The potential health-promoting effects of cocoa products have gained extensive attention in the last few years. Most of these effects are attributed to the polyphenolic fraction of cocoa, especially to the flavonoid group of polyphenols, which in cocoa are mainly flavanols. In fact, cocoa has been defined as a functional food due to its high flavanol content( Reference Selmi, Cocchi and Lanfredini 1 ). Moreover, soluble cocoa products are also a source of dietary fibre (DF). Cocoa powder contains 1·75 % of DF, whereas in chocolate production DF is largely discarded( Reference Jenkins, Kendall and Vuksan 2 ). Nowadays, new soluble cocoa products enriched with dietary components such as DF and polyphenols are being introduced into the food market, and their beneficial health properties need to be assessed.
The cardio-protective properties of cocoa products in humans have been mainly attributed to their polyphenol content. Cocoa flavanols may decrease LDL oxidation, improve plasma antioxidant status, increase the levels of HDL-cholesterol (HDL-C), decrease the levels of biomarkers of lipid oxidation and improve endothelial function by increasing vascular NO synthase activity; the effect of NO synthase has been observed in studies using endothelial cells and in isolated rabbit aortic rings( Reference Rimbach, Melchin and Moehring 3 ). Cocoa flavanols have been the subject of health claims: maintenance of normal blood pressure and protection of lipids from oxidative damage( 4 ). The European Food Safety Authority has recently( 5 ) issued a positive opinion on cocoa flavanols and maintenance of flow-mediated vasodilation, which evaluates the capacity of conduit artery, typically the brachial artery, to respond to an increase in blood flow by dilating, as consistent acute and chronic benefits of chocolate or cocoa on flow-mediated vasodilation have been described regardless of the dose consumed( Reference Davison, Coates and Buckley 6 , Reference Hooper, Kay and Abdelhamaid 7 ). Ried et al. ( 8 ) concluded in their review that flavanol-rich chocolate and cocoa products may have a small but statistically significant effect in lowering blood pressure by 2–3 mmHg in the short term. However, in 2010, the European Food Safety Authority( 4 ) Panel concluded that a cause-and-effect relationship could not be established between the consumption of cocoa flavanols and protection of lipids from oxidative damage based on the review by Ding et al. ( Reference Ding, Hutfless and Ding 9 ). In the review, two long-term studies have reported no effects on F2-isoprostanes( Reference Mursu, Voutilainen and Nurmi 10 , Reference Mathur, Devaraj and Grundy 11 ) and another has reported that plasma oxidised LDL concentrations were decreased in the low-, middle- and high-cocoa groups compared with baseline; however, changes between the intervention groups and the placebo group were not assessed( Reference Baba, Natsume and Yasuda 12 ). Therefore, the antioxidant effects of regularly consuming cocoa products need further clarification. Recently, it has been shown that polyphenols contained in the water-insoluble cocoa fraction exhibit antioxidant actions in the gastrointestinal tract despite being bound to macromolecules using an in vitro digestion model( Reference Fogliano, Corollaro and Vitaglione 13 ). In acute studies, it has been shown that the consumption of a single dose of dark chocolate (40 g of 74 % cocoa) markedly improves endothelium-mediated flow-mediated vasodilation and plasma antioxidant status in young healthy smokers( Reference Hermann, Spieker and Ruschitzka 14 ) and that the intake of flavonoid-rich dark chocolate (40 g) compared with that of cocoa-free control chocolate reduces plasma isoprostane levels in cardiac transplant recipients( Reference Flammer, Hermann and Sudano 15 ).
Furthermore, the natural content of cocoa in DF( Reference Lecumberri, Mateos and Izquierdo-Pulido 16 ) may contribute to the beneficial cardiovascular effects, as it has been proposed that a protective effect of DF against CVD is mediated through direct or indirect effects on serum lipids, possibly through the up-regulation of HDL-C levels( Reference Wu, Dwyer and Fan 17 , Reference Tillotson, Grandits and Bartsch 18 ). Although the health benefits of DF have been mainly attributed to soluble dietary fibre (SDF), insoluble dietary fibre (IDF) through the dilution of gastrointestinal contents may hinder the digestion and absorption of dietary fats( Reference Lecumberri, Goya and Mateos 19 ). In fact, our group observed that a cocoa product rich in IDF induced slight hypocholesterolaemic and marked hypotriacylglycerolaemic effects, also reducing malondialdehyde (MDA) levels in serum and in the liver of hyperlipidaemic rats without inducing changes in serum total antioxidant capacity( Reference Lecumberri, Goya and Mateos 19 ). When the same fibre-rich cocoa product was regularly consumed as part of a non-restrictive, Mediterranean-Spanish diet, blood pressure and serum glucose and MDA levels were decreased in moderately hypercholesterolaemic subjects, without affecting body weight( Reference Sarriá, Mateos and Sierra-Cinos 20 ). Taking these beneficial cardiovascular results into account, a new cocoa product rich in insoluble fibre has been developed and the effects of its regular consumption on cardiovascular risk factors have been assessed in human subjects.
