Se is an essential trace element for animals and humans. Se deficiency is associated with numerous diseases such as Keshan disease and Kashin-Beck disease in humans(1, 2), ‘white muscle disease’ in calves and lambs(Reference Muth, Oldfield and Remmert3) and exudative diathesis in chicks(Reference Patterson, Milstrey and Stokstad4). Supplementation of Se can enhance resistance to oxidative stress(Reference Brenneisen, Steinbrenner and Sies5), reproductive performance(Reference Aréchiga, Ortíz and Hansen6, Reference Kaur and Bansal7) and immune function(Reference McKenzie, Rafferty and Beckett8–Reference Qin, Gao and Huang10), improve the yield and quality of meat in broilers(Reference Choct, Naylor and Reinke11), and protect against certain types of cancers(Reference Schrauzer12–Reference Combs14).
The physiological functions of Se are mediated through various selenoproteins. Se is incorporated into selenoproteins as selenocysteine (Sec). The Sec residue is located at the active centre of the selenoenzymes, and is encoded by theUGA codon. The UGA codon is normally used as a translation termination codon. Incorporation of Sec into selenoproteins involves a read-through of the UGA codon. Se, a Sec incorporation sequence in the mRNA 3′-untranslated region, a Sec-specific transfer RNASec and other translational cofactors are necessary for the read-through of the UGA codon(Reference Stadtman15–Reference Driscoll and Copeland17). The status of Se affects the stability(Reference Baker, Baker and LaRosa18) and translational efficiency of cytoplasmic mRNA of glutathione peroxidase (GPx)(Reference Weiss Sachdev and Sunde19).
The family of GPx plays an important role in the protection of animals and humans against oxidative stress. Five members of the Se-dependent GPx family have been identified and sequenced in mammals(Reference Drevet20). In poultry, the phospholipid hydroperoxide GPx (GPx4, EC 1.11.1.12) gene has been identified and sequenced(Reference Kong, Kim and Foster21). Cellular GPx (GPx1, EC 1.11.1.9) was first identified as a Se-dependent enzyme, and it can detoxify H2O2 and organic peroxides to water and corresponding alcohols using GSH as the hydrogen donor(Reference Arthur22). GPx1 is distributed ubiquitously in various tissues. GPx4 is a membrane-associated GPx which is expressed in various tissues(Reference Drevet20). GPx4 can use phospholipid hydroperoxides as well as H2O2 and other lipid hydroperoxides as substrates(Reference Arthur22). Unlike that in mammalian liver, the percentage of GPx4 activity to total GPx activity in poultry liver is high (28 %)(Reference Miyazaki and Motoi23). Type I deiodinase (D1), belonging to the Se-dependent iodothyronine deiodinase family, is found predominantly in liver, kidneys and thyroid(Reference Köhrle24). D1 can catalyse the production of triiodothyronine from thyroxine by outer-ring deiodination. Triiodothyronine is the most bioactive form of thyroid hormone, which regulates basal metabolism, differentiation and heat production. About 80 % of peripheral triiodothyronine is produced by the catalysis of D1.
In rats, the mRNA levels and activities of GPx and D1 are highly correlated with the Se status(Reference Bermano, Nicol and Dyer25). Previous studies have shown that Se deficiency in the diet leads to significant decreases in the GPx3 activity and triiodothyronine concentration in plasma in chickens, and to a significant increase in thyroxine concentration in plasma(Reference Chang, Combs and Scanes26, Reference Zuberbuehler, Messikommer and Arnold27). Severe deficiency of Se in chickens resulted in depressions in the rate of growth and efficiency of feed utilisation(Reference Root and Combs28). Contrastingly, supplementation of Se in the diet increased the GPx activity in the plasma and tissues of turkeys, and 0·3 mg Se/kg diet was required for maximal GPx activity in the plasma and liver(Reference Fischer, Bosse and Most29).
Supplementation of Se is a common practice in chicken production in China. Presently, addition of sodium selenite (Na2SeO3) to the chicken diets is a prevalent method. Some researchers have suggested that the bioavailability of organic forms of Se (e.g. Se-enriched yeast, which contains selenomethionine (Se-Met)) is higher than that of the inorganic forms of Se such as Na2SeO3(Reference Rayman30–Reference Leeson, Namkung and Caston32). However, there are only a few studies explaining how the mechanism of organic Se is better than that of inorganic Se in the supplementation of Se in the chicken diet. It is known that the chicken liver is the principal organ involved in the storage and metabolism of Se, and that it can synthesise GPx1, GPx4 and D1. However, little is known about the regulation of the activity and expression of selenoenzymes in the chicken liver, which contribute to the mechanism of action of organic Se in chickens. The purposes of the present study were to evaluate the effects of different forms and concentrations of Se on the regulation of GPx activity and GPx4 and D1 mRNA levels in primary cultured chicken hepatocytes, and to determine the optimal doses of different forms of Se for maximal GPx activity and maximal GPx4 and D1 mRNA levels in vitro.
Materials and methods
The experimental use of animals and procedures followed were approved by the Nanjing Agricultural University Animal Care Committee.
