Anaemia and stunting are among the major nutritional problems of children in developing countries( Reference Lopez, Cacoub and Macdougall 1 , Reference Onis and Branca 2 ). Globally, 43 % of children under 5 years of age were anaemic in the year 2013( Reference Lopez, Cacoub and Macdougall 1 ). In the same year, the global prevalence of stunting was estimated to be 25 %( Reference Onis and Branca 2 ). Developing countries bear a disproportionately higher burden of anaemia( Reference Balarajan, Ramakrishnan and Özaltin 3 ) and stunting( Reference Onis and Branca 2 , Reference Black, Victora and Walker 4 ). Because of their negative consequences on health and well-being of children, stunting and anaemia are at the centre of global nutrition policies and targets( 5 ).
Anaemia and stunting are multi-causal conditions with a number of dietary and non-dietary influences. However, Fe deficiency is the leading cause of anaemia accounting for almost half of the global anaemia burden( Reference Lopez, Cacoub and Macdougall 1 , Reference Camaschella 6 ). Anaemia in young children is mainly due to suboptimal infant and young child feeding (IYCF) practices( Reference Magalhaes and Clements 7 , Reference Thejpal 8 ), infection( Reference Magalhaes and Clements 7 ) and poor health care( Reference Camaschella 6 – Reference Thejpal 8 ). Stunting indicates linear growth failure, as well as poor overall child condition( Reference Onis and Branca 2 ). The causes of stunting are diverse but in most cases include suboptimal IYCF practices, infection and poor health care( Reference Onis and Branca 2 , Reference Stewart, Iannotti and Dewey 9 ). Besides, anaemia was reported to be associated with a higher risk of stunting( Reference Soliman, De Sanctis and Kalra 10 ).
The evidence on the relation of Fe supplement use (ISU) with linear growth or Hb is inconsistent. Fe stimulates cell proliferation and physical growth through mechanisms including improving Hb concentration( Reference Soliman, De Sanctis and Kalra 10 ), tissue oxygenation( Reference Soliman, De Sanctis and Kalra 10 ) and insulin-like growth factor-binding protein-1 (IGFBP-1)-induced cell proliferation( Reference Kajimura, Aida and Duan 11 ). Fe deficiency results in low Hb or anaemia( Reference Camaschella 6 , Reference Thejpal 8 ). Anaemia imposes a hypoxic condition in the body( Reference Soliman, De Sanctis and Kalra 10 ), and inhibits the synthesis of hepatic proteins and IGFBP-1( Reference Kajimura, Aida and Duan 11 ), which subsequently may influence linear growth negatively. Thus, it could be assumed that ISU promotes linear growth. However, some studies demonstrated that ISU had no significant effect on linear growth( Reference Vucic, Berti and Vollhardt 12 – Reference Ramakrishnan, Aburto and McCabe 14 ).
In Ethiopia, 44 and 38 % of children under 5 years of age were stunted in the years 2011( 15 ) and 2016( 16 ), respectively. Anaemia prevalence in this age group was 56 % in 2016( 16 ), rising from 44 % in 2011( 15 ). Most of the growth faltering in children occurs in the first 2 years during which anaemia risk is also high( 15 – Reference Victora, de Onis and Hallal 17 ). Evidence on the relationships among ISU, Hb and linear growth is scarce in Ethiopia. In this study, we investigated the relationships between ISU and Hb, ISU and linear growth, and Hb and linear growth. We hypothesised that ISU related positively to Hb or linear growth, and Hb related positively to linear growth. We used a nationally representative sample of children aged 6–24 months from the EDHS 2011 data set.
Methods
Data source, study setting and population
This study was conducted using the data set of children included in the EDHS 2011. The survey was led by the US Agency for International Development (USAID) through its Monitoring and Evaluation to Assess and Use Results Demographic and Health Surveys (MEASURE DHS) project that has been organising over 300 surveys in ninety countries. In Ethiopia, four rounds of DHS were conducted, namely EDHS 2000, EDHS 2005, EDHS 2011 and EDHS 2016. The latest available data set at the time this study was conducted was EDHS 2011 (15) . At that time, the EDHS 2016 data set was not yet released, although preliminary reports were made( 16 ). The full data set of EDHS 2011 is available on the website of the DHS program: http://dhsprogram.com/data/dataset/Ethiopia_Standard-DHS_2011.cfm. The sample in EDHS 2011 was designed to be representative for various health and nutrition indicators at both federal and state administration levels. Administratively, Ethiopia is divided into nine regional states and two city authorities. Children, 6–24 months of age, with complete data on all variables of interest were included in this analysis.
