Folic acid is a water-soluble vitamin (vitamin B9) and is required to be obtained from exogenous sources by man and other mammals. It is essential for the synthesis of amino acids and nucleic acids(Reference Stanger1, Reference Wani, Hamid and Kaur2). There exists a well-developed epithelial folate transport system for the regulation of normal folate homeostasis in the intestine. These systems have been elucidated and mechanisms of carrier-mediated absorption of folic acid have been reported(Reference Qiu, Jansen and Sakaris3–Reference Hamid and Kaur5). Reduced folate carrier (RFC or SLC19A1) and recently discovered proton-coupled folate transporter (PCFT), earlier called haem carrier protein 1, in the intestine have been found to be responsible for the transport of folate across the intestine(Reference Qiu, Jansen and Sakaris3, Reference Hamid, Wani and Kaur6). The RFC is an integral protein of approximately 65 kDa in man and approximately 58 kDa in rats(Reference Moscow, Gong and He7), which mediates cellular uptake of reduced folates and antifolates and is ubiquitously expressed(Reference Matherly and Goldman8). The characteristics of folate transport by the RFC across the intestine, liver, pancreas, kidney and colon include pH dependence, saturable uptake at low folate concentration and competition among various folate derivatives(Reference Said and Mohammadkhani4, Reference Hamid, Wani and Rana9–Reference Hamid and Kaur11). The human RFC shows an amino acid homology of 65·2 and 66·0 % with its rat and mouse counterparts, respectively(Reference Whetstine, Flatley and Matherly12). The transport of folate in the intestine is also mediated by the newly discovered PCFT, which has the characteristics of pH dependence, Na+ independence and similar affinity for reduced and oxidised folates(Reference Qiu, Jansen and Sakaris3). PCFT mRNA expression is also prominent in the liver, an organ that specifically possesses and stores maximum folate, along with the kidney, colon, spleen and placenta(Reference Qiu, Jansen and Sakaris3). It has been found that human PCFT protein has 459 amino acids and eleven putative membrane-spanning domains, and shares 91 % similarity and 87 % identity with both its mouse and rat counterparts(Reference Bosson13). In the kidney and liver, but not in the intestine, a family of high-affinity folate-binding proteins or folate receptors (α and β) also transports the folates(Reference Wang, Shen and Freisheim14). After absorption from the intestine, folic acid undergoes reduction and is methylated to 5-methyltetrahydrofolate before entry into the portal blood system. 5-Methyltetrahydrofolate acts as a methyl group donor for the synthesis of amino acids and nucleic acids(Reference Stanger1, Reference Hamid and Kaur15). Cellular folate concentrations are influenced by the availability of folate, transport efficiency, polyglutamylation and turnover of folate(Reference Hamid, Wani and Kaur6).
Optimisation of body folate homeostasis led to a significant reduction in the incidence of neural tube defects(Reference Czeizel16, Reference Butterworth and Bendich17), and omphalocele(Reference Botto, Mulinare and Erickson18), conditions associated with folate deficiency. Epidemiological studies have suggested that benefits might also accrue from supplements of folic acid for CVD, Alzheimer's disease and certain types of cancers such as colorectal cancer(Reference Glynn, Albanes and Pietinen19–Reference Bailey, Rampersaud and Kauwell21). Considering such benefits, a mandatory fortification of grain products with folic acid was instituted in many countries, including the USA and Canada. The intake of folic acid from fortified food (100–200 μg/d), together with the use of oral supplementation of folic acid (400 μg), standard multivitamin preparations and consumption of nutrition bars, might create a state of folate oversupplementation(Reference Ulrich and Potter22), amounting to an increase in folic acid intake above the upper tolerable level, i.e. 1000 μg folic acid/d(Reference Choumenkovitch, Selhub and Wilson23). This practice is occurring with little knowledge on the potential safety and physiological consequences of chronic intake of such a high dose of folic acid. The most well-known risk of exposure to high doses of folic acid is the possible masking of cobalamin deficiency in pernicious anaemia(Reference Czernichow, Noisette and Blacher24). This is because folic acid may improve haematological indices but not the neurological disease of a prolonged