Isolation and culture of hepatocytes
Male White Leghorn chickens (aged 30–40 d) were used to obtain hepatocytes. Isolation of hepatocytes was performed using the collagenase perfusion method as described previously(Reference Fujii, Yoshino and Suzuki33), with some modifications. Isolated cells were dispersed in 60 ml of serum-free L-15 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 0·2 % (w/v) bovine serum albumin and antibiotics (100 IU/ml penicillin, 100 μg/ml streptomycin and 0·25 μg/ml amphotericin B) at 4°C. Purification of hepatocytes was performed according to the method described by Wu et al. (Reference Wu, Huang and Wei34). The viability of the isolated hepatocytes was 92·3 (sd 1·4) % (n 3), which was determined by the 0·4 % trypan blue dye exclusion method, and the cell yield from each liver preparation was 4·1 (sd 0·82) × 108 hepatocytes (n 3). Evaluation of the cells done using light microscopy indicated that about 95 % of the collected cells were hepatocytes.
Culturing of hepatocytes was performed according to the method described by Fujii et al. (Reference Fujii, Yoshino and Suzuki33), with some modifications. Hepatocytes were seeded into six-well plates at a density of 2 × 106 viable cells per well in 2 ml of L-15 medium (supplemented with 5 % fetal bovine serum, 33 mm-HEPES, 2 mm-l-glutamine, 0·2 % (w/v) bovine serum albumin, 100 nm-dexamethasone, 5 mg/l transferrin, 1 μmol/l insulin and antibiotics, pH 7·65). Cells were cultured at 37°C in a humidified atmosphere. After a plating period of 4 h, cell monolayers were washed twice with Hanks' balanced salt solution, and 3 ml of fresh serum-free L-15 medium were added to each well. Thereafter, the medium was substituted with serum-free L-15 medium every 24 h.
Cell treatments
At 48 h after seeding, the culture medium was removed, and hepatocyte monolayers were washed three times with Hanks' balanced salt solution. Then, hepatocytes were grown in 3 ml of fresh serum-free L-15 medium, and were treated with 0 (control), 0·5, 1, 1·5, 2, 3, 4 or 5 μmol/l of Se supplied as dl-Se-Met (Sigma, St Louis, MO, USA), κ-selenocarrageenan (Se-Car, The First Institute of Oceanography, Qingdao, China) or Na2SeO3 (Sigma). Following incubation for 24 h, the culture medium was collected into 2 ml Eppendorf tubes for assay of lactic dehydrogenase (LDH), and the cells were harvested for the analysis of enzyme activity and mRNA. Each treatment was done in six wells (three wells were used for the measurement of GPx activity, and the other wells were used for mRNA analysis) in three separate experiments.
Preparation of hepatocyte lysates
After 24 h of incubation with different forms and concentrations of Se, hepatocyte monolayers were washed three times with ice-cold Ca2+/Mg2+-free PBS, and were harvested by scraping into 1 ml of cold Tris buffer (20 mm-Tris–HCl, pH 7·5, 2 mm-EDTA and 0·1 % peroxide-free Triton X-100). Hepatocyte lysates were then prepared by ultrasonication for 30 s in ice-cold water, and centrifuged at 12 000 g for 15 min at 4°C. The supernatants were aliquoted and stored at − 20°C for the subsequent analysis of GPx activity.
Analytical methods
Measurement of protein concentration of hepatocyte lysates
Total protein concentration was measured using a Bradford Protein Assay Kit (Beyotime Institute of Biotechnology, Jiangsu, China). The results obtained for GPx activity were corrected for total protein concentration.
Measurement of lactic dehydrogenase activity in the incubation medium
Hepatocyte toxicity was analysed by measuring the LDH activity in the incubation medium. The culture medium was collected into 2 ml Eppendorf tubes at the end of the incubation, and was centrifuged at 12 000 g for 15 min at 4°C. The supernatants were stored at − 20°C until analysis (within 3 d). The measurement of LDH activity in the incubation medium was performed as described previously(Reference Dringen, Kussmaul and Hamprecht35). One unit of enzyme activity was defined as 1 μmol of reduced nicotinamide adenine dinucleotide oxidised per min. The LDH activity in the culture medium was expressed as units per litre. All the samples were measured in duplicate.
Measurement of glutathione peroxidase activity in hepatocyte cytosol
The GPx activity in hepatocyte cytosol was measured according to the method described by Lei et al. (Reference Lei, Evenson and Thompson36), using tert-butyl hydroperoxide as the peroxide substrate. Briefly, 50 μl of hepatocyte lysate were transferred into a 3 ml quartz cuvette containing 1900 μl of the reaction mixture (50 mm-Tris − HCl, pH 7·5, 2 mm-EDTA, 0·1 mm-NADPH, 2 mm-GSH, 1 mm-NaN3 and 0·9 IU of glutathione reductase (Sigma)). The reaction mixture was preincubated for 3 min at 25°C, and the reaction was initiated by adding 50 μl of tert-butyl hydroperoxide (8 mm). The rate of oxidation of NADPH was monitored using a spectrophotometer at 340 nm for 5 min at 25°C. The non-enzymatic reaction rate was determined by substituting water (serving as the blank) with the hepatocyte lysate, and by recording the decrease in NADPH absorbance. One unit of enzyme activity was defined as 1 μmol of NADPH oxidised per min under these conditions. The GPx activity in hepatocyte cytosol was expressed as mU/mg of total cell protein. All the samples were measured in duplicate.