Study design, sample size and sampling methodology
The study was cross-sectional in design. Study participants were selected following a two-stage stratified, cluster sampling. The primary sampling units were census enumeration sites. The survey included 624 sites. The secondary sampling units were households. A representative sample of 17 817 households was selected for EDHS 2011. All children aged under 5 years in the selected households were included in the survey and data were collected on various health and nutrition variables. Of the 11 805 children under 5 years of age who were found eligible, anaemia testing was done for 9798 (83 %). More information about the survey methodology is found in the EDHS 2011 final report( 15 ). In line with the objective of the study, we extracted only the data of children aged 6–24 months (n 2719). Reasons for our interest in the age group 6–24 months include the following: (1) in the EDHS surveys( 15 , 16 ), Hb testing was done only for those aged 6 months and above; (2) the first 2 years of life is the most critical stage during which most growth faltering and anaemia occur( Reference Victora, de Onis and Hallal 17 ); and (3) data on IYCF practices were collected only for those 6–24 months( 15 ) – that is, the other age groups lack data on dietary practices. We thought findings unadjusted for IYCF practices might be more prone to confounding. Of the 2719 children aged 6–24 months, 11 % (n 299) of them with missing data on one or more of the main variables of interest or the covariate were excluded. The remaining 2420 children were weighted by their corresponding state’s sampling weights. To ensure representativeness at both federal and state levels, different sampling fractions were used for different states; that is, the small states were over-sampled and the large states under-sampled. Thus, we weighted the data following the EDHS methodology( 15 , 18 ) to compensate for the unequal probability of selection by the state of residence. The final weighted sample was 2400. All analyses were done based on this weighted sample.
Variables and measurements
Data collection (by EDHS data collectors) was done from January to June 2011, by trained data collectors, using a structured EDHS 2011 questionnaire. USAID/DHS program provided training to data collectors on the DHS questionnaire. The data collectors conducted face-to-face interview with mothers or guardians of eligible children and collected data on anthropometry, IYCF practices, health, nutrition and other variables( 15 ).
Outcome or main variables
Length (cm) was measured using a wooden board (in lying position). Age (months) was obtained from the mother or guardian of the child. On the basis of the length and age data, z scores were calculated for each child using the WHO 2006 Child Growth Standards( 19 ). Because of its more familiarity, height-for-age (HFA) is subsequently used in this work to mean length-for-age. HFA is the preferred indicator of linear growth( Reference Onis and Branca 2 ); thus, it is used in this report interchangeably with linear growth. Hb was measured using blood samples taken from a finger or a heel prick, and then analysed using the HemoCue®Hb 201+ System (HemoCue AB)( 15 ). Hb is a reliable indicator of anaemia at the population level( 19 , 20 ). The altitude adjusted Hb, on a continuous scale, was used in the analyses. ISU in the last 7 d preceding the survey (yes/no) was obtained by asking mothers or guardians. No data were collected on adherence, frequency or dosage of ISU.
Covariates
Child sociodemographic factors namely age (months), birth type (single, twin), birth size or birth weight (as reported subjectively by the mother of the child and grouped into three categories: large, average and small) and sex (boy/girl). History of infection (yes/no) was assessed by whether the child had any one of fever, diarrhoea or cough in the past 2 weeks preceding the survey. Duration of breast-feeding (<6, 6–11, 12–23, 24 months), deworming in the past 6 months preceding the survey (yes/no), vitamin A in the past 6 months preceding the survey (yes/no) and complementary feeding practices (dietary diversity and meal frequency) were also included as covariates. Dietary diversity and meal frequency scores were developed using the 24-h dietary recall data indexed to each child. The dietary recall data were based on seven food groups: (1) flesh foods; (2) eggs; (3) dairy products; (4) grains, roots and tubers; (5) legumes and nuts; (6) vitamin A-rich fruits and vegetables; and (7) other fruits and vegetables. According to the WHO criteria( 21 ), minimum dietary diversity (MDD) is met when a child eats from 4 or more of these food groups a day. Minimum meal frequency (MMF) is met when the frequency of eating a day is at least three times for breast-feeding and four times for non-breast-feeding children.
