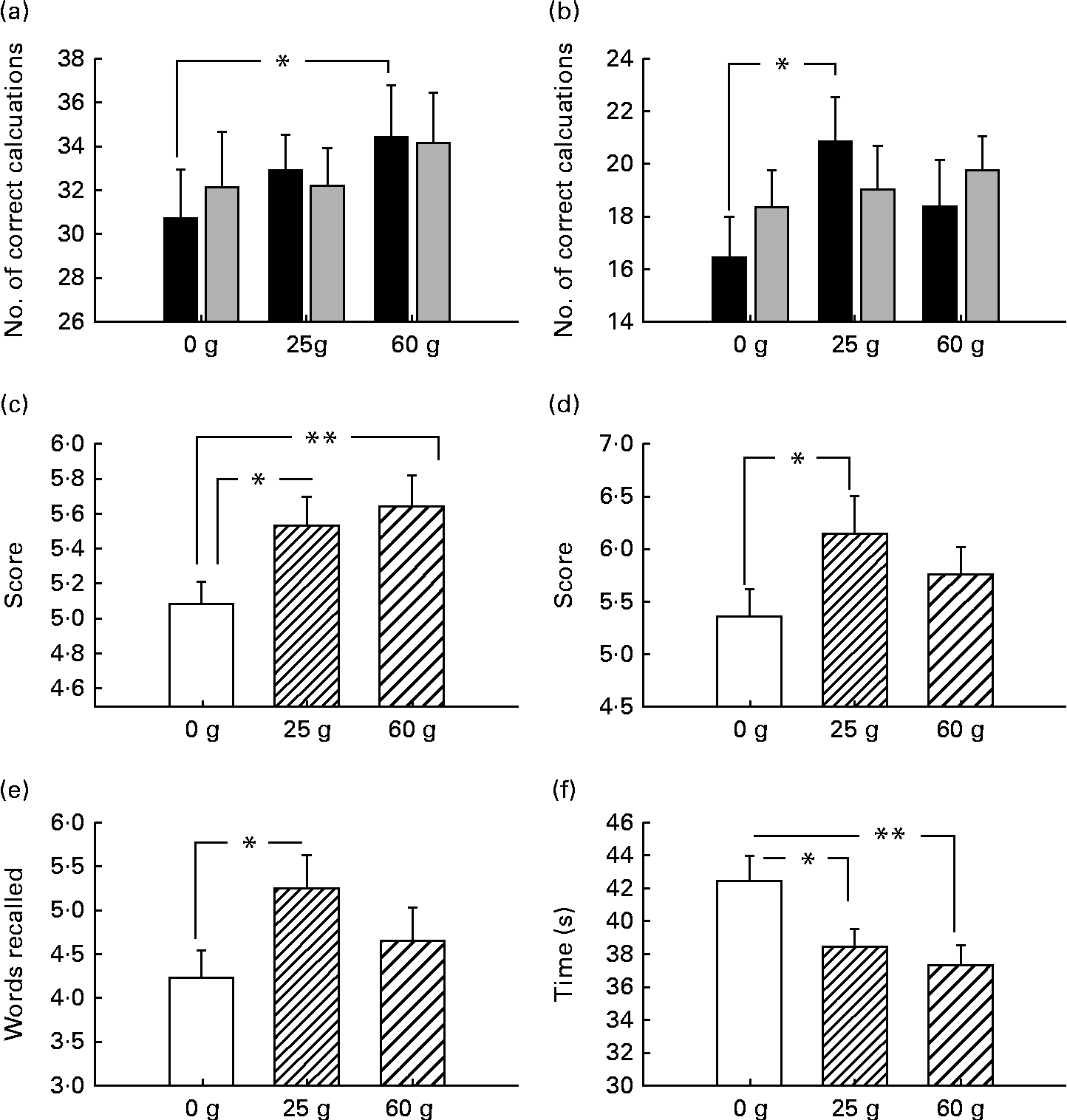
Before examining the effects of moderating variables on cognitive function, the glucose facilitation effect was first established. In terms of cognition, there was improved performance pertaining to working memory, with improved performance following 60 g glucose on the serial threes task (P< 0·05) and 25 g glucose facilitated performance on the serial sevens task (P< 0·05). Both glucose dosages improved spatial working memory performance (Corsi block task: for 25 g, P< 0·05; for 60 g, P< 0·01). Verbal declarative memory was also improved (immediate and delayed word recall: both P< 0·05). Both glucose dosages (25 and 60 g) improved word recognition reaction time (P< 0·05 and P< 0·01, respectively; see Fig. 2(a)–(f)).

