Bioimpedance spectroscopy (BIS) is an evolving method used to predict body water compartments when dilution techniques are not available. Total body water (TBW) can be used to predict body composition(Reference Pace and Rathbun1), or can be used in multiple-compartment models allowing for greater accuracy(Reference Evans, Saunders and Spano2–Reference Wang, Deurenberg and Guo4). Specifically, in some populations, TBW is needed to accurately track changes resulting from diet and exercise(Reference Evans, Saunders and Spano2). Additionally, TBW estimations can be used to monitor nutritional status and identify diseases, such as dehydration and chronic kidney disease(Reference Armstrong5, Reference Dumler and Kilates6).
Since the conception of BIS, there have been many advancements, which have improved the accuracy of BIS TBW measurements, such as utilisation of automatic rejection limits and time delays via an onboard computer. Typically, whole-body BIS analysis requires single electrodes positioned on the dorsal surface of the foot, ankle, wrist and hand. Using a range of frequencies (1–1000 kHz), complex Cole plots are constructed in the shape of a semi-circle allowing for calculations of the resistance of electrical current through the body at both zero and infinite frequencies. These resistance values are used to calculate extracellular and intracellular water, and summed to equal TBW. Past BIS methods have required separate computers to analyse and compute raw impedance values. However, a new battery-powered BIS device (Imp™ SFB7; ImpediMed Limited, Pinkenba, QLD, Australia) with an onboard computer utilising automatic time delays and rejection limits has emerged, allowing for greater portability and practicality than previous BIS devices. More importantly, the Imp™ SFB7 was found to be more accurate than an older BIS device that utilised a separate computer for analysis (4000B; XiTRON Technologies, San Diego, CA, USA)(Reference Moon, Tobkin and Roberts7). Portability of a BIS device allows for fast and accurate body water estimations regardless of the location. Additionally, the Imp™ SFB7 and the BIS methods are preferred over dilution techniques, such as 2H analysis, due to the reduced costs, equipment and time(Reference Moon, Tobkin and Roberts7, Reference Moon, Smith and Tobkin8). Past investigations concluded that the Imp™ SFB7 is a valid tool for estimating and tracking changes in TBW in various populations(Reference Moon, Tobkin and Roberts7, Reference Moon, Smith and Tobkin8). However, the ability of BIS to track changes in TBW is limited to the reproducibility errors(Reference Moon, Smith and Tobkin8). Specifically, changes in TBW may not be accurate or valid when the day-to-day errors associated with the BIS method are not exceeded(Reference Moon, Smith and Tobkin8). Dilution techniques, such as 2H, may require a change in TBW to be greater than 0·91 litres, while BIS may require a larger change (>1·33 litres) before the differences can be considered real(Reference Moon, Smith and Tobkin8).
Since intra-instrument, repeated (back-to-back), standard error of the measurement (sem) values for TBW estimated via the Imp™ SFB7 are as low as 0·04 litres(Reference Moon, Tobkin and Roberts7), external factors must account for the additional 0·44 litres(Reference Moon, Smith and Tobkin8) (sem = 0·48–0·04 litres) error from day to day. An investigation done by Ward et al. (Reference Ward, Byrne and Rutter9) concluded that these errors may be due to the measurement protocol and biological factors. Specifically, intra-individual biological factors could be influenced by fluid and electrolyte balance, body temperature, and skin contact resistance(Reference Ward, Byrne and Rutter9). While having subjects follow strict pre-testing guidelines may reduce intra-individual biological errors, some potentially uncontrollable biological variations may still exist. In contrast, measurement protocol errors can be directly controlled. Even when all measurement protocols are standardised from one testing session to another, methodological errors may exist. It has been suggested that the utilisation of four single-site (SS) electrodes accounts for errors in repeated TBW measurements(Reference Moon, Smith and Tobkin8). Because the typical whole-body BIS method requires four individually placed electrodes, each electrode placement could contribute to reproducibility errors. For instance, a 1 cm difference between electrode placement can alter the resistance values by 2 %(Reference Ellis, Shypailo and Wong10). Furthermore, an investigation (n 5) done by Elsen et al. (Reference Elsen, SIiu, Pineda, Ellis, Yasumara and Morgan11) found that resistance mean values changed by 2·1 % when the wrist or ankle electrodes were moved proximally to 1 cm, with a 4·1 % change at 2 cm. Additionally, Lukaski(Reference Lukaski and Norgan12) discovered that electrode placement could alter resistance values up to 70 Ω, which contributed to a 8·5 kg error in fat-free mass and a 10·2 % error in percentage fat. Nonetheless, these investigations utilised bioelectrical impedance analysis at a low single frequency of 50 kHz. Still, Elsen et al. (Reference Elsen, SIiu, Pineda, Ellis, Yasumara and Morgan11) concluded that bioelectrical impedance analysis could not detect changes in TBW of up to 1·4 % in a 70 kg reference man.
Because of these errors, it has been suggested that fixed-distance electrodes may reduce the reproducibility errors associated with BIS-estimated TBW(Reference Moon, Smith and Tobkin8). Additionally, fixed-distance electrodes (5 cm distal to wrist and 5 cm distal to ankle) have been used in the past with a 5 cm standard distance(Reference Kaysen, Zhu and Sarkar13–Reference Lukaski, Johnson and Bolonchuk15). This distance is considered by the 2004 ESPEN guidelines for the clinical application of bioimpedance analysis to be the minimum acceptable distance between electrodes(Reference Kyle, Bosaeus and De Lorenzo16). However, to date, there have been no investigations that have compared the reproducibility between fixed-distance electrodes and SS electrodes at the standard wrist and ankle positions.