Analysis
Before performing bivariate and multivariate analyses, we checked statistical assumptions, including the normality of distributions of Hb, HFA and multi-collinearity tests among explanatory factors. We presented descriptive statistics by mean values and standard deviations, and 95 % CI, for continuous variables and frequency for categorical variables. The relationships of the three variables of interest, ISU, Hb and HFA, among themselves and with the covariates were checked first by bivariate analyses. Independent t test and one way-ANOVA were done to compare the differences between two and more than two means, respectively. Finally, we run multiple linear regression models on the relationships: ISU with Hb, ISU with HFA and Hb with HFA. All multivariate models were adjusted for covariate demographic and IYCF factors. Covariate selection was done based on the results of the bivariate analyses. Only variables with P≤0·25 in the bivariate analyses were included in the multivariate regression models. Statistical significance, in the final analysis, was determined at P≤0·05. All analyses were based on the weighted sample and performed using SPSS version 23.
Ethical issues
This analysis was based on an existing data set, EDHS 2011, conducted with dual ethical approvals, Ethiopia and USA. The institutional review boards of Ethiopian Health and Nutrition Research Institute and ICF International approved EDHS 2011. Study participants provided consent for data collection( 15 ). For this particular analysis, approval to download and use data was obtained from MEASURE DHS through a project titled ‘Trends and determinants of malnutrition in Ethiopia’. The data were used solely for the purpose requested.
Results
Mean Hb and mean HFA values stratified by weighted demographic and IYCF factors are presented in Table 1. The study included 2400 participants, of whom 1221 (50·88 %) were boys and 1179 (49·12 %) were girls. The mean Hb (g/l) was 103·74 (95 % CI 103·02, 104·54, sd 18·75). The mean HFA (z scores) was −1·40 (95 % CI −1·47, −1·34, sd 0·62). On average, girls had 104·42 g/l (95 % CI 103·51, 105·29) Hb, compared with that of boys, 102·79 g/l (95 % CI 101·69, 103·90); however, the difference was not statistically significant (P=0·537). Boys were significantly shorter than girls (P<0·001) with mean HFA (z score) of −1·59 (95 % CI −1·69, −1·49) and −1·28 (95 % CI −1·37, −1·19) corresponding to boys and girls, respectively.
Table 1 Mean Hb (g/l) and mean height-for-age (HFA, z score) by child demographic and feeding factors – Ethiopian Demographic and Health Survey, 2011 (weighted n 2400) (Mean values and 95 % confidence intervals)
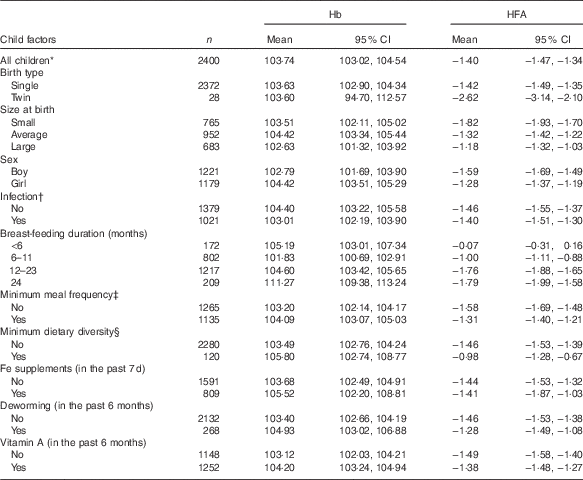
* Values in this particular row refers to descriptive statistics for all children included in the analysis.