Fig. 2 Behavioural (cognitive) response to ingestion of 25 and 60 g glucose compared with placebo. (a) Improved performance following 60 g glucose on the serial threes task and (b) 25 g glucose facilitated performance on the serial sevens task. (c) Both glucose dosages improved spatial working memory performance on the Corsi block task. (d) Immediate and (e) delayed verbal declarative memory was also improved. (f) Both glucose dosages (25 and 60 g) improved word recognition reaction time. □, 0–2 min; ![]() , 2–4 min. *P< 0.05, **P< 0.01.
, 2–4 min. *P< 0.05, **P< 0.01.
Analysis of response variability
Although there was a high correlation between the AUCG and the AUCI (r − 0·867, P< 0·001), the analysis of the data revealed differences in the way they influenced glucose effects on mood and memory. For the AUCG, a significant drink × AUC interaction was observed on the level of alertness (F(2,44) = 5·46, P< 0·008). Further post hoc comparison revealed that for those with poorer glycaemic control, administration of 60 g glucose resulted in a significant decrease in alertness compared with placebo and 25 g glucose (both P< 0·02). In terms of cognitive performance, a significant drink × AUCG interaction on immediate word recall accuracy (F(2,44) = 3·207, P= 0·050) was observed, which was due to those with poorer glycaemic control performing significantly better following the administration of 25 g glucose compared with placebo (P= 0·019). For fasting blood glucose levels, a significant drink × fasting glucose interaction was also observed on immediate recall (F(2,44) = 6·075, P< 0·01) and alertness (F(2,44) = 3·8212, P= 0·029). Post hoc testing revealed that for those with higher fasting blood glucose levels, the administration of 25 g glucose resulted in improved recall performance (P< 0·01), whereas their alertness levels significantly decreased following the administration of 60 g glucose (P< 0·05). Glycaemic response as indexed by the AUCI or blood glucose levels 2 h post-ingestion had no significant and/or meaningful effects on any of the mood or cognitive measures. BC and cortisol responses did not moderate response to glucose drink or cognition and mood per se, i.e. no significant and/or meaningful effects or interactions were observed.
Relationship between body composition glycaemic response and cortisol
For the glycaemic response during the OGTT, a significant interaction between BC and time on blood glucose values was observed (F(6,132) = 3·209, P= 0·006). In addition, a significant time × BC interaction (F(2,44) = 4·884, P= 0·012) was observed at testing. Further post hoc analysis of the interaction showed that during the OGTT, participants with a high BC index displayed a steeper rise in blood glucose levels (30 min after administration, P< 0·001). All other comparisons did not withstand post hoc Bonferroni correction; however, inspection of the means showed that those with a high BC index displayed a steeper rise and a sharper drop in blood glucose levels both at the OGTT and irrespective of the drink at testing. The dose × time × BC interaction failed to reach significance (F(4,88) = 1·954, P= 0·109); however, it is interesting to note that following the administration of 60 g glucose, those with a low BC had falling blood glucose trajectories by the end of the test session while for those in the high BC group, blood glucose levels were still rising (see Fig. 1(e) and (f)). BC did not affect the cortisol response to testing.