Measurement of phospholipid hydroperoxide glutathione peroxidase and type I deiodinase mRNA levels by quantitative RT-PCR
After 24 h of incubation with different forms and concentrations of Se, the medium was removed, and the cell monolayers were washed three times with ice-cold Ca2+/Mg2+-free PBS. Total RNA was isolated from the hepatocyte monolayers using the TRIzol reagent (Invitrogen) according to the manufacturer's protocol. The dried RNA pellets were resuspended in 40 μl of diethyl pyrocarbonate-treated water. The concentration and purity of the total RNA were determined spectrophotometrically at 260/280 nm. The total RNA was immediately used or stored at − 70°C before complementary DNA synthesis.
First-strand complementary DNA was synthesised from 2 μg of total RNA using Oligo-dT primers and SuperScript II RT (Invitrogen) according to the manufacturer's instructions. Synthesised complementary DNA was diluted ten times with sterile water, and stored at − 20°C before use.
Primer Premier software (PREMIER Biosoft International, Palo Alto, CA, USA) was used to design specific primers for β-actin, GPx4 and D1 based on known chicken sequences (Table 1). Quantitative real-time PCR were performed on an ABI PRISM 7300 Detection System (Applied Biosystems, Foster City, CA, USA) according to the method described by Wu et al. (Reference Wu, Huang and Wei34), with some modifications. The PCR procedure for β-actin, GPx4 and D1 consisted of a step performed at 95°C for 2 min followed by forty cycles at 95°C for 15 s, 58°C for 15 s and 72°C for 30 s. The melting curve analysis showed only one peak for each PCR product. Electrophoresis was performed with the PCR products to verify primer specificity and purity of the products. The calculation of the number of copies of each sample was performed from the respective standard curve using the 7300 system software. The ratio of mRNA levels of GPx4 and D1 to that of β-actin internal control was used for statistical comparison of the different treatments.
Table 1 Primers used for quantitative real-time PCR

GPx4, phospholipid hydroperoxide glutathione peroxidase; D1, type I deiodinase.
Statistical analysis
Statistical analysis of the LDH and GPx activities and GPx4 and D1 mRNA levels was performed using the SPSS 11.5 for Windows statistical software package (SPSS, Inc., Cary, NC, USA). When a significant value (P < 0·05) was obtained using one-way ANOVA, further analysis was done. All the data showed a normal distribution and passed equal variance testing. Differences between means were assessed by Tukey's honestly significant difference test of post hoc multiple comparisons. Data were expressed as means and standard deviations. Differences were considered as significant at P < 0·05.
Results
Effect of selenium supplementation on lactic dehydrogenase release