The purpose of the present investigation was twofold: (1) to compare the reproducibility of BIS TBW estimations using fixed-distance and SS electrodes, and (2) to compare the validity and accuracy of fixed-distance and SS electrodes for tracking TBW changes after an exercise intervention using the criterion 2H. Because the utilisation of fixed-distance electrodes eliminates two of the four SS placements, it was hypothesised that the fixed-distance electrodes would decrease reproducibility (day-to-day) errors and track changes more accurately than SS electrodes. Additionally, because of hand and foot length differences and the longer distance between hand to wrist and foot to ankle between men and women, it was hypothesised that both reproducibility and tracking errors in TBW would be greater in men than in women.
Experimental methods
Reproducibility subjects
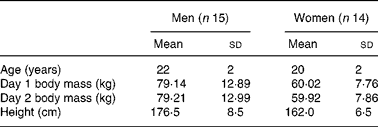
Twenty-nine healthy Caucasian men (n 15) and women (n 14), 18–27 years of age, participated in the 2 d reproducibility investigation. Subject characteristics for the reproducibility study are presented in Table 1. The reproducibility study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the University of Oklahoma Institutional Review Board. Written informed consent was obtained from all subjects/patients.
Table 1 Descriptive characteristics of reproducibility study subjects
(Mean values and standard deviations)
Reproducibility study protocol
Reproducibility testing was conducted on two separate days. All measurements were performed by the same investigator. BIS assessments were performed following a 12 h fast (ad libitum water intake was allowed up to 1 h before testing). Time between visits was 24 (sd 2) h. Participants were instructed to avoid exercise for at least 12 h before testing on day 1 and day 2. Day 1 and day 2 BIS measurements were performed in the same room at a consistent ambient temperature. Subjects wore the same clothing during both testing days. Before all BIS measurements, hydration status was determined using specific gravity via handheld refractometer (Model CLX-1, precision = 0·001 (sd 0·001), VEE GEE Scientific, Inc., Kirkland, WA, USA). Specific gravity values indicated that all subjects were properly hydrated during both day 1 (1·019 (sd 0·005)) and day 2 (1·018 (sd 0·004)) testing sessions with no significant difference between days (mean difference = 0·0004, P = 0·66). On testing days, no women experienced large weight gains due to menstrual status(Reference Bunt, Lohman and Boileau17).
Bioimpedance spectroscopy (reproducibility study)
BIS was used to estimate TBW following the procedures recommended by the manufacturer (Imp™ SFB7; ImpediMed Limited) as reported by Moon et al. (Reference Moon, Tobkin and Roberts7). Briefly, after resting in a supine position for 5–10 min, TBW estimates were taken while the subjects lay supine on a table with their arms ≥ 30° away from their torso with their legs separated. A 5- to 10-minute resting period was selected based on the manufacturer's guidelines and the 2004 ESPEN guidelines for the clinical application of bioimpedance analysis(Reference Kyle, Bosaeus and De Lorenzo16). Electrode placement and subsequent BIS measurements were conducted in a random order within the 5- to 10-minute window with the subjects resting in a supine position. Before analysis, each subject's height, weight and sex were entered into the BIS device. Both SS and fixed-distance electrodes were produced by the same company (ImpediMed Limited), and they were of the same size and shape. Each pair of fixed-distance electrodes was connected by a non-conductive strip, allowing for a distance of 5 cm between electrode centres (Figs. 1 and 2(c)). After hair removal and cleaning with alcohol, proximal, SS and fixed-distance electrodes were placed on the right side of the body at the wrist (dorsal surface at the ulnar styloid process) and ankle (dorsal surface between the malleoli) (Figs. 1 and 2). Distal, fixed-distance electrodes were placed 5 cm from the wrist and ankle (Figs. 1 and 2), and SS electrodes were placed on the hand (dorsal surface of the metacarpal phalangeal joint, 1 cm proximal to the knuckle of the middle finger) and foot (dorsal surface of the metatarsal phalangeal joint, 1 cm proximal to the joint of the second toe) (Figs. 1 and 2(c)). The average of the two trials was used to represent the subject's TBW. TBW was calculated internal to the BIS device using Cole modelling and the Hanai mixture theory(Reference Cornish, Thomas and Ward18, Reference Hanai19). Coefficients used for men (zero/extracellular = 273·9 and infinite/intracellular = 937·2) and women (zero/extracellular = 235·5 and infinite/intracellular = 894·2) were the same as those used in the investigation done by Moon et al. (Reference Moon, Tobkin and Roberts7).

Fig. 1 Electrode placements for the hand: (a) standard site, dorsal surface at the ulnar styloid process and dorsal surface of the metacarpal phalangeal joint, 1 cm proximal to the knuckle of the middle finger; (b) fixed distance (5 cm) without non-conductive strip, dorsal surface at the ulnar styloid process and 5 cm distal; (c) fixed distance (5 cm) with non-conductive strip, dorsal surface at the ulnar styloid process and 5 cm distal. Please note, due to photogenic distortion, actual electrode positions at the ulnar styloid process may not appear accurate. Also, to acquire a 5 cm distance from the middle of the electrodes, measurements can be taken from either the proximal or distal sides of the detecting electrode to the proximal or distal side of the source electrode, respectively.