cobalamin deficiency. Song et al. (Reference Song, Medline and Mason25) suggested that folate oversupplementation in rats accelerates cancer progression, while Achon et al. (Reference Achon, Reyes and Alonso-Aperte26) found no adverse effects of folate oversupplementation on one-carbon metabolism. However, the effect of folate oversupplementation on the regulation of various genes involved in folate metabolism and with one-carbon transfer reactions demands a thorough investigation. It has also been reported that, upon folate oversupplementation, unmetabolised folic acid in the plasma was associated with decreased natural killer cell cytotoxicity among postmenopausal women(Reference Troen, Mitchell and Sorensen27). Results of two very recently conducted randomised, controlled trials have suggested no short- or long-term benefits of folic acid plus vitamin B12 on cardiovascular outcomes in patients with IHD. The authors have suggested that cardiovascular risk prediction by total plasma homocysteine concentration was confined to the homocysteine fraction that does not respond to B vitamins(Reference Ebbing, Bonaa and Arnesen28). As studies have mainly focused on the consequences of folic acid supplementation on one or the other disease, little is known regarding the regulation of the intestinal folate transport process during folate oversupplementation. A recent report has described the down-regulation of folate transporters in human intestinal and renal cells cultured with excess folic acid(Reference Ashokkumar, Mohammed and Vaziri29). More recently, it has been observed that dietary folate oversupplementation decreases the expression of the RFC in an avian system(Reference Jing, Tactacan and Rodriguez-Lecompte30). However, such effects of dietary folate oversupplementation in a mammalian model have not been studied. Therefore, we planned the present study to delineate the regulatory mechanisms of dietary folate oversupplementation on the intestinal folate transport process in rats. We studied the effect of acute and chronic folate oversupplementation on the folate transport process in the intestinal brush-border membrane (BBM), and the mRNA and protein expression of the major folate transporters (PCFT and RFC).
Methods and materials
Chemicals
Radiolabelled [3′,5′,7,9-3H]folic acid, K salt with a specific activity of 24·0 Ci/mmol, was purchased from Amersham Pharmacia Biotech (Hong Kong, China). ColorBurst™ electrophoresis marker (molecular weight 8000–220 000) was purchased from Sigma Chemical Company (St Louis, MO, USA). NP-P Total RNA Extraction Kit was purchased from Taurus Scientific (Cincinnati, OH, USA). Moloney murine leukemia virus reverse transcriptase (RevertAid™ M-MuLV RT) kit was purchased from MBI Fermentas Life Sciences, Glen Burnie, MD, USA. RNAlater (RNA stabilisation solution) was obtained from Ambion, Inc. (Austin, TX, USA). Polyclonal primary antibodies, rabbit anti-rat RFC (rRFC) and anti-rat PCFT (rPCFT), were raised in rabbits in our laboratory. Horseradish peroxidase-labelled goat anti-rabbit IgG secondary antibodies were purchased from G Biosciences (St Louis, MO, USA). Metal-enhanced DAB substrate kit was purchased from Thermo Scientific (Rockford, IL, USA). Cryoprotected Lactobacillus casei bacterial strain (MTCC 1423) was purchased from IMTECH (Chandigarh, India).
Rats and experimental diets
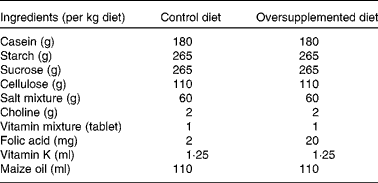
Male albino rats (Wistar strain)(Reference Steinberg, Campbell and Hillman31), 1 month old, weighing 60–80 g (n 24) were obtained from the Central Animal House of the institute. Rats were housed in clean wire mesh cages with controlled temperature (23 ± 1°C) and humidity (45–55 %) and with a 12 h dark–12 h light cycle throughout the study. Rats were randomised into two groups of twelve animals each. Rats in group I were given semisynthetic diets containing a 2 mg folic acid/kg diet (control) and those in group II were given a folate-oversupplemented rat diet, tenfold the requirement for rats, i.e. 20 mg folic acid/kg diet (oversupplemented) (Table 1). Six animals each from groups I and II received the treatment for 10 d (acute treatment) and the remaining six for 60 d (chronic treatment). The animals had free access to water. The body weight of the rats was recorded twice weekly. Animals from both groups were killed under anaesthesia, using sodium pentothal. Starting from the ligament of Treitz, two-thirds of the small intestine was removed, flushed with ice-cold saline and processed for the isolation of cells.