The effects of different forms and concentrations of Se on the release of LDH into the culture medium are shown in Fig. 1. Compared with the control, Se-Met at doses of 1–3 μmol/l (P < 0·05) significantly lowered LDH release in the hepatocytes after incubation for 24 h, but at doses of 0·5, 4 or 5 μmol/l (P>0·05), it did not lower the LDH release. In the hepatocytes incubated with Se-Car, significantly higher LDH release was observed at doses of 3, 4 or 5 μmol/l (P < 0·05, v. control). The release of LDH was significantly increased in the hepatocytes treated with 2, 3, 4 or 5 μmol/l of Na2SeO3 (P < 0·05, v. control). The response occurred in a dose-dependent manner. The release of LDH induced by Na2SeO3 at doses of 2, 3, 4 or 5 μmol/l was significantly higher than that induced by Se-Met and Se-Car at equivalent doses, respectively. Furthermore, the release of LDH induced by Se-Car at doses of 3, 4 or 5 μmol/l was significantly higher than that induced by Se-Met at equivalent doses. Cell detachments were not observed during light microscopy in the hepatocytes incubated with Na2SeO3 at a dose of 5 μmol/l.
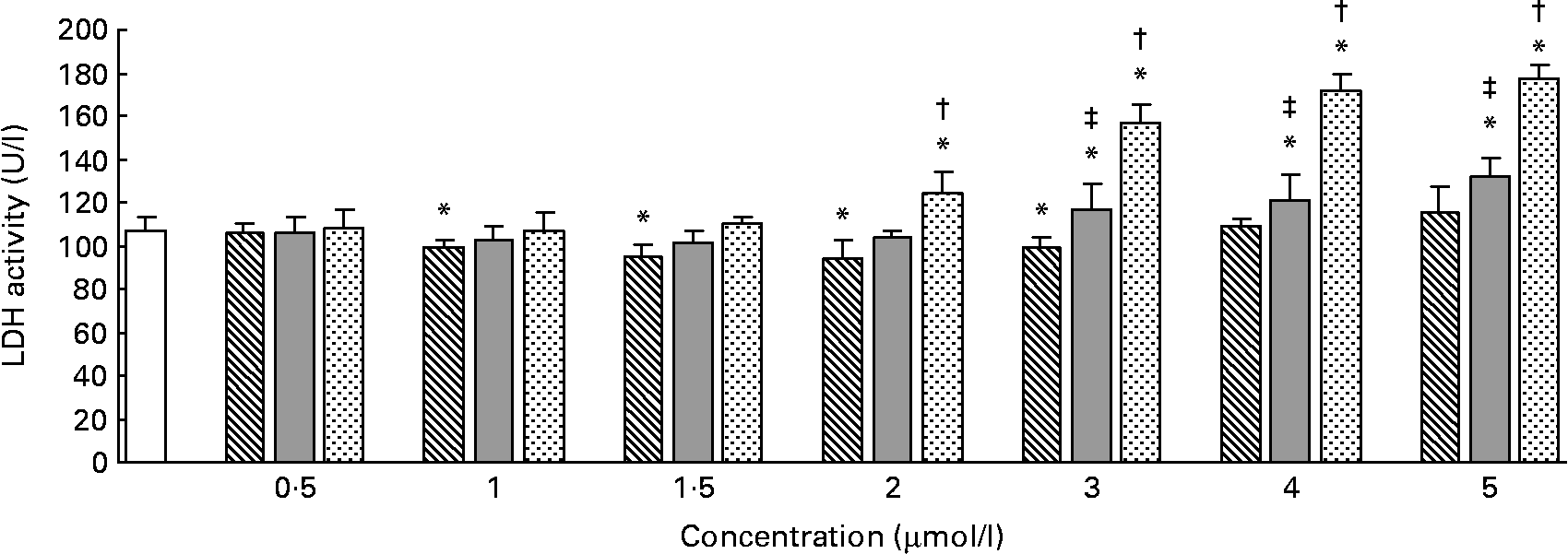
Fig. 1 Effects of different forms and concentrations of Se on lactic dehydrogenase (LDH) release from hepatocytes. The primary cultured chicken hepatocyte monolayers were incubated with 0 (control), 0·5, 1, 1·5, 2, 3, 4 or 5 μmol/l of Se supplied as dl-selenomethionine (Se-Met), κ-selenocarrageenan (Se-Car) or sodium selenite (Na2SeO3) for 24 h. The LDH activity in the culture medium was used to evaluate the integrity of cell membrane. Bars represent means and standard deviations of triplicate culture media. * Mean values were significantly different from the control assessed by one-way ANOVA and then by Tukey's multiple comparison test (P < 0·05). † Mean values were significantly different from Se-Met groups and Se-Car groups at equivalent doses (P < 0·05). ‡ Mean values were significantly different from Se-Met groups at equivalent doses (P < 0·05). □, Control; ▧, Se-Met; ![]() , Se-Car;
, Se-Car; ![]() , Na2SeO3.
, Na2SeO3.
Effect of selenium supplementation on glutathione peroxidase activity
The effects of different forms and concentrations of Se on the GPx activity are shown in Fig. 2. Compared with the control, Se-Met or Se-Car at doses of 0·5–5 μmol/l and Na2SeO3 at doses of 0·5–4 μmol/l significantly increased the GPx activity in the hepatocytes, but Na2SeO3 at a dose of 5 μmol/l did not increase the GPx activity (P < 0·05). In the hepatocytes treated with Se-Met or Se-Car, a significant dose-dependent increase in GPx activity was observed (v. control), up to a maximum at a dose of 2 μmol/l. For Na2SeO3 supplementation, 1·5 μmol/l was found to be the most effective dose. After resulting in a maximal GPx activity, higher Se supplementation led to a dose-dependent reduction in GPx activity in all the groups treated with Se. The increases in GPx activity induced by Se-Met at doses of 0·5, 1, 2, 3 or 5 μmol/l were significantly higher than those induced by Se-Car and Na2SeO3 at equivalent doses (P < 0·05), respectively. At 1·5 μmol/l of added Se, Na2SeO3 had a relatively greater effect on GPx activity than Se-Car (P < 0·05), but the effect was not greater than that of Se-Met. However, Se-Car at doses of 2, 3, 4 or 5 μmol/l had a relatively greater effect on GPx activity than Na2SeO3 at equivalent doses (P < 0·05). Moreover, 2 μmol/l of Se from Se-Met resulted in the highest GPx activity in all the groups treated with Se.