Fig. 2 Electrode placements for the foot: (a) standard site, dorsal surface between the malleoli and dorsal surface of the metatarsal phalangeal joint, 1 cm proximal to the joint of the second toe; (b) fixed distance (5 cm) without non-conductive strip, dorsal surface between the malleoli and 5 cm distal; (c) fixed distance (5 cm) with non-conductive strip, dorsal surface between the malleoli and 5 cm distal. Please note due to photographic distortion, actual electrode positions between the malleoli may not appear accurate. Also, to acquire a 5 cm distance from the middle of the electrodes, measurements can be taken from either the proximal or distal sides of the detecting electrode to the proximal or distal side of the source electrode, respectively.
Statistical analysis (reproducibility study)
Data were analysed using a custom-built LabVIEW Program version 8.2.1 (National Instruments, Austin, TX, USA) and Microsoft® Excel® 2007 version 12.0.6504.5001, SP1 MSO 12.0.6320.5000 (Microsoft Corporation, Redmond, WA, USA). Reproducibility of BIS TBW estimates was based on the evaluation of day-to-day values from SS and fixed-distance electrode placements. Each analysis was performed on men, women, and men and women combined. Day-to-day, intra-electrode mean placement differences (constant errors, CE = day 2 − day 1) were calculated using a single-factor within-subject design ANOVA(Reference Keppel and Wickens20) as described and suggested by Weir(Reference Weir21). Intraclass correlation coefficients (ICC) were calculated using a two-way fixed model as described by Shrout & Fleiss(Reference Shrout and Fleiss22) and by Weir(Reference Weir21) (model 3,1). Standard error of the measurement (sem) values were calculated using the mean square error (MSE) from the ANOVA model (sem = √MSE). This calculation for the sem is suggested because it only considers random errors and not systematic deviations(Reference Weir21, Reference Hopkins23). Size of 95 % CI was calculated using the following equation: 95 % CI = 2 (sem (1·96)). The percentage CV was calculated using the following equation: % CV = 100 (sem/X), where X is the grand mean of both days. Considering that measurement errors (sem) exist both before and after testing, the minimal difference (MD) statistic(Reference Weir21), sometimes referred to as the smallest detectable change(Reference de Vet, Terwee and Knol24), was used to determine the MD needed to be considered real for both electrode placements when used for repeated measurements. MD was calculated using the following equation(Reference Weir21):
Validity subjects
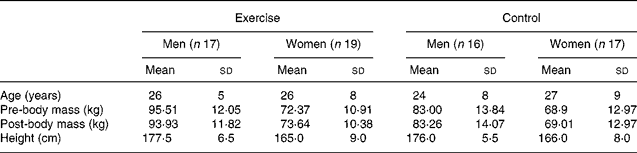
Sixty-nine sedentary ( < 30 min physical activity/week) men (n 33) and women (n 36), 18–45 years of age, participated in the 12-week investigation. Subjects were given the choice to be in the exercise group or control group. Starting body mass in men was the only significant difference between groups (control group = − 12·5 kg, P < 0·05). However, body mass has been shown not to influence delta values(Reference Moon, Smith and Tobkin25). The control group (no exercise) consisted of thirty-three subjects, while the exercise group included thirty-six subjects. Subject characteristics for the validity study are presented in Table 2. Participants included fifty-two Caucasians, five African Americans and twelve Asians. Each participant was assessed by routine medical screening for inclusion. None of the participants reported or exhibited a history of medical or surgical events that may have significantly affected the study outcome, including metabolic, renal, hepatic or musculoskeletal disorders. Additionally, the present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the University of Oklahoma Institutional Review Board. Written informed consent was obtained from all subjects/patients.
Table 2 Descriptive characteristics of validity study subjects (n 69)
(Mean values and standard deviations)
Validity study protocol
TBW assessments (BIS and 2H) were performed during weeks 1 and 12 of the 12-week investigation. Measurements were performed on the same day following a 12 h fast (ad libitum water intake was allowed up to 1 h before testing). Pre- and post-intervention BIS measurements were performed in the same room at a consistent ambient temperature. Subjects wore the same clothing both before and after testing. Participants were instructed to avoid exercise for at least 12 h before testing. Before all measurements, hydration status was determined using specific gravity via handheld refractometer (Model CLX-1, precision = 0·001 (sd 0·001), VEE GEE Scientific, Inc.). Specific gravity values indicated that all subjects were properly hydrated during both pre-testing (1·020 (sd 0·008)) and post-testing (1·019 (sd 0·007)) sessions with no significant difference between days (mean difference = 0·001, P = 0·37). On testing days, no women experienced large weight gains due to menstrual status(Reference Bunt, Lohman and Boileau17).
Training protocol
The exercise programme was designed using the American College of Sports Medicine-recommended guidelines for apparently healthy adults(Reference Balady, Berra and Golding26). Progressive endurance training on cycle ergometers was conducted 3 d per week. Resistance training was conducted 2 d per week, providing at least 24 h recovery between sessions. Participants completed nine single-joint and multi-joint exercises, including bench press, lat pulldown, seated military press, biceps curl, triceps pushdown, leg press, lying leg curl, low-back extension and abdominal crunch. Each exercise was performed once per session, with participants completing 8–12 repetitions per exercise until volitional fatigue. Weight was increased when participants performed>10 repetitions at the same resistance during two consecutive lifting sessions. All exercises were performed in the University laboratory, and were evaluated and monitored by a certified trainer.
