Zn is an essential mineral for normal growth and development, by participating in many enzyme reactions and biological functions including the synthesis of proteins and nucleic acids(Reference McCall, Huang and Fierke1), bone growth(Reference Hadley, Newman and Hunt2), and the initiation of transcription processes, gene expression and cell division(Reference Cousins3). The requirement for Zn is increased during periods of rapid growth, such as pregnancy(Reference Swanson and King4). Earlier studies have reported that Zn is one of the most important nutrients for pregnancy(Reference Swanson and King4–Reference Tamura and Goldenberg8); maternal Zn deficiency during pregnancy causes fetal growth retardation, pre-term delivery, low birth weight and effects on postnatal growth and development(Reference Swanson and King4–Reference Danesh, Janghorbani and Mohammadi11).
It is well established that both the total intake of Zn and its bioavailability, usually measured by the molar ratio of phytate:Zn(Reference Oberleas and Harland12), are important contributing factors to Zn nutrition(Reference Hunt, Beiseigel and Johnson13). Zn present in animal foods is known to exhibit a greater degree of bioavailability than that in plants because of the possible presence of phytate, which inhibits Zn absorption in the intestine(Reference Oberleas and Harland12, Reference Joung, Nam and Yoon14). However, a high amount of phytate is present in cereals, legumes and nuts, which are often staple foods in Asian countries(Reference Ma, Li and Jin15, Reference Alloway16), including Korea; approximately 80 % of the total food consumed is of plant origin, the majority being from foods with a relatively low bioavailability, such as white rice, grain products, legumes and vegetables(17). Although processing, such as milling and/or cooking methods that remove both phytate and Zn from grain products, can improve Zn bioavailability, the total amount of Zn in the foods may be very low(Reference Ma, Li and Jin15, Reference Gibson, Yeudall and Drost18).
Despite the well-known variability in the bioavailability of dietary Zn and the importance of maternal Zn nutrition for fetal growth, there have been very limited studies. Velie et al. (Reference Velie, Block and Shaw19) have reported the positive relationship between maternal Zn intake from animal sources and the risk of neural tube defects; however, they did not include anthropometric measurements, which is a major indicator of fetal growth. Yokoi et al. (Reference Yokoi, Sandstead and Egger20) have reported that consumption of beef and other red meats measured by qualitative food frequency was positively associated with Zn pool size in premenopausal women.
We hypothesised that the food source of maternal Zn intake and the molar ratio of phytate:Zn during pregnancy are associated with fetal growth, and the positive association may exist in animal sources only. Moreover, to test this hypothesis, we evaluated the relationship between maternal dietary Zn intake from different food sources (namely plant and animal) and fetal growth using a large sample from the Mothers and Children's Environmental Health study(Reference Kim, Ha and Park21).
Subjects and methods
Subjects
The Mothers and Children's Environmental Health study is a prospective cohort study designed to investigate the effect of pre- and postnatal environmental exposures on growth, development and health from early fetal life to young adulthood. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all subjects provided a written informed consent. The study was reviewed and approved by three institutional review boards at Ewha Womans University School of Medicine, Dankook University Hospital and Ulsan University Hospital, and informed consent for participation was obtained from all subjects. The present investigation formed part of a multicentre prospective cohort study of women in mid-pregnancy (12–28 weeks of gestation) conducted in Seoul (a metropolitan area), Ulsan (an industrial area) and Cheonan (a medium-sized urban area), Republic of Korea, as described previously(Reference Kim, Ha and Park21, Reference Kim, Lee and Hwang22). Of the 1610 women who participated in the Mothers and Children's Environmental Health study between August 2006 and October 2009, we excluded twenty-five women who were pregnant with twins, twenty-two with spontaneous abortion, twenty-six with pregnancy complications (hypertension and/or diabetes), sixty-six whose pregnancy lasted less than 37 or greater than 42 weeks, 106 without dietary intake data and 447 who had not delivered babies at the time of this analysis. Among the remaining 918 subjects, fifty-six were excluded because they had insufficient data for the covariates that may affect fetal growth. Thus, 862 and 784 subjects were eligible for inclusion in birth-weight and -height analyses, respectively (since birth-height data were missing from seventy-eight subjects).