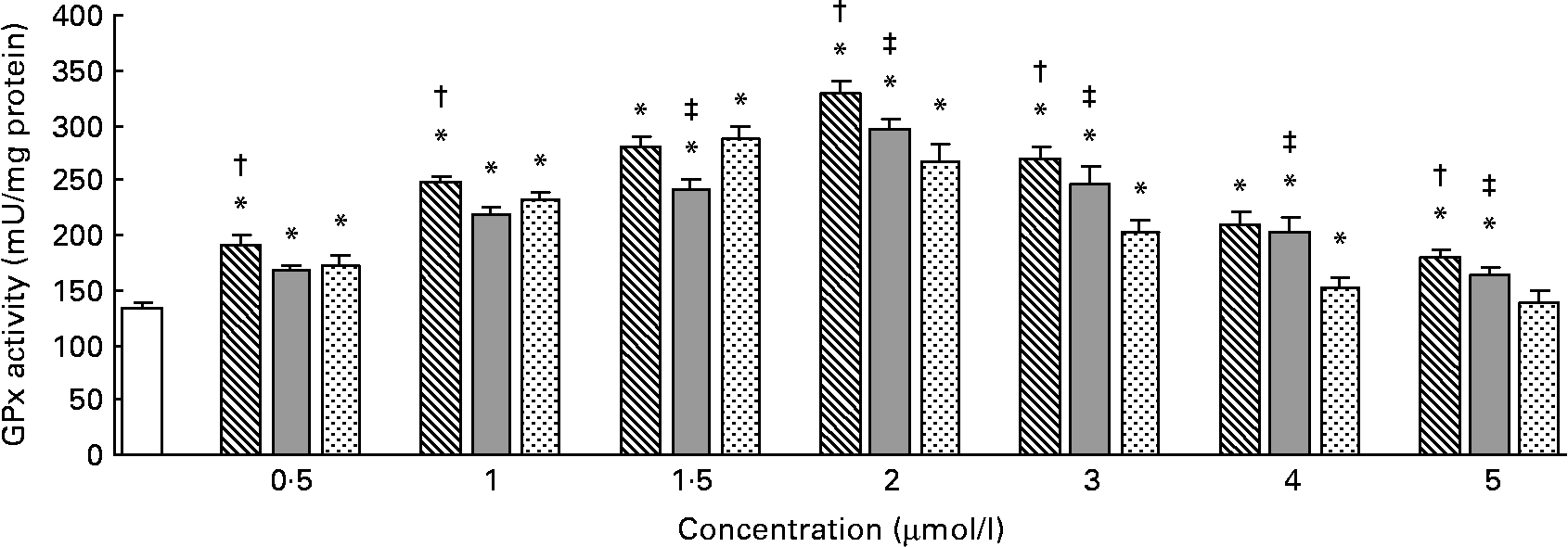
Fig. 2 Effects of different forms and concentrations of Se on glutathione peroxidase (GPx) activity in chicken hepatocytes. The primary cultured chicken hepatocyte monolayers were treated with 0 (control), 0·5, 1, 1·5, 2, 3, 4 or 5 μmol/l of Se supplied as dl-selenomethionine (Se-Met), κ-selenocarrageenan (Se-Car) or sodium selenite (Na2SeO3) for 24 h. The GPx activity in hepatocyte cytosol was measured using a spectrophotometric method, and expressed as mU/mg protein. Bars represent means and standard deviations of triplicate cultures. * Mean values were significantly different from the control assessed by one-way ANOVA and then by Tukey's multiple comparison test (P < 0·05). † Mean values were significantly different from Se-Car groups and Na2SeO3 groups at equivalent doses (P < 0·05). ‡ Mean values were significantly different from Na2SeO3 groups at equivalent doses (P < 0·05). □, Control; ▧, Se-Met; ![]() , Se-Car;
, Se-Car; ![]() , Na2SeO3.
, Na2SeO3.
Effect of selenium supplementation on phospholipid hydroperoxide glutathione peroxidase mRNA levels
The GPx4 mRNA levels measured by quantitative RT-PCR are shown in Fig. 3. Compared with the control, Se-Met, Se-Car or Na2SeO3 significantly decreased GPx4 mRNA levels in a dose-dependent manner in all the groups (P < 0·05). At 0·5–1·5 μmol/l of added Se, the GPx4 mRNA levels were not significantly different between the groups treated with Se-Met, Se-Car or Na2SeO3 (P>0·05). The GPx4 mRNA levels in the hepatocytes treated with Se-Met at doses of 2–5 μmol/l were higher than those in the hepatocytes treated with Se-Car or Na2SeO3 at equivalent doses.
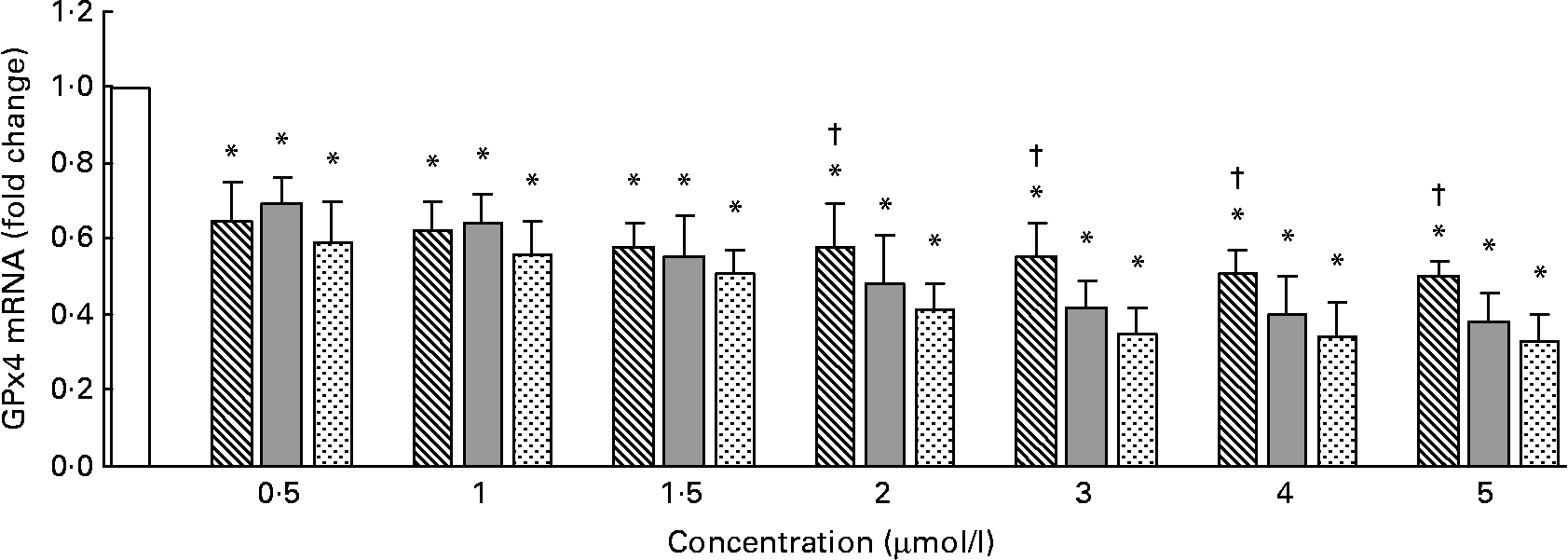
Fig. 3 Effects of different forms and concentrations of Se on phospholipid hydroperoxide glutathione peroxidase (GPx4) mRNA of chicken hepatocytes. The primary cultured chicken hepatocyte monolayers were treated with 0 (control), 0·5, 1, 1·5, 2, 3, 4 or 5 μmol/l of Se supplied as dl-selenomethionine (Se-Met), κ-selenocarrageenan (Se-Car) or sodium selenite (Na2SeO3) for 24 h. GPx4 mRNA of chicken hepatocytes was measured by quantitative real-time RT-PCR, and the ratio of mRNA level of GPx4 to that of β-actin internal control was used for statistical comparison. Bars represent means and standard deviations of triplicate cultures. * Mean values were significantly different from the control assessed by one-way ANOVA and then by Tukey's multiple comparison test (P < 0·05). † Mean values were significantly different from Se-Car groups and Na2SeO3 groups at equivalent doses (P < 0·05). □, Control; ▧, Se-Met; ![]() , Se-Car;
, Se-Car; ![]() , Na2SeO3.