Bioimpedance spectroscopy (validity study)
BIS procedures were the same as those performed in the reproducibility study with the exception of electrode brand. Both SS and fixed-distance electrodes were of the same size and shape (23 × 24 mm) (SENSI-TABS, Unomedical Limited, Stonehouse, UK). Fixed-distance electrodes were positioned using a constant of 5 cm between electrode centres (Figs. 1 and 2(b)). Electrodes for the reproducibility and validity studies were of the same size and shape (23 × 24 mm). A single-subject, repeated measurement comparison between the electrode brands and placements used in the reproducibility and validity studies indicated no difference between the brands of electrodes or placement techniques (Figs. 1 and 2(b) v. (c)) (P>0·05).
2H technique
Criterion TBW estimations were conducted using 2H (99·8 % 2H, Cambridge Isotope Laboratories, Inc., Andover, MA, USA) following the standard procedures reported by Moon et al. (Reference Moon, Tobkin and Roberts7). Before 2H ingestion, urine samples were collected from all subjects. Subjects were instructed to void their bladders as much as possible. After voiding the bladder completely, subjects ingested approximately 11 g of 2H along with a 100 ml rinse of tap water. The exact amount of 2H ingested for each subject was recorded. After a 4 h equilibration period, subjects were instructed to provide a post-urine sample. Urine-diluted 2H was analysed in triplicate using an isotope ratio mass spectrometer. Isotope abundances in the urine were calculated following the method of Wong et al. (Reference Wong, Lee and Klein27). TBW was then calculated from the dilution of isotopic water, and corrected for the exchange of 2H with non-aqueous tissue(Reference Schoeller, van Santen and Peterson28). Reproducibility measurements from eleven men and women for 2H in one urine sample measured in triplicate resulted in an sem value of 0·33 litres.
Statistical analysis (validity study)
Data were analysed using a custom-built LabVIEW Program version 8.2.1 (National Instruments) and Microsoft® Excel® 2007 version 12.0.6504.5001, SP1 MSO 12.0.6320.5000 (Microsoft Corporation). Pre- and post-TBW differences in fixed-distance (5 cm) and SS electrode placements were analysed using dependent t tests. However, since the focus of this part of the investigation was to compare the validity of the different electrode placements for tracking changes, and due to the potential issues regarding pre- and post-BIS TBW assessments(Reference Moon, Smith and Tobkin8), only delta values were fully analysed using the statistical procedures below. Additionally, BIS TBW delta values have been shown to be unbiased, regardless of age, sex, race, fat mass, fat-free mass and BMI(Reference Moon, Smith and Tobkin8). All groups (exercise and control) and sexes (men and women) were analysed individually as well as together. The validity of BIS TBW estimates measured using different electrode placements was based on the evaluation of predicted delta values v. the criterion delta or actual delta values obtained using 2H by calculating the constant error (CE = actual (2H) − predicted (BIS)), r value (Pearson's product-moment correlation coefficient), standard error of estimate and total error (![]() )(Reference Heyward and Wagner29). The mean differences (CE) between BIS delta values using both electrode placements and 2H delta TBW values were analysed using dependent t tests with the Bonferroni α adjustment (P ≤ 0·025). The method of Bland and Altman was used to identify the 95 % limits of agreement between 2H delta and BIS delta TBW values using both electrode placements(Reference Bland and Altman30).
)(Reference Heyward and Wagner29). The mean differences (CE) between BIS delta values using both electrode placements and 2H delta TBW values were analysed using dependent t tests with the Bonferroni α adjustment (P ≤ 0·025). The method of Bland and Altman was used to identify the 95 % limits of agreement between 2H delta and BIS delta TBW values using both electrode placements(Reference Bland and Altman30).
Results
Reproducibility study
Reproducibility results are presented in Tables 3 and 4. One-way ANOVA revealed no significant interactions between sex and electrode placement on day 1 and day 2, or the mean differences between day 1 and day 2 (P>0·675). Mean differences between days were not significantly different for all groups (men, women, and men and women) for the 5 cm or SS electrode positions. ICC values were larger and sem values were lower for men and for men and women combined when comparing the 5 cm electrode placement with the SS electrode placement for TBW. However, only women produced identical ICC and sem on comparing the electrode placements. The fixed-distance electrode placement (5 cm) only improved the reproducibility for men and for men and women combined (Table 3). Additionally, the 5 cm electrode placement did not improve the reproducibility in women. Regression results indicated that BMI, TBW, height and body mass did not influence day-to-day errors for men, women, or men and women combined (slope < − 0·12, P>0·085).
Table 3 Reproducibility of electrode placement for predicting total body water compared with 2H (litres)
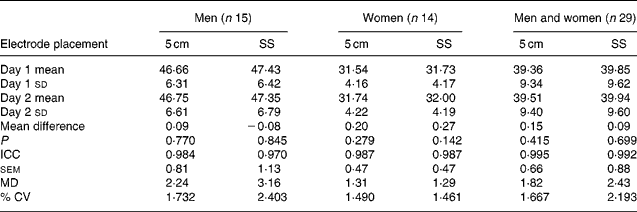
5 cm, Fixed-distance bioimpedance spectroscopy electrode placement; SS, single-site bioimpedance spectroscopy electrode placement; ICC, intraclass correlation coefficients; MD, minimal difference.