Using a structured questionnaire, trained personnel interviewed the participants to obtain demographic and socio-economic data, including local centres (Seoul, Ulsan or Cheonan), age (years), weight (kg), height (cm), education (less than high school, less than university or university or higher), family income ( < US$2000, US$2000–4000 or >US$4000; median monthly household income per two or more persons in Korea is approximately 3400 000 Korean won = approximately US$2900 in 2009), exercise (yes or no), ever smoked (yes or no), current smoking (yes or no), alcohol consumption (current or none) and parity (n). Pre-pregnancy BMI was calculated using self-reported height and weight. Gestational age at delivery (weeks) was estimated on the basis of the self-reported date of the last menstrual period. Birth weight (g) and height (cm) at delivery were measured by trained nurses, and neonatal sex (girl or boy) was obtained from medical records.
Dietary assessment
Dietary intake data were obtained from participants by a well-trained dietary interviewer using the 24 h recall method. Dietary intakes of food groups and nutrients were analysed using a computerised nutrient-intake assessment software program (CAN-Pro 3.0; Korean Nutrition Society, Seoul, Korea). Zn intakes from animal (meat, fish, eggs and dairy products), plant (grains, potatoes, legumes, mushrooms, fruit and vegetables, seaweeds, and nuts) and other (sweets, carbonated beverages, fats and oils, and condiments) food sources were calculated. Percentages of Zn intake from animal or plant foods were defined as (Zn intake from animal or plant foods/total dietary Zn intake) × 100. To estimate Zn intake from supplements, information on self-reported supplement use was collected by asking the type (vitamins, minerals and others) and brand name of the supplements, and the amount and frequency of their use.
Phytate intakes were calculated using the phytate content of each food component from the literature(Reference Joung, Nam and Yoon14, Reference Ma, Li and Jin15, Reference Harland and Oberleas23). Adjustments were made, when necessary, to take into account any known changes in phytate content arising from food processing and preparation methods. The molar ratio of phytate:Zn was calculated as the mole of phytate intake (molecular weight, 660·1) divided by the mole of Zn intake (molecular weight, 65·4). We covered 80·5 % (range 42·9–100 %) of phytate intake of the subjects.
Statistical analysis
Statistical analyses were performed using the SAS statistical package (SAS 9.1; SAS Institute, Cary, NC, USA). Nutrient intake data were logarithmically transformed in order to normalise their distributions. Sociodemographic and behavioural characteristics are expressed as means and standard deviations (continuous variables) or as numbers and percentages (categorical variables). Total Zn intake from food and supplements, total dietary Zn intake and Zn intake from animal and plant food sources, individually, were divided into quartiles. For the trend test, the categorical variables (i.e. quartiles of Zn and phytate intakes, and phytate:Zn ratio) were treated as continuous variables assigned with the median value within each category, after adjustment for covariates, such as maternal age, pre-pregnancy BMI, neonatal sex, gestational age at delivery, parity, level of education, local centres and interaction between level of education and local centres, that may affect fetal growth(Reference Kim, Lee and Hwang22). Multiple regression analysis was used to examine the relationship between maternal Zn intakes from animal and plant sources and fetal growth. Differences were considered significant at the 5 % level.
Results
Our subjects were 30·1 (sd 3·7) years old and had a pre-pregnancy BMI of 21·7 (sd 3·3) kg/m2 (Table 1). Approximately, 70 % of the subjects had a university or higher education, and about 56 % were primiparous. Gestational age at delivery was 39·3 (sd 1·1) weeks, and birth weight and height were 3297 (sd 385) g and 50·7 (sd 2·3) cm, respectively.
Table 1 General characteristics of mothers and newborns
(Mean values, standard deviations, number of subjects and percentages)

Energy intake during pregnancy was 1775 (sd 482) kcal/d (7421 (sd 2016) kJ/d; Table 2). Total Zn intake was 9·9 (sd 5·6) mg/d (8·4 (sd 3·5) mg/d from foods and 1·5 (sd 4·4) mg/d from supplements). Zn intakes from animal and plant food sources (% total Zn intake) were 3·4 (sd 3·0) mg/d (37·1 %) and 4·8 (sd 1·8) mg/d (60·2 %), respectively. Average phytate intake was 626·0 (sd 331·8) mg/d, and the molar ratio of phytate:Zn was 7·7 (sd 3·8).
Table 2 Daily energy and zinc intake of pregnant women
(Mean values, standard deviations, medians and ranges, n 918)
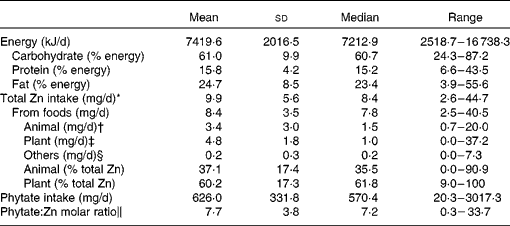
* Includes food and supplements.