The influence of cocoa on inflammatory parameters seems to depend on the amount of cocoa consumed and the health status of the consumer. Regular consumption of one serving of dark chocolate for 3 d significantly decreases the low-grade inflammation biomarker C-reactive protein (CRP) levels, whereas with higher intakes this effect is not maintained( Reference Di Giuseppe, Castelnuovo and Centritto 21 ). Hypercholesterolaemic postmenopausal women who consumed a high-flavanol cocoa beverage for 6 weeks had significantly lower levels of soluble vascular cell adhesion molecule 1 (VCAM-1) than those who consumed a low-flavanol cocoa beverage( Reference Wang-Polagruto, Villablanca and Polagruto 22 ). In contrast, the consumption of moderate amounts of cocoa-related products induced platelet-inhibiting effects, although the levels of inflammation biomarkers did not change( Reference Ostertag, O'Kennedy and Kroon 23 ). DF has also been reported to exert anti-inflammatory effects. In postmenopausal women, a greater intake of total, soluble and insoluble DF was inversely associated with IL-6 and TNF-α receptor 2 (TNF-α-R2) levels in plasma, but not with CRP levels( Reference Ma, Hebert and Li 24 ). In contrast, in women with type 2 diabetes, the highest intake of whole grains was associated with both lower CRP and TNF-α levels compared with the lowest intake of whole grains( Reference Qi, van Dam and Liu 25 ).
In conclusion, intermediate and long, well-controlled, human studies that evaluate the effects of realistic, moderate consumption of cocoa products on cardiovascular health are necessary. Bearing this in mind, we conducted a study in healthy and hypercholesterolaemic subjects to examine the influence of regularly consuming for 4 weeks a commercially available cocoa product rich in fibre in milk v. consuming only milk (control) on a broad range of cardiovascular biomarkers: serum lipid and lipoprotein levels; serum oxidation and antioxidant biomarker levels; plasma inflammation biomarker levels; blood pressure; heart rate. As cocoa products are relatively high-energy foods, their addition to a diet may lead to weight gain, which is an undesirable confounding factor that may affect the studied parameters; therefore, a wide range of anthropometric parameters were controlled, as well as physical activity, which is a well-known protective factor against the occurrence and progression of CVD.
Experimental methods
Subjects
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures were approved by the Clinical Research Ethics Committee of Hospital Universitario Puerta de Hierro Majadahonda in Madrid (Spain). Written informed consent was obtained from all subjects. Volunteer recruitment was carried out by placing advertisements in the Universidad Complutense campus as well as by giving short talks between lectures. The inclusion criteria were as follows: having total cholesterol concentrations < 2000 and >2000 mg/l for the normocholesterolaemic and hypercholesterolaemic groups, respectively; being non-vegetarian and non-smoker for both men and women and being non-pregnant for women; aged between 18 and 55 years; not suffering from any chronic pathology or gastrointestinal disorder. None of the subjects had taken dietary supplements, laxatives or antibiotics 6 months before the start of the study. Their BMI was less than 30 kg/m2.
On the whole, fifty subjects initially accepted to participate in the study and gave informed written consent; however, only forty-four completed it.