Table 4 Reproducibility of electrode placement for measuring raw resistance values

5 cm, Fixed-distance bioimpedance spectroscopy electrode placement; SS, single-site bioimpedance spectroscopy electrode placement; ICC, intraclass correlation coefficients; MD, minimal difference.
Compared with TBW results, further analysis revealed that the raw resistance (R) values at zero (R zero) and infinite (R inf) frequencies from the Cole model (used for calculating TBW) produced similar reproducibility results when comparing electrode placements (Table 4). However, both R zero and R inf results in women revealed a significant difference from day to day for only the SS electrode placement (P < 0·05), and compared with women, men had lower ICC, sem and MD values with the exception of R inf on day 1 obtained using the 5 cm electrode placement. Larger differences (more than double) were observed for R zero when comparing men with women for either electrode placement. Both electrode placements appeared to be similar and reliable from day to day; however, utilisation of the 5 cm electrode placement slightly reduced the mean differences between days. When intracellular resistance (R i = (R zero × R inf)/(R zero − R inf)) was calculated, which utilises both R zero and R inf, the 5 cm electrode placement resulted in lower sem, higher ICC, lower MD and smaller mean differences for all groups compared with the SS electrode placement (Table 4).
Validity study
Significantly larger raw resistance values (Ω) were observed from the Cole model at both zero (pre-CE = 12·79 Ω) and infinite (CE>6·87 Ω) frequencies comparing the 5 cm electrode placement with the SS electrode placement during both pre and post visits (P < 0·001). One-way ANOVA revealed significant differences in TBW between men (CE>0·55 litres) and women (CE < 0·36 litres) when comparing the differences between electrode placements for pre- and post-measurements (P < 0·0003). However, the TBW mean differences between electrode placements were not significantly different between men (CE = 0·04 litres) and women (CE = 0·07 litres) when comparing the delta values (P = 0·61). Delta TBW values were not significantly different between electrode placements (CE = 0·052 litres, P = 0·08). Pre- and post-TBW values were significantly different between electrode placements for men, women, and men and women combined (P < 0·05). Utilisation of the 5 cm electrode placement increased the TBW estimates in men by 0·872 litres and by 0·606 litres in women compared with that of the SS electrode placement. Delta validation results are presented in Tables 5–7. All groups (control, exercise, men, women, and men and women) produced similar and accurate validity results. The control group SS electrode placement for men and for men and women combined produced the only significant mean differences (P < 0·025). Significant trends to underestimate changes in TBW were observed for both electrode placements in women in the exercise group (P < 0·05). No trends were observed in men in either the exercise or control group or in women who participated as controls. Further analysis identified an outlier in the women's exercise group with a difference of more than double two 2sd from the CE for both electrode placements. Therefore, her data did not influence the comparisons between electrode placements, and the significant trends in the exercising women should be interpreted with caution, as they may not be meaningful. Since the focus of the present study was to compare electrode placements, and both placements produced trend slopes between 70 and 74, it appears that electrode placement had no influence in this trend to underestimate TBW as TBW increased. Regardless of electrode placement, BIS accurately tracked changes in TBW for all groups with slightly better precision with the 5 cm electrode placement in the control group.
Table 5 Validation of bioimpedance spectroscopy (BIS) electrode placement for predicting total body water compared with 2H2O presented as delta values (entire validation study, men and women)
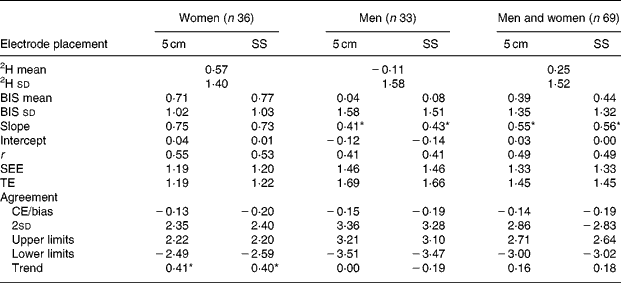
5 cm, Fixed-distance bioimpedance spectroscopy electrode placement; SS, single-site bioimpedance spectroscopy electrode placement; CE/bias, constant (mean) error; TE, total error; SEE, standard error of estimate; r, Pearson's product-moment correlation coefficient; limits, 95 % limits of agreement (CE ± 1·96 sd of residual scores (2H2O-BIS)); trend, relationship (r) between the difference of 2H2O and BIS methods, and the mean of both the methods.
* Significance at P ≤ 0·05. Slope significance is compared with a slope of 1.