Discussion
The main aim of the present study was to further explore the previously observed response variability to glucose administration. In terms of general drink effects, these were observed on a number of tasks at both dosages. The finding that verbal declarative memory was facilitated by 25 g glucose is in line with previous research suggesting that glucose effectively improves performance on the measures of verbal recall tasks at this dosage(Reference Sünram-Lea, Foster and Durlach5, Reference Foster, Lidder and Sünram7, Reference Sünram-Lea, Owen and Finnegan12, Reference Sünram-Lea, Foster and Durlach32–Reference Sünram -Lea, Foster and Durlach34). Furthermore, while no effect of word recognition accuracy was observed, speed of recognition was significantly improved following the administration of both 25 and 60 g glucose. Green et al. (Reference Green, Taylor and Elliman35) reported faster reaction times for recognition following the administration of 50 g glucose. The effects of glucose on reaction time have been somewhat inconsistent; however, the data suggest that this might be due to the necessity for higher glucose loads in order to elicit enhancement effects. Alternatively, the lack of robust findings might be due to word recognition speed not being a ‘pure’ measure of reaction time, but representing a proxy measure for more complex functions. Improved performance also was observed on numeric working memory at both dosages. Serial threes task performance was augmented at the 60 g glucose dose, whereas serial sevens performance by 25 g glucose. It is interesting to note that performance on the more demanding serial sevens task was improved following a 25 g glucose dose, whereas performance of the less demanding serial threes task was improved following 60 g glucose. The fact that, in the present study, the task that supposedly requires less effortful processing (and potentially less metabolic resources) was facilitated by the larger glucose load is surprising. However, we have previously observed this same pattern of effects(Reference Owen, Finnegan and Hu36). Kennedy & Scholey(Reference Kennedy and Scholey4) found that 25 g glucose significantly improved performance on serial sevens but not on serial threes. They suggested that supplemental glucose preferentially targets tasks with a relatively high cognitive load. The suggestion that effortful cognitive processes may be ‘fuel limited’ and therefore augmented by the simple provision of supplemental metabolic substrates has previously been theorised(Reference Moss, Scholey and Wesnes37–Reference Scholey, Sünram-Lea and Greer41). However, the findings of the present study suggest that the notion that only highly demanding tasks are ‘fuel limited’ may require some revision, as we have demonstrated that improved performance of the less demanding serial threes task can be observed following 60 g glucose.
Spatial working memory in the form of the Corsi block task was improved following the administration of both glucose drinks. The observation that both dosages of glucose appear to be having improving effects on cognitive performance may be due to the moderation of the glucose effects by individual physiological factors, especially given that recruitment of participants was carried out in order to include a wide variation of body types.
When looking at the glycaemic response during the OGTT, blood glucose levels peaked 30 min post-glucose administration and fell over the course of the 3 h period, returning to baseline within the last 30 min. There was a significant rise in blood glucose from 0 to 30 min; however, there was no significant difference in blood glucose values between 30 and 60 min. In our previous studies where testing lasted up to an hour, we have observed a significant rise and fall of blood glucose within this time frame. Failure to observe a fall in blood glucose levels between 30 and 60 min during the OGTT was most probably due to a higher glucose load at 75 g or might reflect the differences between increased cognitive demand (in the case of a cognitive testing session) and a period of no cognitive demand, as is the case for the OGTT where the participants simply rest. It has been shown that cognitive processing can be associated with a moderate drop in blood glucose levels(Reference Donohoe and Benton2). If effortful processing is indeed a moderator of ambient blood glucose levels, then the need to use a more standardised assessment of glycaemic response through an OGTT rather than the assessment of blood glucose levels during a testing session is further highlighted.
During the testing session, both glucose drinks (25 and 60 g) raised blood glucose levels significantly compared with placebo, but there was no significant difference between the two glucose drinks. It is interesting to note that the differential task effects occurred, despite there being no significant difference in the glycaemic response to the two doses. Failure to observe differences in the blood glucose response to the two glucose doses is probably due to the short overall testing time (47 min), and suggests that the effects may not be a direct effect of increased energy from available blood glucose, but rather due to differences in downstream physiological processes, most probably insulin levels(Reference Gerozissis42).
However, further analysis revealed that blood glucose trajectories differed depending on BC. Participants within the high BC group (higher BMI, higher body fat percentage, lower muscle mass and higher metabolic basal age) displayed a steeper rise in blood glucose levels following glucose ingestion. Those with a low BC index had an overall lower glycaemic response over time. It has been argued that obese individuals develop resistance to the cellular actions of insulin, characterised by an impaired ability of insulin to inhibit glucose output from the liver and to promote glucose uptake in fat and muscle(Reference Saltiel and Kahn43, Reference Hribal, Oriente and Accili44). Both effects of insulin insensitivity on the liver and muscle tissue cause elevations in blood glucose levels. Our data tentatively suggest that some degree of insulin resistance or insensitivity in participants with higher body composite scores might be responsible for the failure to observe different blood glucose trajectories between 25 and 60 g glucose; an effect not observed for those participants with low BC scores.