† Includes meat, fish, eggs and dairy products.
‡ Includes grains, potatoes, legumes, fruit and vegetables, mushrooms, seaweeds, and nuts.
§ Includes sweets, carbonated beverages, fats and oils, and condiments.
∥ Phytate:Zn molar ratio = (phytate/phytate molecular weight (660·1))/(Zn/Zn molecular weight (65·4)).
As shown in Table 3, multiple regression analysis indicated that maternal Zn intake from animal food sources and their proportions relative to total Zn intake were positively associated with birth weight (P = 0·034 and 0·045, respectively) and height (P = 0·020 and 0·032, respectively), whereas the percentage of Zn intake from plant food sources was negatively associated with birth height (P = 0·026). Phytate intake was not associated with birth weight and height, but the molar ratio of phytate:Zn was negatively associated with birth weight (P = 0·037).
Table 3 Multiple regression analysis for the association between maternal zinc and phytate intakes, and birth weight and height*
(β Coefficients with their standard errors)
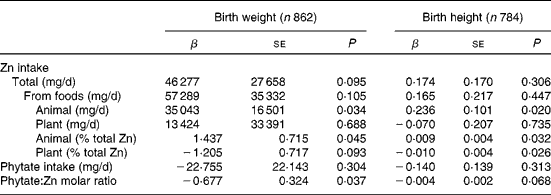
* Multiple regression analysis adjusted for maternal age, pre-pregnancy BMI, neonatal sex, gestational age at delivery, parity, level of education, local centres and level of education × local centres.
A general linear model adjusted for covariates revealed that birth weight increased from the lowest to the highest quartiles of total Zn intake from foods (P for trend = 0·039), as did that from animal sources (P for trend = 0·002) and their proportion (%) to total Zn intake (P for trend = 0·026; Table 4). Birth height increased from the lowest to the highest quartiles of Zn from animal sources (P for trend = 0·002) and their proportion to total Zn intake (P for trend = 0·002), whereas it decreased with proportions of Zn from plant sources (P for trend = 0·006). Birth weight and height increased from the lowest to the highest quartiles of the molar ratio of phytate:Zn (P for trend = 0·001 and 0·021, respectively).
Table 4 Birth weight and height according to the quartiles of zinc and phytate intakes of pregnant women*
(Mean values and standard deviations)

* General linear models adjusted for maternal age, pre-pregnancy BMI, neonatal sex, gestational age at delivery, parity, level of education, local centres and level of education × local centres.
Discussion
We found that maternal Zn intake from animal food sources was positively associated with birth weight and height, but the percentage of Zn intake from plant food sources was negatively associated with birth height. The phytate:Zn ratio was negatively associated with birth weight in the present study.
Such beneficial effects of Zn from animal sources on fetal growth can be explained by the bioavailability of Zn differing with its food sources(Reference Oberleas and Harland12, Reference Etcheverry, Hawthorne and Liang24). Zn present in animal foods such as meat, fish, eggs and milk is generally known to have a greater bioavailability than that found in plants because of the possible presence of phytate, a chelator of Zn that decreases its availability for absorption in the intestine(Reference Joung, Nam and Yoon14, Reference Kim, Paik and Joung25). Since diets that comprise mostly plant foods with a high phytate:Zn ratio result in low Zn absorption in humans(Reference Oberleas and Harland12, Reference Kim, Paik and Joung25, Reference Liang, Han and Nout26), the issue of Zn bioavailability is important for populations with plant-dominated diets. The bioavailability of Zn is influenced by different foods, and Zn from animal foods is believed to be most bioavailable. Yokoi et al. (Reference Yokoi, Sandstead and Egger20) reported that consumption of beef and other red meat measured by qualitative food frequency was positively associated with Zn pool size, and Velie et al. (Reference Velie, Block and Shaw19) found that increased total Zn intake and Zn intake from animal products were associated with a reduced risk for neural tube defects, suggesting that dietary Zn exerts differential effects on pregnancy outcome depending on the food source. It becomes even more important for pregnant women, because during pregnancy, the requirement for Zn is increased for the demands by fetal growth(Reference Tamura and Goldenberg8, Reference Goldenberg, Tamura and Neggers9, Reference Shah and Sachdev27).
Another possible mechanism for the positive association between the high consumption of Zn from animal food sources and fetal growth is the high consumption of animal food itself, because of its protein content and an enhancing role of animal protein on Zn absorption. Zn absorption is reported to increase in a linear fashion with increasing protein consumption(Reference Sandström, Almgren and Kivistö28). Animal protein with a higher content of amino acids, such as histidine, cysteine and methionine, has been shown to counteract the inhibitory effects of phytate on Zn absorption(Reference Lönnerdal29). However, we found no association between the intakes of either total or animal protein and fetal growth (data not shown).