Table 6 Validation of electrode placement for predicting total body water (litres) compared with 2H presented as delta values (validation study exercise group only)
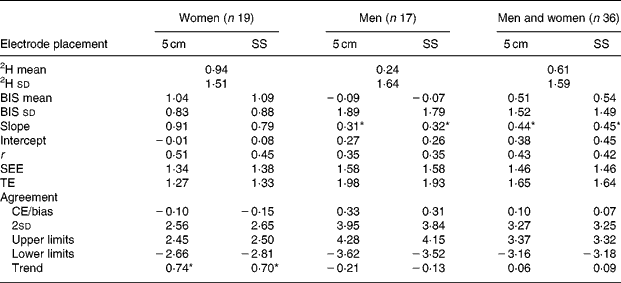
5 cm, Fixed-distance bioimpedance spectroscopy electrode placement; SS, single-site bioimpedance spectroscopy electrode placement; BIS, bioimpedance spectroscopy; CE/bias, constant (mean) error; TE, total error; SEE, standard error of estimate; r, Pearson's product–moment correlation coefficient; limits, 95 % limits of agreement (CE ± 1·96 sd of residual scores (2H-bioimpedance spectroscopy)); trend, relationship (r) between the difference of 2H and bioimpedance spectroscopy methods, and the mean of both the methods.
* Significance at P ≤ 0·05. Slope significance is compared with a slope of 1.
Table 7 Validation of electrode placement for predicting total body water (litres) compared with 2H presented as delta values (validation study control group only)
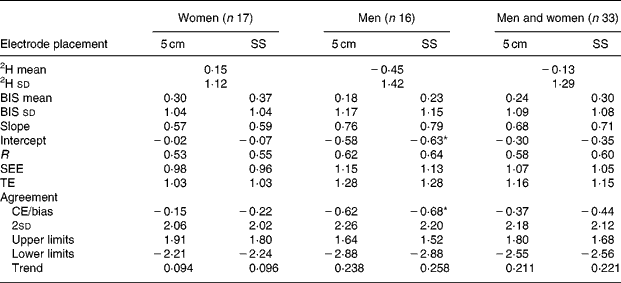
5 cm, Fixed-distance bioimpedance spectroscopy electrode placement; SS, single-site bioimpedance spectroscopy electrode placement; BIS, bioimpedance spectroscopy; CE/bias, constant (mean) error; TE, total error; SEE, standard error of estimate; r, Pearson's product–moment correlation coefficient; limits, 95 % limits of agreement (CE ± 1·96 sd of residual scores (2H-bioimpedance spectoscopy)); trend, relationship (r) between the difference of 2H and BIS methods, and the mean of both the methods.
* Significance at P ≤ 0·05. Intercept significance is compared with an intercept of 0.
Discussion
In agreement with our hypothesis, fixed-distance electrodes moderately decreased TBW reproducibility (day-to-day) errors. In accordance with our hypothesis, fixed-distance electrodes tracked TBW changes more accurately than SS electrodes. Results obtained from raw resistance values (R zero, R inf and R i) indicated both similar and dissonant findings compared with TBW results. It appears that TBW reproducibility errors are influenced by both sex and electrode placement, and that these errors are influenced during the calculation of TBW. As a result, if TBW is used in practice or research, reproducibility should be calculated using the final TBW results.
Reproducibility study
To the best of our knowledge, this is the first investigation that has compared the reproducibility of BIS TBW estimations using fixed-distance and SS electrode placements. Utilisation of the 5 cm electrode placement reduced the day-to-day TBW reproducibility errors in men, and produced no improvement in women. This was not observed when comparing raw resistance values, but when R i was calculated, the 5 cm electrode placement outperformed the SS electrode placement. Both electrode placements produced more precise reproducibility results (ICC, sem, MD and % CV) for women than for men when looking at R inf, R zero and TBW. However, this was not the case for R i. These results suggest that BMI, sex and/or the amount of TBW may affect BIS reproducibility results, and that errors may be introduced during the calculation of TBW. However, day-to-day TBW errors were not influenced by BMI, TBW, height or body weight in men or women (slope < − 0·12, P>0·085). Therefore, it appears that individual day-to-day errors are not influenced by electrode placement alone. One possible source of error could be due to the Cole modelling within the device. However, exactly the same Cole model parameters were used for each measurement. Though more research is needed to investigate this source of error, it appears that errors are compounded when resistance values are converted to TBW values, and that the larger the resistance sem values are, the larger the sem values for TBW are. This can be observed by the larger raw resistance sem values in men (R zero>20·58 Ω and R inf>9·53 Ω) than in women (R zero < 10·60 Ω and R inf < 10·23 Ω). Specifically, R zero produced an sem in men (>20·58) that was twice as large as that in women ( < 10·23 Ω). It appears that the variability in R zero influences TBW more than R inf. This can be explained, in part, by the fact that R zero is equal to extracellular resistance (R e), while R inf is used for the calculation of intracellular resistance (R i). Both R i and R e are used for the next step for calculating TBW. Since R i is calculated using the following equation: R i = R zero(R inf)/R zero − R inf, it is apparent that R zero influences both R e and R i. However, R i results revealed that women had larger sem and MD values and lower ICC values. When comparing the final resistance values (R e and R i) used to calculate TBW, there appears to be no agreement between raw resistance values and the final TBW reproducibility results. Because raw resistance values do not completely explain the reproducibility of TBW, reproducibility calculations for laboratories and clinics should be performed using TBW alone. It appears that multiple factors influence the final TBW results, which appear to stem from the initial raw resistance values. However, the impact of the reproducibility of raw resistance values on TBW reproducibility remains unclear.