In terms of the hypothalamic–pituitary–adrenal axis response, a fall in cortisol levels was observed irrespective of the drink over the course of the cognitive testing sessions. However, this drop was only significant following placebo, suggesting that glucose administration somewhat truncated the fall in cortisol. Our data do not support the notion that cognitive tasks act as a psychosocial stressor. However, it is important to note that failure to observe a rise in cortisol levels does not preclude the activation of other stress systems. The endocrine stress system has two broad components with a considerable central anatomic interconnection. The stress response is mediated by both the hypothalamic–pituitary–adrenal axis and sympathetic adrenal medullary axis activation. While activation of the hypothalamic–pituitary–adrenal axis was not demonstrated in the present study, the possibility of sympathetic adrenal medullary axis activation still remains. The increase in alertness reported by participants may be indicative of brief acute stress responses mediated by sympathetic adrenal medullary axis activation. It is also worth noting that previous research has examined circadian differences in cognitive performance between young and old people(Reference Hasher and Goldstein45), with younger people tending to perform less well in the morning. Since the glucose facilitation effect appears to be mediated by ‘task difficulty’, it may be the case that a morning cognitive deficit in this sample may increase margins for improvement and subsequent attenuation by glucose supplementation. To our knowledge, only one study has addressed the effect of glucose on the time of day(Reference Sünram-Lea, Foster and Durlach32) and found that glucose facilitation still persists into the afternoon; however, further support of this finding is required.
In terms of the moderation of behavioural response to glucose drink, glucoregulation as indexed by the AUCG and fasting glucose levels were found to moderate mood and cognitive performance, whereas there were no effects of glucoregulation as indexed by the AUCI or 2 h glucose levels. The observation that only the AUCG index and fasting glucose levels predicted performance suggests that not only participants' responsiveness to a glucose drink, but also overall circulating glucose levels are important factors in determining individuals' glucoregulation. In terms of mood measures, 60 g glucose decreased alertness in those with poorer glycaemic control. Furthermore, those with poorer glycaemic control also reported the strongest decrease in calmness after consumption of a 25 g glucose drink, whereas those with a better glucoregulation rated themselves as more calm after consumption of 25 g glucose. Moreover, those with higher fasting blood glucose levels, the administration of 60 g glucose led to a significant decrease in alertness levels. These data demonstrate that for mood measures, those with poorer glucoregulation and ultimately more circulating blood glucose actually felt worse following a glucose load. However, those with better glycaemic tolerance, and ultimately lower circulating blood glucose, experienced improved feelings of calmness due to glucose ingestion. Extremely high doses of glucose have been observed to make participants feel nauseous and ill (Messier(Reference Messier8), cited unpublished results). It seems likely or at least possible that mood may be modified by oversaturation of glucose in those with overall higher blood glucose levels. Taken together, this may suggest that for mood, there is an optimal level of circulating blood glucose.
In terms of cognition, poorer glucoregulation predicted improved immediate word recall accuracy following the administration of 25 g glucose compared with placebo. Furthermore, participants with better glucoregulation produced significantly more errors following 60 g glucose compared with 25 g glucose on delayed word recall. Those with better glucoregulation also showed performance decrements following the administration of 25 g compared with placebo on word recall accuracy. These findings are in line with accumulating evidence that glucose load may preferentially enhance cognition in those with poorer glucoregulation(Reference Kennedy and Scholey4, Reference Sünram-Lea, Owen and Finnegan12–Reference Kaplan, Greenwood and Winocur14, Reference Hall, Gonder-Frederick and Chewning46). Furthermore, the finding that for those with better glucoregulation, administration of a glucose load may in fact impair performance is interesting. Several glucoregulatory indices have been previously evaluated for their relationship with cognitive performance in younger and older participants. These include various estimates of glucoregulation such as fasting levels, peak glucose levels, recovery and evoked glucose to baseline levels and AUC(Reference Donohoe and Benton2, Reference Messier, Desrochers and Gagnon10, Reference Craft, Murphy and Wernstrom13–Reference Vanhanen and Soininen18), assessed as incremental AUC(Reference Awad, Gagnon and Desrochers11). This calculation corresponds to what is termed AUCincrease by Pruessner et al. (Reference Pruessner, Kirschbaum and Meinlschmid31). In the present study, AUCground predicted cognitive performance, whereas the most commonly used incremental AUC did not. Taken together, the moderation of cognitive enhancement by glucoregulation suggests that optimal dosing is heavily dependent upon individuals' glucoregulation, and that overall circulating glucose levels may be an important factor in the assessment of glucoregulation in populations with normal glucose tolerance, as defined by the WHO. It is noteworthy that nine participants in our sample fulfilled the WHO criteria for impaired fasting glucose and/or impaired glucose tolerance (see Fig. 3). These diagnostic glucose concentration thresholds were derived from estimates of the level at which they are associated with an increased risk of disease, including cardiovascular and retinal complications.