Table 1 Composition of the diets
The protocol of the study was approved by the Institutional Animal Ethics Committee and the Institutional Biosafety Committee.
Isolation of intestinal epithelial cells
Intestinal epithelial cells were isolated following the method of Weiser(Reference Weiser32), with modifications(Reference Hamid, Wani and Rana9). The upper two-thirds of the small intestine was cut and flushed two to three times with 0·9 % saline. One end of the intestine was tied with a thread and filled with rinsing buffer containing 1 mm-dithiothreitol in normal saline. The rinsing buffer was then replaced with a solution consisting of 1·5 mm-KCl, 96 mm-NaCl, 27 mm-sodium citrate, 8 mm-KH2PO4 and 8 mm-Na2HPO4, and incubated at 37°C for 15 min in a beaker containing PBS. The intestine was then filled with a solution containing 1·5 mm-EDTA and 0·5 mm-dithiothreitol in PBS, and incubated at 37°C in a shaker at 100 rpm for 30 min; the solution was then collected for the isolation of total enterocytes. The collected cells were centrifuged at 800 g for 15 min. The pellet contents were mixed with a Pasteur pipette and centrifuged at 800 g for 10 min after the addition of 5 ml of cold PBS. Two more PBS washings were performed. These cells were then used for BBM isolation.
Preparation of brush-border membrane vesicles from isolated intestinal epithelial cells
BBMV were prepared from the isolated total intestinal cells from the control and folate-oversupplemented rats by the method of Kessler et al. (Reference Kessler, Acuto and Storelli33), with some modifications(Reference Hamid, Kaur and Mahmood34). The final pellet obtained was suspended in loading buffer (280 mm-mannitol, 20 mm-HEPES–Tris, pH 7·4) so as to obtain a protein concentration of approximately 5 mg/ml. These BBMV were used to determine [3H]folic acid uptake and to analyse the PCFT and RFC protein levels.
Transport of [3H]folic acid
Uptake studies were performed at 37°C, using the incubation buffer of 100 mm-NaCl, 80 mm-mannitol, 10 mm-HEPES, 10 mm-2-morpholinoethanesulphonic acid (MES), pH 5·5, and 0·5 μm-[3H]folic acid, unless otherwise mentioned. Isolated BBMV (10 μl; 50 μg protein) from the control and folate-oversupplemented diet-fed rats for different specific assays were added to the incubation buffer containing [3H]folic acid of known concentration. Reaction was stopped by adding an ice-cold stop solution containing 280 mm-mannitol, 20 mm-HEPES–Tris, pH 7·4, followed by rapid vacuum filtration. Non-specific binding to the filters was determined by residual filter counts after filtration of the incubation buffer and labelled substrate without vesicles(Reference Hamid, Kaur and Mahmood34). The radioactivity retained by the filters was determined by liquid scintillation counting (Beckman Coulter LS 6500, Kingsmead, London, UK). For the determination of the kinetic constants K m and V max, transport of [3H]folic acid was measured by varying the concentration of [3H]folic acid from 0·125 to 3·0 μm and transforming the data according to the Lineweaver–Burk plot, as described earlier(Reference Hamid, Kiran and Rana35).