† Infection defined as history of cough, diarrhoea or fever in the last 2 weeks preceding the survey (yes, any one of the three conditions).
‡ Minimum meal frequency defined as, according to the WHO criteria( 21 ), when a child ate at least three or four times a day for breast-feeding and non-breast-feeding, respectively.
§ Minimum dietary diversity defined as, according to the WHO criteria( 21 ), eating four or more of the seven food groups: (1) flesh foods; (2) eggs; (3) dairy products; (4) grains, roots and tubers; (5) legumes and nuts; (6) vitamin A-rich fruits and vegetables; and (7) other fruits and vegetables.
Results of bivariate and multivariate analyses between ISU and Hb are shown in Table 2. In the bivariate analysis, Fe supplement users had a higher Hb compared with those who did not use, although the difference was not significant (β=1·02; 95 % CI −0·54, 2·46, P=0·207). In the multivariate analysis, ISU did not demonstrate a significant association with Hb (β=1·09; 95 % CI −2·73, 5·01, P=0·567). The model was adjusted for the child demographic and IYCF factors shown in Table 2. We also examined whether ISU was related to HFA but did not find a significant association during bivariate analysis (β=0·04; 95 % CI −0·10, 0·19, P=0·242) or multivariate analysis (β=0·07; 95 % CI −0·06, 0·21, P=0·217). As shown in Table 3, the multivariate model on the relation of ISU with HFA was adjusted for child demographic and IYCF factors.
Table 2 Bivariate and multivariate analysis of factors associated with Hb (g/l) – Ethiopian Demographic and Health Survey, 2011 (weighted n 2400) (β-Coefficients and 95 % confidence intervals)

* Reference category.
† Infection defined as history of cough, diarrhoea or fever in the last 2 weeks preceding the survey (yes, any one of the three conditions).
‡ Minimum meal frequency defined as, according to the WHO criteria( 21 ), when a child ate at least three or four times a day for breast-feeding and non-breast-feeding, respectively.
§ Minimum dietary diversity defined as, according to the WHO criteria( 21 ), eating four or more of the seven food groups: (1) flesh foods; (2) eggs; (3) dairy products; (4) grains, roots and tubers; (5) legumes and nuts; (6) vitamin A-rich fruits and vegetables; and (7) other fruits and vegetables.
Table 3 Bivariate and multivariate analysis of factors associated with height-for-age (HFA, z score) – Ethiopian Demographic and Health Survey, 2011 (weighted n 2400) (β-Coefficients and 95 % confidence intervals)
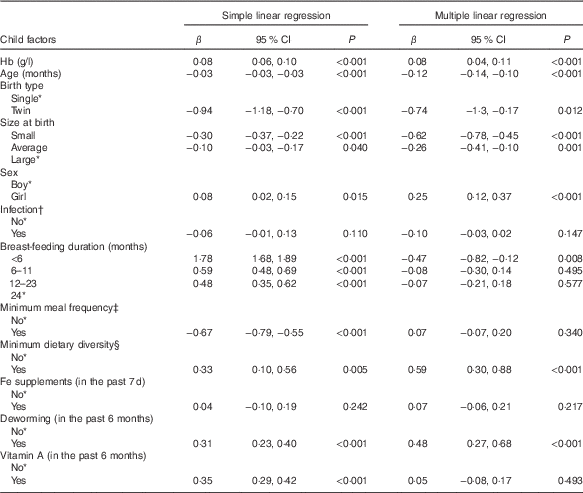
* Reference category.
† Infection defined as history of cough, diarrhoea or fever in the last 2 weeks preceding the survey (yes, any one of the three conditions).
‡ Minimum meal frequency defined as, according to the WHO criteria( 21 ), when a child ate at least three or four times a day for breast-feeding and non-breast-feeding, respectively.
§ Minimum dietary diversity defined as, according to the WHO criteria( 21 ), eating four or more of the seven food groups: (1) flesh foods; (2) eggs; (3) dairy products; (4) grains, roots and tubers; (5) legumes and nuts; (6) vitamin A-rich fruits and vegetables; and (7) other fruits and vegetables.