Study design
This was a randomised, controlled, cross-over, free-living study. After a 2-week run-in stage, in which consumption of the fruit, vegetables and beverages mentioned below was restricted, the subjects consumed two sachets of soluble cocoa powder per d, one for breakfast and the other as a snack between lunch and dinner (in order to reduce interferences with other food items) in 200 ml of semi-skimmed milk (total intake: 400 ml/d in the cocoa+milk (C+M) intervention stage) or two 200 ml servings of semi-skimmed milk for 4 weeks (400 ml/d in the milk (M) intervention stage) in a random order. Blood samples and blood pressure, heart rate and anthropometric measurements were taken at baseline and at the end of each intervention. The trial was conducted during autumn months. The soluble cocoa product was provided by Nutrexpa S.L. It contained 33·9 % of total dietary fibre (TDF) and 13·88 mg/g of soluble polyphenols; therefore, the two servings of cocoa provided 10·17 g/d of TDF and 416·4 mg/d of polyphenols. The servings used (15 g/sachet) in the present study corresponded to the quantity of the product that can reasonably be expected to be consumed. During the run-in stage and the intervention periods, consumption of other cocoa products, oranges, mandarins, apples, grapes, strawberries, berries in general, beets and onion, as well as their derived beverages, including wine and juices, and tea was restricted in order to reduce inter-individual differences in the intakes of DF and polyphenols.
Dietary control and compliance
The subjects were asked to maintain the same dietary habits throughout the study. Their dietary intake was regularly evaluated to control any possible changes. The volunteers were instructed on how to fill the dietary records before starting the study. In the run-in stage and at the end of the two intervention periods, the volunteers were asked to complete a 72 h detailed food intake report, specifying the ingredients and amounts of food consumed, including serving weights (when possible) and household measurements. Compliance was controlled by counting the number of cocoa servings provided to the volunteers before and after the interventions, as well as by weekly calling the volunteers. In order to assess energy intake and dietary composition, the programme DIAL (Department of Nutrition and Bromatology I, Pharmacy Faculty, Complutense Universidad of Madrid (UCM), Spain) was used. The intake of polyphenols was estimated using the program www.phenol-explorer.eu taking into account the Folin–Ciocalteu method.
Cocoa fibre and polyphenol analysis
The TDF of the cocoa product was analysed in triplicate defatted samples following the Association of Official Analytical Chemists (AOAC) method modified in our laboratory( Reference Saura-Calixto, García-Alonso and Goñi 26 ). Briefly, the cocoa samples were treated with heat-stable α-amylase (A-3306; Sigma), protease (P-3910) and amyloglucosidase (A-9913), followed by centrifugation (15 min, 3000 g ) instead of filtration to separate the soluble and insoluble fractions obtained after the enzymatic hydrolysis of digestible compounds. Supernatants were quantitatively collected and pellets were washed twice with 10 ml of distilled water and centrifuged, and all the supernatants were combined. These were transferred into dialysis tubes (12 000–14 000 molecular weight cut-off (MWCO), Dialysis Tubing Visking; Medicell International Limited) and dialysed against water for 48 h at 25°C (water flow 7 litres/h in a 43-litre reservoir). Dialysates (SDF) were hydrolysed with 1 m-H2SO4 at 100°C for 90 min and NSP was determined in the hydrolysates. The residues obtained after enzymatic hydrolysis of the samples (IDF) were dried overnight at room temperature and hydrolysed with 12 m-H2SO4 for 1 h at 30°C and then diluted with 1 m-H2SO4 at 100°C for 90 min with shaking. After acid hydrolysis, the samples were centrifuged (15 min, 3000 g ), pellets were washed twice with distilled water, and the combined supernatants were collected for the determination of NSP. The residues were dried at 105°C overnight and gravimetrically quantified as Klason lignin.
Uronic acids (UA) in the hydrolysates from both SDF and IDF were quantified spectrophotometrically by the Scott( Reference Scott 27 ) method using galacturonic acid as a standard. Neutral sugars (NS) were analysed by spectrophotometry following the Southgate method( Reference Southgate 28 ). TDF was calculated as IDF+SDF; IDF was calculated as NSP+Klason lignin and SDF as NSP (NSP = UA+NS). Polyphenols were extracted following a procedure set up by our group( Reference Bravo and Saura-Calixto 29 ) and were analysed spectrophotometrically as total polyphenols using the Folin–Ciocalteu reagent and gallic acid as a standard, and the extracts were characterised by HPLC-diode array detection (DAD) using an Agilent 1200 series liquid chromatograph( Reference Cienfuegos-Jovellanos, Quiñones and Muguerza 30 ).
Blood samples
Blood samples were drawn after an overnight fasting for 8–10 h at baseline and after the consumption of milk or milk+cocoa for 4 weeks. Serum (without an anticoagulant) and plasma (EDTA-coated tubes) were separated by centrifugation and frozen at − 80°C until analysis.