RT-PCR analysis
Total RNA from all animals was isolated from the upper 1 cm of jejunal tissues, using the total RNA extraction kit, and complementary DNA synthesis was carried out from the purified and intact total RNA according to the manufacturer's instructions. Primarily, the quality of RNA was assessed by electrophoresis using 1·5 % formaldehyde agarose gel. Analysis of RNA revealed the 28S and 18S RNA species and depicted the RNA to be in the intact form. Expressions of rRFC, rPCFT and anti-rat glyceraldehyde 3-phosphate dehydrogenase (rGAPDH) were evaluated using sequence-specific primers corresponding to the sequence in the open reading frame. PCR mixture (20 μl) was prepared in 1 × PCR buffer consisting of 0·6 U (10·06 nkatal) of Taq polymerase, 2 μm of each primer for rGAPDH, rPCFT and rRFC along with 200 μm of each deoxynucleotide triphosphate. In optimised PCR, the initial denaturation step was carried out for 2 min at 95°C. The denaturation, annealing and elongation steps were carried out, respectively, for 1 min at 94°C, 45 s at 64°C (PCFT) or 56°C (GAPDH) and 1 min at 72°C for thirty-five cycles. In the case of the RFC, the denaturation, annealing and elongation steps were carried out, respectively, for 30 s at 94°C, 30 s at 52·1°C and 30 s at 72°C for forty-five cycles. The final extension step was carried out for 10 min at 72°C. The primers were designed using Primer3 Input (version 0.4.0; USA). The sequences of the primers used are as follows(Reference Yasuda, Hasui and Yamamoto36):
Direction Primer sequence (5′ to 3′)
RFC
Forward CATGCTAAGCGAACTGGTGA
Reverse TTTTCCACAGGACATGGACA
PCFT
Forward AAGCCAGTTATGGGCAACAC
Reverse GGATAGGCTGTGGTCAAGGA
GAPDH
Forward CCTTCATTGACCTCAACTACAT
Reverse CCAAAGTTGTCATGGATGACC
The expected PCR products of size 120, 300 and 400 bp were obtained for rRFC, rPCFT and rGAPDH, respectively, when electrophoresed on 1·2 % agarose gel. The densitometric analyses of the products were determined by using Scion image software (Frederick, MD, USA).
Western blot analysis
For protein expression studies, BBMV (100 μg) isolated from epithelial cell preparations were resolved on 10 % SDS-PAGE and transferred to a polyvinylidene fluoride membrane for 20 min at 15 V. Western blotting was performed using the procedure described by Towbin et al. (Reference Towbin, Staehelin and Gordon37), using polyclonal primary antibodies as rabbit rRFC (1:500 dilution) raised against a specific region of rat RFC synthetic peptide corresponding to amino acids 494–512(Reference Said, Chatterjee and Haq38). The polyclonal antibodies against rPCFT (1:500 dilution) were raised against a specific region of rat PCFT synthetic peptide corresponding to amino acids 442–459. The immunogenic property of this peptide corresponding to PCFT protein was determined by using DNA STAR software (Madison, WI, USA). The primary antibodies against β-actin (1:400 dilution) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The secondary antibodies used were goat anti-rabbit IgG horseradish peroxidase-labelled antibodies (1:2000 dilutions). The quantification of blots was carried out by using Scion image.
Estimation of folate by microbiological assay
Folate estimations were determined by a microtitre plate assay using L. casei, as described earlier(Reference Hamid, Wani and Rana9). All the steps were carried out in aseptic conditions.
Statistical analysis
Each uptake assay was performed three times with four to six independent preparations from each group. Data were computed as means with their standard errors. Group means were compared using Student's t test. The acceptable level of significance was P < 0·05 for each analysis. The power of the study was 0·80.
Results
There was no significant change in the body weight of the folate-oversupplemented rats compared with the controls, during the course of the experiment both in acute and chronic treatments. At the time of killing, the mean body weights of the control and folate-oversupplemented rats in the acute group were 103 (sem 2·4) and 105 (sem 3·2) g, whereas those in the chronic folate oversupplementation group were 236 (sem 6·5) and 245 (sem 9·4) g, respectively.
Serum folate levels
Serum folate represents present folate intake, influenced by the diet, so it was important to determine the folate levels in all groups. The results showed that acute folate oversupplementation significantly increased the serum folate levels (63·5 (sem 15·5) ng/ml in the control group v. 140 (sem 9·89) ng/ml in the oversupplememented group; P < 0·01). However, when folate oversupplementation was carried out for a longer period (60 d), there was no further change in the folate level of the folate-oversupplemented group. However, the serum folate levels in the folate-oversupplemented group (146 (sem 20) ng/ml) remained significantly higher compared with the control group (65 (sem 10·5) ng/ml).