, Na2SeO3.
Effect of selenium supplementation on type I deiodinase mRNA levels
The D1 mRNA levels measured by quantitative RT-PCR are shown in Fig. 4. Compared with the control, Se-Met, Se-Car or Na2SeO3 significantly increased the D1 mRNA levels in all the groups (P < 0·05). The maximal D1 mRNA levels were observed in the hepatocytes treated with 1·5 μmol/l of Se-Met and 0·5 μmol/l of Se-Car or Na2SeO3 (v. control). After resulting in a maximal D1 mRNA level, higher Se supplementation led to a dose-dependent reduction in D1 mRNA levels in all the groups treated with Se. The degree of reduction in D1 mRNA levels was relatively small in the groups treated with Se-Met than in the groups treated with Se-Car or Na2SeO3. At 0·5 μmol/l of added Se, Se-Car and Na2SeO3 had a relatively greater effect on D1 mRNA than Se-Met (P < 0·05). There were no differences between the hepatocytes treated with Se-Met, Se-Car and Na2SeO3 at a dose of 1 μmol/l. At 1·5–5 μmol/l of added Se, however, Se-Met had a relatively greater effect on D1 mRNA than Se-Car and Na2SeO3 (P < 0·05). Moreover, Se-Car at doses of 4 or 5 μmol/l had a relatively greater effect on D1 mRNA than Na2SeO3 at equivalent doses (P < 0·05).
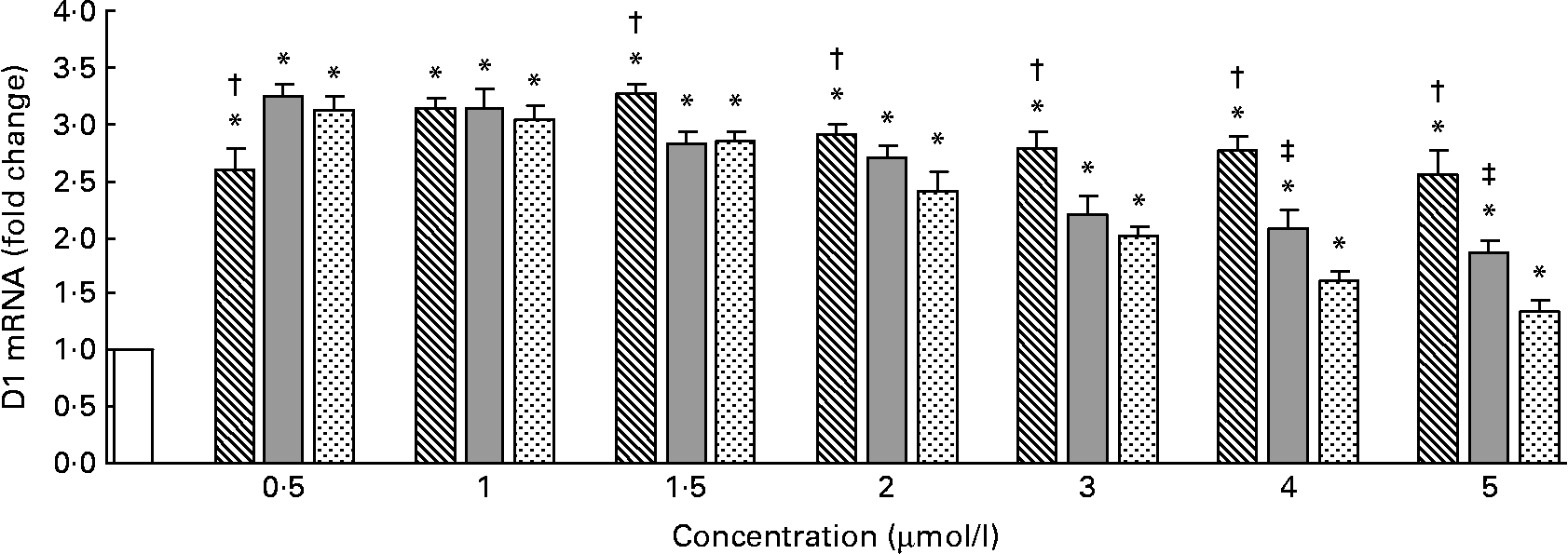
Fig. 4 Effects of different forms and concentrations of Se on type I deiodinase (D1) mRNA of chicken hepatocytes. The primary cultured chicken hepatocyte monolayers were treated with 0 (control), 0·5, 1, 1·5, 2, 3, 4 or 5 μmol/l of Se supplied as dl-selenomethionine (Se-Met), κ-selenocarrageenan (Se-Car) or sodium selenite (Na2SeO3) for 24 h. D1 mRNA of chicken hepatocytes was measured by quantitative real-time RT-PCR, and the ratio of mRNA level of D1 to that of β-actin internal control was used for statistical comparison. Bars represent means and standard deviations of triplicate cultures. * Mean values were significantly different from the control assessed by one-way ANOVA and then by Tukey's multiple comparison test (P < 0·05). † Mean values were significantly different from Se-Car groups and Na2SeO3 groups at equivalent doses (P < 0·05). ‡ Mean values were significantly different from Na2SeO3 groups at equivalent doses (P < 0·05). □, Control; ▧, Se-Met; ![]() , Se-Car;
, Se-Car; ![]() , Na2SeO3.