Fig. 3 Frequency distribution of the participants in the study with impaired fasting glucose (IFG) and impaired glucose tolerance (IGT). Fifteen participants showed normal glucose tolerance (NGT), two showed IFG and five showed IGT. ![]() , NGT (finger prick glucose (FPG) < 6·1; 2 h PG < 7·8);
, NGT (finger prick glucose (FPG) < 6·1; 2 h PG < 7·8); ![]() , IFG (FPG = 6·1–6·9; 2 h PG < 7·8); ■, IGT (FPG < 7·0; 2 h PG = 7·8–11·0); □, type 2 diabetes (T2D) (FPG >7·0; 2 h PG >11·0).
, IFG (FPG = 6·1–6·9; 2 h PG < 7·8); ■, IGT (FPG < 7·0; 2 h PG = 7·8–11·0); □, type 2 diabetes (T2D) (FPG >7·0; 2 h PG >11·0).
However, analysis of the data revealed no differences in response to glucose administration and cognitive performance (defined by cognitive performance under placebo conditions) by the WHO-defined criteria of impaired fasting glucose and impaired glucose tolerance. Our data therefore tentatively suggest that at least in young adults with normal or mildly impaired glucose tolerance, the WHO criteria are less suitable to predict cognition.
When looking at the blood glucose data in terms of BC, the present data showed that those with poorer BC scores had overall higher blood glucose levels than those with low BC scores. The data suggest that poor BC predicts impaired performance on tasks of word recall and recognition in the absence of a glucose drink. However, this effect disappears when glucose is administered.
The potential moderating effects of cortisol were also examined in the present study. There is a growing body of research demonstrating robust links between cortisol and cognition. For example, short-term memory performance(Reference Brandenberger, Follenius and Wittersheim47) and declarative memory task performance(Reference Kirschbaum, Wolf and May48) have been found to be strongly inversely correlated with the size of cortisol rise induced by a challenging or stressful task. Moreover, acute administration of cortisol has been shown to impair working memory performance(Reference Lupien and Lepage49), declarative memory and spatial thinking tasks, but not procedural memory(Reference Hall, Gonder-Frederick and Chewning46). These effects are thought to involve the action of cortisol on hippocampal neuronal function. Receptors binding cortisol are abundant in the hippocampus(Reference Kloet, Oitzl and Joëls50), a brain region strongly implicated in declarative memory(Reference Newcomer, Craft and Hershey51). There is also evidence for rapid, non-genomic, membrane receptor modulation of memory by glucocorticoids(Reference Tasker, Di and Malcher-Lopes52), which may be affected by neural energy supply as glucocorticoids have also been found to inhibit glucose uptake into the hippocampus(Reference Homer, Packan and Sapolsky53). A rise in cortisol levels was not observed, suggesting that participants were not greatly stressed by the cognitive testing, although, as mentioned earlier, acute stress and activation of the sympathetic adrenal medullary axis cannot be ruled out. Given that cortisol levels fell over the course of the testing session, participants' responsiveness in relation to the cortisol fall was assessed. This analysis of fall with the addition of glucose loading has not been examined in previous work. Although cortisol response did not predict behavioural response to glucose loading, administration of a glucose load did truncate the fall in cortisol levels. The mechanisms of this relationship are not entirely understood, and it remains unclear whether glucose has an impact on cortisol or vice versa; however, glucose levels have previously been associated with cortisol levels. Specifically, plasma glucose levels after a 100 g glucose load predicted the extent of a stress-induced rise in cortisol (using the Trier Social Stress Test), whereas in the absence of stress, cortisol did not change following the glucose load(Reference Kirschbaum, Gonzalez Bono and Rohleder54). Kirschbaum et al. (Reference Kirschbaum, Wolf and May48, Reference Kirschbaum, Gonzalez Bono and Rohleder54) concluded from their research that that free cortisol response to stimulation is under significant control of centres responsible for monitoring energy availability and that low glucose levels appear to inhibit adrenocortical responsiveness in healthy subjects. The work by Kirschbaum et al. (Reference Kirschbaum, Wolf and May48, Reference Kirschbaum, Gonzalez Bono and Rohleder54) assessed plasma glucose with respect to the cortisol rise following a stressor, not cortisol fall, as in the present study. However, the data of the present study support the notion that cortisol response at testing may be under some control from systems that monitor energy availability. In terms of the relationship between BC and cortisol, we found that those with a higher BC index had higher basal cortisol levels compared with those with a lower BC index. It has been suggested that hypercortisolism can result in visceral obesity and insulin resistance(Reference Rosmond55), although the order of cause and effects is as yet unclear. Taken together, the data tentatively suggest that even in a young, healthy adult population, a degree of physiological inefficiency can be observed in overweight individuals.