In the present study, no association was observed between fetal growth and either total Zn intake from food only or that from food and supplements together, a finding that is consistent with the Glover & Hogstrand study(Reference Glover and Hogstrand30), but contrasts with others who found a positive association(Reference Merialdi, Caulfield and Zavaleta10, Reference Yang, Chen and Lin31). Our finding of no association between total Zn intake from food and supplements on fetal growth might be due to only a small proportion (15 %) of our subjects taking dietary supplements containing Zn, rendering the contribution of Zn from supplements negligible (1·5 mg/d). The results of prenatal Zn supplementation trials on fetal growth have been equivocal. Garg et al. (Reference Garg, Singhal and Arshad32) found that Zn supplementation exhibited positive effects on birth weight and gestational age. However, Goldenberg et al. (Reference Goldenberg, Tamura and Neggers9) found a positive effect of Zn supplementation on fetal growth only for women with a BMI of < 26 kg/m2.
The likelihood of mild-to-moderate Zn deficiency(Reference Caulfield, Zvaleta and Shankar33) and lower plasma levels of Zn(Reference Swanson and King34) is higher in pregnant women than in non-pregnant women. Despite a recent increasing trend of animal food consumption and a subsequent improvement in overall Zn bioavailability(Reference Kwun, Do and Chung35), approximately 54 % of the Korean population has an inadequate Zn intake due to the traditionally high intake of white rice and vegetables(Reference Joung, Nam and Yoon14). The situation could be more critical for pregnant women, because the nutritional requirement for Zn is increased during pregnancy to meet the demands of fetal growth and the maintenance of maternal health. The mean dietary Zn intake from foods was 8·4 mg/d in our subjects, which is comparable with that among pregnant women in both China(Reference Cheng, Dibley and Zhang36) and England(Reference Mouratidou, Ford and Prountzou37), but lower than that reported in the USA(Reference Litonjua, Rifas-Shiman and Ly38) and New Zealand(Reference Watson and McDonald39). The estimated mean Zn intakes from animal and plant food sources of our subjects were 3·4 and 4·8 mg/d, respectively, indicating that about 40 % of the dietary Zn was derived from animal foods. Although several studies have found that both the total amount of Zn intake and its bioavailability are important for fetal growth during pregnancy(Reference Hunt, Beiseigel and Johnson13–Reference Ma, Li and Jin15), our findings suggest that fetal growth is influenced more by the bioavailability of Zn than by the absolute amount of Zn intake.
The present study has a few limitations. We did not measure plasma Zn concentrations that are considered to be a more objective means to assess Zn status than dietary Zn intake data alone, and a 1 d, 24 h recall may not be sufficient to assess usual daily intake due to large intra-individual variability in food and nutrient intake, but trained dietitians using standard protocols were employed to help the subjects reflect on their daily diet to minimise variation if it existed. There are other possible mechanisms of a positive effect of a higher consumption of animal foods for better fetal growth. These include the increased intakes of other amino acids (methionine, etc.), of which adequate intakes are known to be important for fetal growth(Reference Dasarathy, Gruca and Bennett40); however, we were not able to distinguish such a possibility. Further studies are warranted to clarify these issues. Nonetheless, to our knowledge, this is the first study involving a large prospective cohort to investigate the relationship between maternal intake of Zn from different food sources and fetal growth among pregnant women in Korea, where the food source is predominantly of plant origin with high phytate content.
In conclusion, we found that the maternal dietary Zn intake from animal foods may have a positive effect on fetal growth. Therefore, an adequate Zn intake from animal foods that are particularly rich sources of Zn should be recommended for pregnant women to promote optimal fetal growth. The findings of the present study could help in the further development of public health policies to improve nutritional status of Zn during pregnancy.
Acknowledgements
The present study was supported by the Mothers and Children's Environmental Health project of the Ministry of Environment, Republic of Korea. None of the authors has any conflicts of interest to declare. Y. A. L. conducted the statistical analyses and wrote the manuscript. J.-Y. H. assisted in the study design and analyses, and wrote the manuscript. Y. A. L. and H. K. collected the dietary data. E.-H. H., H. P., M. H., Y. K. and Y.-C. H. conducted the research. N. C. designed the study and supervised all aspects of its implementation. All authors contributed to the preparation of the manuscript and approved the final version submitted for publication.