Nonetheless, when utilising BIS to estimate TBW in men, the 5 cm electrode placement appeared to improve reproducibility by as much as 0·32 litres (sem), which results in a reduction of the MD value of 0·92 litres. Therefore, fixed-distance electrodes appear to be more sensitive for tracking a real change in TBW. Additionally, the present MD findings (>1·28 litres) are in agreement with those reported by Elsen et al. (Reference Elsen, SIiu, Pineda, Ellis, Yasumara and Morgan11), who concluded that a change in TBW may need to exceed 1·4 %. However, the present results indicate that a 1·4 % change would be < 0·7 litres, which is less than the MD but similar to the sem values for men and women combined (5 cm = 0·66 and SS = 0·88). Therefore, in contrast to past literature, a percentage change greater than 1·4 % may be required for BIS to track changes in TBW. Specifically, for men and women, the percentage change required based on the MD would be 6·55 % (5 cm) and 8·61 % (SS) based on day 1 TBW values for men and women combined. While utilisation of the MD is the conservative approach, repeated measurements must account for the errors (sem) in both pre- and post-measurements. Therefore, it is suggested that the MD statistic, rather than the sem statistic, be used to identify the required changes in TBW.
Because electrode placements produced different sem and MD values, we analysed the agreement between the two methods using the Bland & Altman method(Reference Bland and Altman30). The agreement between electrode placements is depicted in Fig. 3. Results indicated a lack of agreement between CE values obtained through both electrode placements. Specifically, if a 5 cm electrode placement produced a difference of 1 litre from day 1 to day 2, chances of the SS electrode placement producing the same change in the same subject were not good (Fig. 3). Only 55 % of the subjects produced differences between methods that were < 1 litre. Two obvious outliers are depicted in Fig. 3 from the reproducibility study (representing 6·9 % of the data). During the utilisation of the SS electrode placement, two men produced TBW values that were 3·5 and 3·1 litres greater on day 1 than on day 2, while the 5 cm electrode placement showed an increase of only 0·8 and 0·2 litres, respectively. However, results indicated no trend between methods (slope = − 0·35, P = 0·06). The results also indicated no significant CE values between methods (CE = 0·05, P = 0·77). While CE values may not show differences between electrode placements, individual variations could be much larger depending on the electrode placement technique. When comparing the differences between days and electrode placements, absolute values resulted in a much larger mean, standard deviation and range for the SS electrode placement (CE = 0·84 (sem 0·90) and 0·09–3·50 litres) than for the 5 cm electrode placement (CE = 0·72 (sem 0·58) and 0·08–2·68 litres), confirming that day-to-day reproducibility is better when using the 5 cm electrode placement than when using the SS electrode placement. Absolute values for men and women when comparing the CE between days and electrode placements indicated a larger mean, standard deviation and range for men (CE>0·86 (sem >0·72) and>0·17–2·66 litres) than for women (CE < 0·58 (sem < 0·53) and < 0·09–1·22 litres), and for the SS electrode placement (CE = 1·18 (sem 1·05) and 0·26–3·50 litres) than for the 5 cm electrode placement (CE = 0·87 (sem 0·72) and 0·17–2·68 litres) in men.

Fig. 3 Bland and Altman plots comparing mean differences from day 1 and day 2 using either a fixed-distance (5 cm) electrode placement or a standard-site (SS) electrode placement. —, The upper and lower limits of agreement ( ± 1·96 scd); – – –, the constant error (CE) or mean bias; \, the trend between the differences of methods and the mean of both methods.
Because of the differing TBW reproducibility results for men and women, it may be necessary to report reproducibility for the sex of subjects involved in the investigation or diagnosis. If both men and women are participating in a study or being evaluated in a clinic, two approaches may be followed. One approach is to interpret results based on the sex-specific reproducibility data. The other approach would be to interpret the results based on the highest sem and MD value, which appears to be associated with men. While the second approach is more conservative, if accurate reproducibility data can be collected for both men and women, the first approach could also provide valid interpretations without disregarding women who do not reach the MD for men. Regardless of the approach used, reproducibility results are not only dependent on sex.
Current sem values are in contrast (sem>0·65) to those reported in the investigation done by Moon et al. (Reference Moon, Smith and Tobkin8), who reported an sem of 0·48 litres for men and women when utilising the same BIS device and the SS electrode placements. Thus, the reproducibility obtained in the investigation done by Moon et al. (Reference Moon, Smith and Tobkin8) using the SS electrode placement resulted in more reliable measurements than the present investigation using either SS or 5 cm electrode placement. Since the device was the same for both the studies, subjects were from the same population and the testing location was the same, reproducibility differences can only be explained by tester error. This suggests that experience and training may independently contribute to BIS TBW reproducibility. Therefore, individual laboratories, clinics and individual investigators should calculate internal reproducibility for BIS TBW estimations. Knowledge of individual investigator reproducibility may aid in accurate interpretations of TBW, as different investigators may produce varying reproducibility results.
For example, if two investigators are collecting BIS TBW data for the same investigation, client or patient, and one produced an sem of 0·43 litres for TBW and the other produced an sem of 0·78 litres, results based on sem values < 0·78 litres may not be accurate. While this is the most conservative approach, investigators can feel confident that interpretations based on reproducibility data are accurate. More importantly, individual TBW changes that do not meet the MD may be due to tester and biological errors, and if the reproducibility results used to calculate the MD are not accurate, then correct interpretations are unattainable. This understanding is crucial for estimating changes in TBW with BIS, as individual changes in TBW estimated via BIS are accurate 90 % of the time when the MD is reached(Reference Moon, Smith and Tobkin8).