Folic acid transport across intestinal brush-border membrane vesicles
Folic acid uptake across the intestinal BBM was significantly decreased (26·9 %; P < 0·05) in acute folate-oversupplemented group compared with the control group (Fig. 1). However, in the chronic folate-oversupplementated group, there was no significant change in folate transport compared with the control group. Furthermore, the kinetic characterisation of the transport of folic acid across intestinal BBMV was carried out in the acute folate-oversupplemented group only.

Fig. 1 [3H]Folic acid transport in intestinal brush-border membrane vesicles after acute and chronic folate oversupplementation. The incubation buffer (100 mm-NaCl, 80 mm-mannitol, 10 mm-HEPES, 10 mm-2-morpholinoethanesulphonic acid, pH 5·5) containing 0·5 μm-[3H]folic acid was used for uptake measurements. Data are mean values with their standard errors (n 4), carried out in duplicate. * Mean value was significantly different from that of the control group (P < 0·05). ░, Control; ■, oversupplemented.
Kinetic characterisation of folic acid uptake across intestinal brush-border membrane vesicles
BBMV from the control and folate-oversupplemented rats were incubated with [3H]folic acid, and the transport was studied at various time intervals, i.e. 10, 20, 30, 60, 120 and 240 s at 37°C. The transport increased with an increase in time and an initial peak was observed within 20–30 s in both groups of rats. At different time intervals studied, the uptake was 27·2 to 56·5 % less in the folate-oversupplemented rats (P < 0·01) (Fig. 2). In order to determine the driving force for folate transport across the intestinal BBM, the pH of the incubation buffer was varied from 5 to 8, keeping the intravesicular pH constant at 7·5. As shown in Fig. 3, upon decreasing the incubation buffer pH from 7 to 5, an increase in folic acid uptake was observed in both groups, with maximum uptake at pH 5·5 in both groups of rats. Moreover, at the different pH points studied, the folic acid uptake was 15·8 to 41·8 % (P < 0·01) less in the oversupplemented group. Notably, no such uniform trend was observed in the pH range 7–8. Furthermore, since the saturable kinetics is a notable characteristic feature of carrier-mediated transport, kinetic studies were performed in the presence of increasing concentrations of the substrate from 0·125 to 3·0 μm. The initial velocity determined at 30 s and at pH 5·5 showed that, in both groups, the saturation phenomenon with a plateau at 1·0 μm of the substrate concentration was indicative of the Michaelis–Menten kinetics (Fig. 4). At the physiological range of folic acid (0–1·0 μm), the uptake was 12·9–29 % less in the folate-oversupplemented group (P < 0·05). Further from the data, the kinetic constants K m and V max values were determined by the Lineweaver–Burk plot. K m values for the control and folate-oversupplemented groups were found to be the same, i.e. 2·85 (sem 0·12) μm. However, the values of V max for the control and folate-oversupplemented rats was 667 (sem 17·3) and 500 (sem 18·8) pmol/30 s per mg protein, respectively (P < 0·01).

Fig. 2 [3H]Folic acid transport in intestinal brush-border membrane vesicles at different time intervals after acute folate oversupplementation. The incubation buffer (100 mm-NaCl, 80 mm-mannitol, 10 mm-HEPES, 10 mm-2-morpholinoethanesulphonic acid, pH 5·5) containing 0·5 μm-[3H]folic acid was used for uptake measurements at different time intervals. Data are mean values with their standard errors of four separate uptake determinations, carried out in duplicate. Mean values were significantly different from that of the control group: *P < 0·05, **P < 0·01. –♦–, Control; –■–, oversupplemented.

Fig. 3 [3H]Folic acid uptake in rat intestinal brush-border membrane vesicles as a function of pH optimum after acute folate oversupplementation. Uptake was measured by varying the incubation buffer (100 mm-NaCl, 80 mm-mannitol, 10 mm-HEPES and 10 mm-2-morpholinoethanesulphonic acid) with pH from 5·0 to 8·0, keeping intravesicular pH 7·4 at 0·5 μm-[3H]folic acid concentration for 30 s. Data are mean values with their standard errors of four separate uptake determinations, carried out in duplicate. Mean values were significantly different from that of the control group: *P < 0·05, ***P < 0·001. –♦–, Control; –■–, oversupplemented.