In summary, blood glucose levels during an OGTT rose in response to glucose loads. Furthermore, blood glucose levels during the test were to some degree moderated by inter-individual differences in BC, in that those with a low BC score showed different glucose trajectories following the differing glucose loads, whereas those with high BC scores did not. In terms of cortisol levels, a fall in cortisol levels was observed. However, glucose drinks administered truncated the effects of falling cortisol levels. Furthermore, BC appeared to affect cortisol levels, in that those with high BC scores had higher basal cortisol levels. The overall findings of the study were that in a sample of participants representing a wide range of body types, glucose facilitation of cognition can be observed at both 25 and 60 g dosages. For working memory, spatial memory and reaction time, both 25 and 60 g glucose facilitated performance. In contrast, for the measures of verbal declarative memory, 25 g glucose facilitated performance. In terms of moderating factors, glycaemic response appeared to exert both direct effects and moderate the glucose facilitation effect. Participants with poor glucoregulation performed worse on declarative memory tasks compared with those with better glucose regulation and BC. It was in participants with poor glucoregulation that performance enhancement by glucose was observed. Consequently, in the present study, the most prominent response modifiers appear to be glucoregulation as indexed by AUCG and fasting blood glucose levels. A few caveats should be noted in the present study: (1) while the sample size was sufficient to detect the effects of improved cognitive function by glucose administration, a greater sample size would have allowed for more sophisticated analysis of the individual factors as well as the potential to examine the upper and lower quartiles of those participants who were termed as ‘good’ or ‘poor’ glucoregulators; (2) while cortisol was examined at the same time each morning, the evaluation of cortisol effects might be more evident with a tight control over the timing of wakening response; ideally this would be evaluated in a sleep laboratory. Such studies are expensive and was not possible in the present experiment, but the data represent an avenue for further study in this area.
There is a large body of evidence demonstrating that cognitive decline accompanies certain metabolic health conditions such as type 2 diabetes, the metabolic syndrome and obesity. However, there is less research examining the potential modification of cognitive function by metabolic activity in relatively ‘normal’ healthy young samples. The very fact that glucose is capable of moderating cognitive performance and that these effects are moderated by individual differences demonstrates how susceptible brain function is to even small metabolic fluctuations. In health terms and for diagnostic purposes, diseases such as these are categorised by whether an individual's weight or glucoregulation falls between various ranges and cut-off points. However, these disease states are progressive, and thus it seems important to think of our metabolic profile in terms of a continuum rather than whether an individual fits within diagnostic ranges, particularly since these disease states are preventable. A possible extension of the present study would be to examine whether poor glucoregulation could be reversed by a whole dietary intervention such as the Mediterranean diet or a low-glycaemic index diet, and to examine whether glucose facilitation is moderated differentially in the same individual following the dietary intervention. Recently, we have conducted the first study in which a whole dietary intervention of the Mediterranean diet was implemented for 10 d. Following 10 d on the Mediterranean diet, participants lost central adipose tissue mass and reported a range of improved mood measures(Reference McMillan, Owen and Kras56). Whole dietary interventions are costly, time consuming and difficult to implement; however, in light of the growing epidemic of metabolic illness in the UK and evidence of increased neurological dysfunction in these individuals, this research is timely and necessary.
Acknowledgements
Conflicts of interest: the present research was funded by GlaxoSmithKline and partially supported by the Australian Research Council (grant no. DP1093834). The authors' contributions are as follows: L. O. was the investigator of the study and the primary author of the paper; A. S. contributed to the writing of the manuscript; Y. F. was an external industry supervisor of the study and contributed to the writing of the manuscript; S. I. S.-L. was the senior investigator of the study and contributed to the writing of the manuscript.