In conclusion, BIS TBW estimations in men are more reliable when using a fixed-distance (5 cm) electrode placement. BIS TBW reproducibility in women was not affected by electrode placement, which is most likely due to the decrease in distance from the hand and wrist positions, as well as from the ankle and foot positions, when using SS electrodes. Because of the 90 % accuracy of BIS to track changes in TBW when the MD is met, it is suggested that individual BIS TBW results be based on the reproducibility of individual devices, laboratories, clinics and investigators, as well as on men and women alone. Furthermore, when interpreting group changes in TBW estimated via BIS, reproducibility should be considered, and results that include subjects who have not met the MD should be interpreted with caution unless no change in TBW is desired. Additionally, the fixed-distance (5 cm) electrode placement may allow for smaller changes in TBW to be considered real, thus allowing for shorter study durations when attempting to modify TBW values.
Validity study
Pre- and post-raw resistance values at both zero and infinite ohms were significantly (P < 0·001) larger when utilising the 5 cm electrode placement than when utilising the SS electrode placement. Consequently, pre- and post-TBW values were significantly (P < 0·05) larger when utilising the 5 cm electrode placement than when utilising the SS electrode placement. These findings are in agreement with those reported in the investigation done by Evans et al. (Reference Evans, McClagish and Trudgett31), who discovered that moving the proximal electrodes proximally on the hand and foot independently resulted in a significant decrease in impedance (P < 0·001). In contrast, the present investigation moved both hand and foot distal electrodes proximally closer to the ankle and wrist electrodes. Changing the SS electrode placement to a fixed-distance (5 cm) electrode placement caused resistance values at both zero and infinity to increase, causing a decrease in TBW values. Therefore, when interpreting or comparing fixed-distance electrode (5 cm) TBW values with SS electrode values, it may be necessary to add 0·872 litres to TBW values obtained for men and 0·606 litres to those obtained for women. This correction may become useful when utilising the BIS correction equations based on SS electrode placement followed in the investigation done by Moon et al. (Reference Moon, Smith and Tobkin8). However, this correction would only be needed when utilising BIS for a single measurement, as the delta TBW values between the 5 cm and SS electrode placements were not significantly different. Specifically, if BIS is utilised to predict TBW for the estimation of body fat alone or in a multiple-compartment model for validation analyses or an individual assessment and not in a longitudinal study, the 5 cm corrections for men and women may provide more accurate results. Nonetheless, future research is needed to identify whether the 5 cm corrections for men and women are accurate for single BIS TBW estimations.
While the pre- and post-TBW values are different between electrode placements, the delta values were nearly identical. CE values were more accurate for the 5 cm electrode placement in men, women, and men and women combined, as well as in the control group (Tables 5 and 7). Nonetheless, both electrode placements resulted in valid delta results. Therefore, either SS or 5 cm electrode position can provide accurate BIS TBW estimations. However, when a single measurement is desired and until further research is conducted, the SS electrode placement is suggested. However, implementing the suggested sex-specific corrections when using the 5 cm electrode placement should produce results that are similar to those produced when using SS electrode placement.
Conclusion
In conclusion, when attempting to track changes in TBW, the use of fixed-distance electrodes appears to moderately reduce the reproducibility errors and allows for more accurate delta TBW values than SS electrodes. Accurate interpretation of delta TBW values requires reproducibility calculations including the sem and MD statistics. These reproducibility calculations should be specific to the sample population, laboratory, clinic, investigator, device and final values (such as TBW values). While there will always be variability in BIS TBW estimations, appropriate pre-testing guidelines may reduce intra-individual biological errors, and utilisation of a fixed-distance electrode placement may reduce methodological errors. Therefore, if an attempt is made to track changes in TBW, fixed-distance electrodes may produce more accurate results with less of a change required to be considered real. Finally, SS electrodes appear to be valid for tracking changes in TBW via BIS; however, a greater change may be required before the changes can be considered real. Clinics and laboratories should feel confident in TBW changes after an intervention when using either electrode placement technique. However, it is suggested that internal reproducibility analysis be conducted to accurately determine whether the change in TBW is real. More importantly, men and women may require different amounts of change in TBW before accurate interpretations can be made. When conducting TBW assessments using bioimpedance, it is strongly encouraged that the sex of the patient, client and/or subjects and the reproducibility of subsequent devices be considered.
Acknowledgements
We would like to thank all the men and women who participated in the present investigation, and ImpediMed Limited (Pinkenba, QLD, Australia) and Celsius, Inc. (Delray Beach, FL, USA) for funding the present study. The authors declare no conflict of interest. All authors made substantial contributions and gave the final approval for the concepts, drafting and final version. Specifically, J. R. M., A. E. S., S. E. T., C. M. L., K. L. K. and J. L. G. participated in data collection and subject training for the validity study, and also aided in drafting and editing of the manuscript, as well as assisted in the study design and execution. D. H. F., P. B. C., M. S. S., K. C. Y., P. S. T., E. K., T. J. H., A. A. W., S. L. F., V. D. S., J. T. C. and J. R. S. participated in drafting and editing of the manuscript along with aiding in data collection for the reproducibility study, and also assisted in the development of the study design, execution and interpretation.