Result of the Pearson’s correlation test showed Hb directly related with HFA, albeit the association was weak (r 0·09; 95 % CI 0·06, 0·11, P<0·001) (data not shown). Table 3 presents results of bivariate and multivariate analyses between Hb and HFA. In the bivariate analysis, Hb and HFA demonstrated a significant positive association (β=0·08; 95 % CI 0·06, 0·10, P<0·001). Furthermore, Hb maintained its relationship with HFA after controlling covariates (β=0·08; 95 % CI 0·04, 0·11, P<0·001), such that each unit increase in Hb was independently associated with 0·08 z score unit increase in HFA.
Although not the aim of this study, we noted some covariates significantly linked with Hb or HFA. Age (β=0·37, P=0·004) and <6 months duration of breast-feeding (β=−5·54, P=0·006) were significantly associated with Hb. Age was negatively associated with HFA (β=−0·12, P<0·001). In twin siblings, mean HFA was significantly lower compared with that of single births (β=−0·74, P=0·012). Compared with children of large birth size, mean HFA was significantly lower in those with average (β=−0·26, P=0·001) or small (β=−0·62, P<0·001) birth sizes. Girls, compared with boys, had a significantly higher mean HFA (β=0·25, P<0·001). Children who met the MDD had a significantly higher HFA (β=0·59, P<0·001) compared with those who did not meet the MDD. Children who took deworming in the past 6 months had a significantly higher mean HFA (β=0·48, P<0·001) compared with those who did not take deworming.
Discussion
In this study, we reported on the relationships among ISU, Hb and linear growth in children aged 6–24 years, in Ethiopia. We found that ISU was associated with neither Hb nor HFA. Hb was significantly associated with HFA, although the association was weak.
WHO recommends Fe supplementation for under-5 children, particularly in communities with high anaemia burden( 20 ). In Ethiopia, Fe supplementation is one of the strategies to combat anaemia in young children( Reference Lemma and Matji 22 ). In this study, we did not find a significant relationship between ISU and Hb, in contradiction with our hypothesis that ISU would be positively associated with Hb. The evidence on the impact of routine ISU for the prevention of anaemia is not consistent. Given the proven roles of Fe in the production of erythrocytes and treatment of anaemia( Reference Lopez, Cacoub and Macdougall 1 , Reference Camaschella 6 ), higher Hb could be expected among Fe supplement users, albeit Hb is of multiple influences( Reference Thejpal 8 , 20 ) and ISU is not an exclusive way of Fe intake. The lack of association between ISU and Hb in this work might be due to: (1) the high prevalence of chronic undernutrition and multiple micronutrient deficiencies in Ethiopia( 15 , 16 ). Chronic undernutrition or multiple micronutrient deficiencies could influence Hb response to ISU( Reference Ramakrishnan, Aburto and McCabe 14 , Reference Allen, Rosado and Casterline 23 ); (2) limitations related with the way ISU was assessed in EDHS 2011. No information was collected on frequency, dose and duration of ISU, albeit they could substantially influence Hb response to ISU; (3) ISU might be ineffective in resulting in Hb response because of the quality of the supplement or related implementation challenges. A study in Mexico showed that ISU alone did not improve Hb( Reference Allen, Rosado and Casterline 23 ). Another study in Vietnam also showed that weekly ISU improved only serum Fe level, not Hb( Reference Hieu, Sandalinas and de Sesmaisons 24 ); (4) poor adherence to routine Fe supplementation reported by other studies( Reference Balarajan, Ramakrishnan and Özaltin 3 , Reference Bilenko, Yehiel and Inbar 25 ); or (5) other factors out of our speculations.
We did not find a significant link between ISU and linear growth. The finding was in disagreement with our hypothesis that ISU would be positively related to linear growth. Despite ISU not meaning total Fe intake, our finding contradicted with the evidence that Fe intake stimulates cell proliferation and linear growth( Reference Soliman, De Sanctis and Kalra 10 , Reference Kajimura, Aida and Duan 11 ). However, our result agreed with meta-analysis reports of Fe supplementation trials( Reference Sachdev, Gera and Nestel 13 , Reference Ramakrishnan, Aburto and McCabe 14 ), which reported null ISU effect on children’s anthropometric indices, including HFA. Given the existence of nutrient to nutrient interaction( Reference Allen, Rosado and Casterline 23 ), the effect of Fe on linear growth may be indirect, moderated by other nutrients, or not necessarily on child growth but rather on other health parameters. The limitations of the DHS data set, specifically the lack of data on the frequency and dose of ISU, might have also influenced our finding. These factors were reported to substantially influence Hb response to ISU( Reference Bilenko, Yehiel and Inbar 25 ).