, Na2SeO3.
Discussion
The population doubling time of the chicken hepatocytes during the logarithmic phase of growth was 46 h when they were cultured in the serum-free L-15 medium (data not shown).
The compounds of Se are generally classified into inorganic and organic forms. The primary metabolic pathway of Se in animals and cells is the reduction of the elemental form followed by methylation, producing methylselenol, dimethylselenide and trimethylselenonium cation(Reference B'Hymer and Caruso37, Reference Zeng and Combs38), which are excreted through lungs or kidneys. Selenide plays an intermediary role in the process of Se metabolism, and is either used for selenoprotein synthesis or used for methylation(Reference Zeng and Combs38). There are some differences in the metabolism of inorganic and organic forms of Se. Inorganic forms of Se (e.g. Na2SeO3) are reduced to the selenide state using reducing equivalents from GSH and NADPH. Organic forms of Se (e.g. Se-Met) release Se in the selenide state as a result of catabolism. Moreover, Se-Met can be incorporated non-specifically into proteins as a substitute for sulphur-containing methionine. Greater tissue retention of organic Se than of inorganic Se from Na2SeO3 from Se-enriched yeast was observed in broilers(Reference Leeson, Namkung and Caston32, Reference Payne and Southern39) and swines(Reference Kim and Mahan40). The distribution of Se in different organs of laying hens fed Se-supplemented diets varied, and the liver was found to be the principal organ involved in the storage of Se(Reference Pan, Huang and Zhao41). The results of the present study showed that the regulations of the GPx activity and GPx4 and D1 mRNA levels by different forms of Se varied in primary cultured chicken hepatocytes. This may, in part, be caused by the metabolic differences of these forms of Se in the cultured cells.
Previous studies have suggested that organic forms of Se (e.g. Se-Met) are less toxic than inorganic forms of Se such as Na2SeO3(Reference Mihajlović42, Reference Tiwary, Stegelmeier and Panter43). LDH is a stable cytosolic enzyme, which becomes extracellular when the cell membrane is damaged. Therefore, the hepatocyte toxicity of the different forms of Se used in the present study can be analysed by measuring the LDH activity in the culture supernatant. The results of the present study are similar to those of our previous report(Reference Wu, Huang and Wei34), and suggest that Se-Met is less toxic than Na2SeO3 and Se-Car, and that Se-Car is slightly less toxic than Na2SeO3. Earlier research has shown that incubation of isolated rat hepatocytes for 2 h with Se supplied as Na2SeO3 at concentrations higher than 6·3 μmol/l led to a decrease in cell viability compared with the controls(Reference Bell, Nonavinakere and Soliman44). Cytotoxicity was not observed in the primary cultured rabbit hepatocytes treated with Na2SeO3 at a dose of 100 ng/ml (about 1·27 μmol/l)(Reference Müller and Pallauf45). The results of the present study were similar to these findings.
Recent research has shown that supplementation with Se supplied as Na2SeO3 led to a significant increase in GPx activity in growing male turkey livers, and that 0·3 mg Se/kg diet was required for the maximal GPx activity in the liver(Reference Fischer, Bosse and Most29). Lei et al. (Reference Lei, Dann and Ross46) also reported that Se supplementation at a dose of 0·2 mg/kg diet was required to support the full expression of GPx1 in pig livers. The results of the study done by Salman et al. (Reference Salman, Muğlali and Selçuk47) indicated that the supplementation of organic Se to the diets containing adequate Se increased GPx activity in broiler livers and other tissues. In the present study, significant increases in GPx activity were observed in all the hepatocytes treated with Se except in the hepatocytes treated with 5 μmol/l of Na2SeO3. These results are similar to those of our previous research(Reference Wu, Huang and Wei34). After resulting in a maximal effect, higher Se supplementation led to a dose-dependent decreasing trend of GPx activity in all the hepatocytes treated with Se. We believe that the decreases in GPx activity are related to the excess supplementation and cytotoxicity of Se. Toxicity associated with Se leads to the inhibition of cell growth(Reference Spyrou, Björnstedt and Skog48), DNA fragmentation(Reference Garberg, Ståhl and Warholm49) and reduction in protein synthesis(Reference Uezono, Toyohira and Yanagihara50). Ebert et al. (Reference Ebert, Ulmer and Zeck51) reported that GPx1 activity was enhanced by 1·8-fold in the bone marrow stromal cells cultured in the presence of 100 nm Se supplied as Na2SeO3. Supplementation of Se as Na2SeO3 (1 μmol/l) led to significant increases in GPx1 activity in mouse hepatoma cells (about 2-fold), and then GPx1 activity exhibited a decreasing trend at a dose of 2 μmol/l(Reference Keck and Finley52). In the present study, the increases in GPx activity induced by Se-Met at doses of 0·5, 1, 2, 3 or 5 μmol/l were significantly higher than those induced by Se-Car and Na2SeO3 at equivalent doses (P < 0·05). Se-Car at doses of 2, 3, 4 or 5 μmol/l had a relatively greater effect on GPx activity than Na2SeO3 at equivalent doses (P < 0·05). These results are similar to those of our previous report(Reference Wu, Huang and Wei34), and suggest that different forms and concentrations of Se have varied effects on the regulation of the GPx activity in primary cultured chicken hepatocytes.