Fig. 4 [3H]Folic acid uptake in intestinal brush-border membrane vesicles as a function of substrate concentration after acute folate oversupplementation. Uptake was measured by varying [3H]folic acid concentration from 0·125 to 3·0 μm in the incubation medium (100 mm-NaCl, 80 mm-mannitol, 10 mm-HEPES and 10 mm-2-morpholinoethanesulphonic acid, pH 5·5) after incubating brush-border membrane vesicles for 30 s. Data are mean values with their standard errors of four separate uptake determinations, carried out in duplicate. ![]() , Control;
, Control; ![]() , oversupplemented.
, oversupplemented.
Expression of mRNA corresponding to rRFC and rPCFT
The findings that the folic acid uptake process is markedly decreased in intestinal BBMV with an appreciable decrease in V max, i.e. the number of transporters in the folate-oversupplemented group compared with the control group, led us to determine the mRNA expression of folate transporters. For this purpose, RT-PCR analysis was performed using gene-specific primers for rRFC, rPCFT and rGAPDH (as an internal control). The RT-PCR products were resolved on 1·2 % agarose gel to observe the expression, and the band densities were quantified by densitometric analysis. It was deduced that there was no significant change in the expression of mRNA coding for rRFC and rPCFT, both in the acute and chronic folate-oversupplemented groups compared with the control group (Figs. 5(a), (b) and 6(a), (b)).

Fig. 5 RT-PCR analysis of anti-rat reduced folate carrier (rRFC), anti-rat proton-coupled folate transporter (rPCFT) and anti-rat glyceraldehyde 3-phosphate dehydrogenase (rGAPDH) as an internal control in jejunal tissues (a) resolved on 1·2 % agarose gel electrophoresis and (b) densitometric analysis representing a relative change in rRFC and rPCFT mRNA expressions. Data shown are mean of four separate sets of experiments. (a) Lanes 1–3: control (![]() ); 4–6: acute folate-oversupplemented (■); Western blot analysis of intestinal BBM vesicle (c) using anti-rRFC (58 kDa), anti-rPCFT (54 kDa) and anti-rat β-actin (43 kDa) antibodies and (d) densitometric analysis representing a relative change in rRFC and rPCFT protein levels. Data shown are mean of four separate sets of experiments. (c) Lanes 1 and 2: control (
); 4–6: acute folate-oversupplemented (■); Western blot analysis of intestinal BBM vesicle (c) using anti-rRFC (58 kDa), anti-rPCFT (54 kDa) and anti-rat β-actin (43 kDa) antibodies and (d) densitometric analysis representing a relative change in rRFC and rPCFT protein levels. Data shown are mean of four separate sets of experiments. (c) Lanes 1 and 2: control (![]() ); 3 and 4: acute folate-oversupplemented (■). Mean values were significantly different from that of the control group: *P < 0·05, ***P < 0·001.
); 3 and 4: acute folate-oversupplemented (■). Mean values were significantly different from that of the control group: *P < 0·05, ***P < 0·001.

Fig. 6 RT-PCR analysis of anti-rat reduced folate carrier (rRFC), anti-rat proton-coupled folate transporter (rPCFT) and anti-rat glyceraldehyde 3-phosphate dehydrogenase (rGAPDH) as an internal control in jejunal tissues (a) resolved on 1·2 % agarose gel electrophoresis and (b) densitometric analysis representing a relative change in rRFC and rPCFT mRNA expressions. Data shown are mean of four separate sets of experiments. (a) Lanes 1–3: control (![]() ); 4–6: chronic folate-oversupplemented (■); Western blot analysis of intestinal BBM vesicle (c) using anti-rRFC (58 kDa), anti-rPCFT (54 kDa) and anti-rβ-actin (43 kDa) antibodies and (d) densitometric analysis representing a relative change in rRFC and rPCFT protein levels. Data shown are mean of four separate sets of experiments. (c) Lanes 1 and 2: control (
); 4–6: chronic folate-oversupplemented (■); Western blot analysis of intestinal BBM vesicle (c) using anti-rRFC (58 kDa), anti-rPCFT (54 kDa) and anti-rβ-actin (43 kDa) antibodies and (d) densitometric analysis representing a relative change in rRFC and rPCFT protein levels. Data shown are mean of four separate sets of experiments. (c) Lanes 1 and 2: control (![]() ); 3 and 4: chronic folate-oversupplemented (■).