Hb was significantly associated with linear growth, independent of a variety of dietary or non-dietary factors. The finding was in agreement with epidemiological studies that showed a higher risk of stunting among anaemic children( Reference Stewart, Iannotti and Dewey 9 – Reference Kajimura, Aida and Duan 11 ). It was also in agreement with the nutrition situation in Ethiopia where both stunting and anaemia were highly prevalent among children under 5 years of age( 15 , 16 ). Studies on the relation of Hb with linear growth are generally scarce. The mechanism by which Hb may be related to linear growth is largely unknown. One possible mechanism could be that Hb improves tissue oxygenation, which subsequently enhances optimal cell proliferation and physical growth( Reference Soliman, De Sanctis and Kalra 10 , Reference Kajimura, Aida and Duan 11 ). Hb is of multiple influences. It may indicate intake, as well as body pool of Fe and some other nutrients. Thus, the association of Hb with linear growth may also be because of other nutrients. We did not rule out this possibility because of the nature of the data set we used. Poor IYCF practices were associated with both low Hb and growth faltering( Reference Magalhaes and Clements 7 – Reference Stewart, Iannotti and Dewey 9 ), implying that Hb may intermediate the link between IYCF practices and linear growth. However, as we adjusted the multivariate models for IYCF practices, we believe that the possibility of Hb as an intermediate factor might be low.
Some of the covariates demonstrated independent associations with Hb or linear growth. Age and breast-feeding duration were independently associated with Hb. Age, sex, birth type, size at birth (birth weight), breast-feeding duration, dietary diversity and deworming were independently associated with linear growth. These findings were consistent with the evidence that Hb and linear growth are multi-causal, influenced by both nutritional and non-nutritional factors( Reference Onis and Branca 2 , Reference Balarajan, Ramakrishnan and Özaltin 3 , Reference Magalhaes and Clements 7 ).
The main strengths of this study were the use of a large and nationally representative sample, adjustment for both dietary and non-dietary covariates and objectively measured biomarkers (height, weight and laboratory-confirmed Hb). The study design, cross-sectional, precluded making causal inference. The collection of data on some variables based on respondents’ memory of past events might have introduced recall bias. We did not take into account the effects of dose and frequency of ISU on Hb or HFA. This could be problematic because these factors can substantially influence the effectiveness of ISU. Besides, we did not account for dietary Fe intake, which might have confounded our findings; however, the inclusion of MDD and MMF in all models might have minimised the possibility of confounding by dietary Fe intake. Despite these limitations, we believe that the findings of this study may serve as input for further studies and for designing evidence-based anaemia or stunting prevention interventions.
Conclusion
Fe supplementation was not associated with either Hb or linear growth in this study; however, Hb was positively associated with linear growth. Duration of breast-feeding, deworming, dietary diversity, sex, age and birth weight were independently associated with Hb or linear growth, highlighting the multifactorial natures of Hb and linear growth in young children.
Acknowledgements
The authors thank ICF International team and MEASURE DHS for granting us free access to the EDHS 2011 data set, and Ms Johanna Ehrenstein who assisted in the proof-reading of the final manuscript. The authors are also grateful for the EDHS study participants and staff members involved in the collection and processing of the data set.
S. H. M. is a recipient of a postgraduate study scholarship from Tehran University of Medical SciencesInternational Campus. This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
S. H. M. conceptualised the study, extracted the data set, designed the analysis plan, performed the analysis and prepared the manuscript. A. E. supervised the work and critically reviewed the manuscript. Both authors reviewed and approved the final manuscript.
The authors declare that there are no conflicts of interest.