Sneddon et al. (Reference Sneddon, Wu and Farquharson53) reported that Se supplied as Na2SeO3 at a dose of 0·114 μmol/l led to an optimal GPx4 mRNA level in human umbilical vein endothelial cells. In HPL1D cells from human lung peripheral epithelium, a maximal GPx4 mRNA level was observed at 0·1 μmol/l of added Se supplied as Na2SeO3(Reference Romanowska, Kikawa and Fields54). Because the mRNA sequences of GPx1 gene of poultry are not available in the GenBank and the primers of GPx1 cannot be designed, GPx1 mRNA levels in the cultured cells cannot be measured by quantitative RT-PCR. In the present study, significant decreases in GPx4 mRNA levels were observed in all the hepatocytes treated with Se-Met, Se-Car or Na2SeO3 compared with those treated with the control (P < 0·05). These results suggest that the concentration of Se required for the maximal expression of GPx4 mRNA in the primary cultured chicken hepatocytes is relatively low, despite the different forms of Se (speculated < 0·5 μmol/l). Using in vivo experiments, it was found that Se requirement needed to reach the plateau level of GPx4 mRNA in rat livers was about at 0·033 mg/kg diet(Reference Lei, Evenson and Thompson36). In addition, some researchers believed that Se deficiency had no effect on the expression of GPx4 mRNA in rat livers(Reference Weiss Sachdev and Sunde19, Reference Bermano, Nicol and Dyer25, Reference Sunde, Evenson and Thompson55).
Supplementation of Se as Na2SeO3 (0·1 mg/kg diet) led to significant increases in the mRNA level and activity of D1 in rat livers, but Se supplementation above a nutritionally adequate level (2 mg/kg diet) reduced the enzyme activity(Reference Christensen, Cammack and Wray56). The results of the study done by Bermano et al. (Reference Bermano, Nicol and Dyer25) showed that mRNA level and activity of D1 in rat livers required 0·104 mg Se/kg diet to reach plateau levels. However, Sunde et al. (Reference Sunde, Evenson and Thompson55) reported that dietary Se requirement needed to reach the plateau levels of D1 mRNA in the livers of pregnant and lactating rats was less than 0·01 mg/kg diet. In the present study, the maximal D1 mRNA levels were observed in the primary cultured chicken hepatocytes treated with 1·5 μmol/l of Se-Met (about 3·26-fold v. control) and 0·5 μmol/l of Se-Car (about 3·24-fold v. control) or Na2SeO3 (about 3·12-fold v. control). These results suggest that concentrations of different forms of Se required for a maximal D1 mRNA level vary in chicken hepatocytes. After resulting in a maximal D1 mRNA level, higher Se supplementation led to a dose-dependent reduction in D1 mRNA levels in all the groups treated with Se, but the degree of reduction in D1 mRNA levels was different between the groups treated with these three forms of Se. As mentioned earlier, the reduction in D1 mRNA levels may be related to excess supplementation and cytotoxicity of Se. At 0·5 μmol/l of added Se, Se-Car and Na2SeO3 had a relatively greater effect on D1 mRNA than Se-Met (P < 0·05), while Se-Met at doses of 1·5–5 μmol/l had a relatively greater effect on D1 mRNA than Se-Car and Na2SeO3 at equivalent doses (P < 0·05). These results suggest that regulations of D1 mRNA levels by different forms and concentrations of Se differ in primary cultured chicken hepatocytes.
In summary, these results demonstrate that supplementation of Se in the culture medium can increase GPx activity and D1 mRNA level in primary cultured chicken hepatocytes, while excess supplementation of Se causes cytotoxicity and negative effects. The regulations of GPx activity and D1 mRNA by different forms and concentrations of Se vary in chicken hepatocytes. The concentration of Se required for the maximal expression of D1 mRNA in chicken hepatocytes is higher than that of Se required for the maximal expression of GPx4 mRNA.
Acknowledgements
The final manuscript was read and approved by all the authors, and the authors declare no conflicts of interest. The study was sponsored by the National Natural Science Foundation of China (grant nos 30671547 and 30871892). The contribution of the authors were as follows: X. W. isolated and cultured the chicken hepatocytes, measured the mRNA levels and wrote the paper. C. W. isolated and cultured the chicken hepatocytes, and measured the enzyme activity. C. P. measured the LDH activity. Y. D. measured the protein concentration. K. H. was involved in technical direction, statistical analysis and writing of the paper.