); 3 and 4: chronic folate-oversupplemented (■).
Expression of anti-rat reduced folate carrier and anti-rat proton-coupled folate transporter in the brush-border membrane of the intestine
Since there was no significant change in the mRNA expression of folate transporters, the study was further extended to determine the regulation of folate transporters at the protein level. Western blot analysis was performed using specific polyclonal anti-rRFC and anti-rPCFT antibodies. The results showed a significant decrease in rRFC (P < 0·001) and rPCFT (P < 0·05) protein levels in BBMV isolated from the folate-oversupplemented rats compared with the control rats during acute folate oversupplementation (Fig. 5(c) and (d)). The decreased expression in the folate-oversupplemented group was to the extent of 33 % in rRFC and 37 % in rPCFT in intestinal BBMV. However, there was no significant change in the protein expression of rRFC and rPCFT in the chronic folate-oversupplemented group compared with the control group (Fig. 6(c) and (d)).
Discussion
The present study was carried out to determine the effect of short (acute) and long (chronic) term dietary folate oversupplementation on intestinal folic acid uptake. The study was carried out on the Wistar strain of rats, which were used earlier for folate deficiency studies(Reference Hamid and Kaur39) and for determining the in vivo folate kinetics(Reference Steinberg, Campbell and Hillman31). Folate oversupplementation in rats was carried out by feeding ten times of normal folic acid, i.e. 20 mg/kg diet for 10 d (acute group) and 60 d (chronic group). The folic acid level in the oversupplemented group corresponds to 900 μg/d of folic acid intake in humans(Reference Choumenkovitch, Selhub and Wilson23). The duration of acute and chronic treatments was chosen as per the previous reports(Reference de Oliveira, Silvestrin and Mello e Souza40–Reference Reul, Stec and Soder43). Folate oversupplementation in rats for a period of 10 and 60 d did not significantly change their body weight. This is in agreement with previous reports(Reference Achon, Reyes and Alonso-Aperte26), where weanling rats were administered 40 mg folic acid/kg body weight for 4 weeks and no change in growth was observed compared with controls. Our feeding regimen was useful in inducing the folate-oversupplemented state in rats, as indicated by a significant increase (2·2-fold) in serum folate levels of the folate-oversupplemented rats compared with the controls, in agreement with some previous results observing the same serum folate levels in controls(Reference Hamid, Wani and Rana9, Reference Vitale and Hegsted44). Earlier, Achon et al. (Reference Achon, Reyes and Alonso-Aperte26) have shown a threefold increase in serum folate levels upon feeding 40 mg folic acid to rats for a period of 3 weeks. Such an increase in folic acid concentration has been shown to decrease natural killer cell cytotoxicity and to reduce the response to antifolate drugs used against malaria and other diseases(Reference Smith, Kim and Refsum45). Besides, this increase in serum folate levels might also have detrimental effects on one-carbon metabolism, leading to an increase in S-adenosylmethionine, an important contributor of methyl group for methylation reactions, and thereby might down-regulate the expression of tumour-suppressor genes.
Folate uptake in intestinal BBMV follows saturable kinetics and has a pH optimum at 5·5, in agreement with the previous reports from our laboratory(Reference Hamid, Kaur and Mahmood34) as well as from other laboratories(Reference Qiu, Jansen and Sakaris3, Reference Chiao, Roy and Tolner46), suggesting that acidic pH is a potential driving force of folate transport across the BBM surface. The present results on folate uptake in the intestinal BBMV isolated from the folate-oversupplemented rats showed that acute folate oversupplementation leads to a significant down-regulation in intestinal folate uptake at acidic pH optima. However, during chronic folate oversupplementation, there was no significant change in intestinal folate uptake. This observed decrease in folate uptake during acute folate oversupplementation was associated with a decrease in V max without any significant change in the K m of folate uptake process, indicating that folate oversupplementation decreased folate uptake by decreasing the number or activity of folate transporters without altering the affinity of transporters towards its substrate. Moreover, the K m values obtained were similar to the values observed earlier(Reference Hamid, Kaur and Mahmood34, Reference Hamid, Kiran and Rana35).
In order to evaluate the mechanism of the down-regulation of folate uptake, the expression of the folate transporters RFC and PCFT was of prime importance, as both are suggested to have a role in intestinal folate uptake(Reference Matherly and Goldman8, Reference Matherly, Hou and Deng47). We found that there was no significant change in rRFC and rPCFT mRNA levels in the small intestine between the folate-oversupplemented rats and control rats during acute as well as chronic folate oversupplementation. Therefore, the results deduce that the expression of transporters, i.e. rRFC and rPCFT, are not regulated at the transcriptional level, suggesting a different regulatory point for decreased folate uptake during acute folate oversupplementation. The down-regulation of intestinal folate uptake during acute folate oversupplementation was found to be associated with a parallel decrease in the protein levels of both RFC and PCFT transporters, suggesting the possible involvement of a post-transcriptional or translational regulatory mechanism in regulating intestinal folate uptake. However, during chronic folate oversupplementation, there was no significant change in the protein expression of rRFC and rPCFT. Earlier studies by Ashokkumar et al. (Reference Ashokkumar, Mohammed and Vaziri29) have shown that the carrier-mediated uptake of [3H]folic acid at pH 5·5 by Caco-2 and HK-2 cells was significantly lower when cells were cultured in folate-oversupplemented condition for five generations. This reduction in folic acid uptake was associated with a significant decrease in the mRNA and protein levels of the hRFC and was associated with a decrease in mRNA levels of PCFT. The present results are not in complete agreement with those of Ashokkumar et al. (Reference Ashokkumar, Mohammed and Vaziri29). The differences might be due to the differences in models studied, as they have used an in vitro model for their study in comparison with our in vivo rat model. Recently, a study by Jing et al. (Reference Jing, Tactacan and Rodriguez-Lecompte30) has found that jejunal mRNA levels of RFC were decreased in hens fed with 5-methyltetrahydrofolate (11·30 mg/kg diet for 21 d), but not in hens fed with a folic acid diet (10 mg/kg diet for 21 d). The variations in the amount of folic acid in the diet, duration of treatment and species studied seem to be responsible for the different results achieved in different studies. In the present study, folic acid supplementation increased the serum folate levels, which could be sensed by the folate transport regulatory systems that led to a decrease in the expression of folate transporters.
Conclusions
The results of the present investigation showed that, initially, folate oversupplementation for a short period resulted in a significant down-regulation of intestinal folate uptake, by decreasing the number of transporters without any change in the specificity of folate transporters towards its substrate. The observed down-regulation was associated with a significant decrease in rRFC and rPCFT expressions, suggesting a post-transcriptional or translational regulation of folate uptake during folate oversupplementation. When oversupplementation was continued for a longer period, the intestinal folate transport and the expression of transporters remained unaltered compared with the control. Notably, the serum folate levels remained 2·2-fold higher under these conditions, thus exposing the cells to a high concentration of unmetabolised folic acid. The question whether the presence of unmetabolised folic acid in blood could interfere with various folate-dependent biochemical reactions needs to be addressed in future studies.
Acknowledgements
There is no funding agency supporting this work. S. D. carried out the animal study, transport study, prepared BBMV from animals for gene expression analyses and performed the statistical analysis. N. A. W. carried out RT-PCR, Western blotting and helped to draft the manuscript. J. K. conceived of the study and participated in its design and coordination. All authors declare that there are no potential conflicts of interest. All authors read and approved the final manuscript.