Biochemical parameters
The lipid profile of the serum samples was determined following reference methods or methods recommended by Sociedad Española de Bioquímica Clínica y Patología Molecular (SEQC) using the Roche Cobas Integra 400 plus analyser (Roche Diagnostics). The concentrations of uric acid, creatinine and glucose were determined according to standardised spectrophotometric techniques, and the concentration of CRP was determined using an automatised ultrasensible turbidimetric method (AU2700 Biochemistry analyser; Olympus). Reference ranges used for all the biochemical parameters were those indicated by SEQC.
Inflammation biomarkers
The concentrations of cytokines IL-1β, IL-6, TNF-α, IL-10 and IL-8 in the plasma samples were determined using the High Sensitivity Human Cytokine MILLIPLEX MAP kit (Millipore Corporation), and those of monocyte chemoattractant protein-1, VCAM-1 and intracellular cell adhesion molecule 1 (ICAM-1) were determined using the Human Cardiovascular disease MILLIPEX MAP kit on a Luminex equipment (Luminex-100/200; Luminex Corporation). High- and low-concentration quality controls were used with all the biomarkers.
Oxidation and antioxidant biomarkers
The concentration of MDA was determined as that of its hydrazone by HPLC using dinitrophenylhydrazine for derivatisation( Reference Mateos, Lecumberri and Ramos 31 ). Serum samples were analysed directly, and standard MDA was prepared by acidic hydrolysis of 1,1,3,3-tetraethoxypropane in 1 % sulphuric acid. The concentrations are expressed as nmol MDA/mm in serum. Protein oxidation of the serum samples was measured as carbonyl group content according to the method of Richert et al. ( Reference Richert, Wehr and Stadtman 32 ). Absorbance was measured at 360 nm, and carbonyl content is expressed as nmol/mg protein using an extinction coefficient of 22 000 nmol/l per cm. Protein content of the serum samples was determined using the Bradford reagent.
The antioxidant capacity of the fibre-rich cocoa powder was evaluated in the soluble polyphenol extract and serum samples using the ferric reducing/antioxidant power assay( Reference Pulido, Bravo and Saura-Calixto 33 ). Free radical-scavenging capacity of the cocoa product and serum samples was also measured using the radical monocation 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) discolouration method( Reference Re, Pellegrini and Proteggente 34 ) with some modifications. The oxygen radical absorbance capacity of the serum samples was determined according to the method of Huang et al. ( Reference Huang, Ou and Hampsch-Woodill 35 ). For the three parameters, Trolox was used as a standard, and results are expressed as μmol of Trolox equivalents/g DM of the product and per ml of serum.
Blood pressure parameters
Blood pressure and heart rate were measured using an automatic arm sphygmomanometer (BS 150; Pic Indolor Diagnostic). At baseline and after 4 weeks, the volunteers were asked to rest on a chair before the cuff was placed on their left arm. After they were rested for 5 min, a second reading was taken on the same arm. Readings were compared, and if not in agreement within 10–15 mmHg, a third reading was taken.
Anthropometric measurements
At baseline and the two intervention periods, the total body and trunk fat percentage of the volunteers was assessed from tetrapolar bioimpedance measurements using a Tanita segmental body composition analyser BC-418 MA (Tanita Corporation). The device had a weighing system, which was used to weigh the volunteers, and their height was determined using a Holtain precision mechanical stadiometer (Holtain Limited). With these data, BMI was calculated according to the following formula: weight (kg)/height (m)2. Brachial, waist, abdominal, hip and thigh circumferences were measured using a SECA 203 flexible tape (SECA UK Ltd) (Table 6). Tricipital and subscapular skin folds were measured using an anthropometric calliper (Harpender Anthropometer; Holtain Limited). By means of these biometry data, body density( Reference Siri, Brozeck and Henschel 36 ) and the percentage of body fat( Reference Durnin and Womersley 37 ) were calculated.
Physical activity
The participants were asked to maintain their usual level of physical activity during the study. The volunteers filled out a questionnaire before the start of the study to report on their occupation and consequently the level of physical activity involved. At the beginning and end of the study, physical activity levels were calculated using an adapted version of the Minnesota Leisure Time Physical Activity Questionnaire by Martínez-González et al. ( Reference Martínez-González, López-Fontana and Varo 38 ). Total energy expenditure from leisure time was obtained by making the assumption that one metabolic equivalent is approximately 4·2 kJ/min (1 kcal/min) for a 70 kg man. Taking this into account and knowing the duration that the activities were performed per day, the data are expressed as kJ/d.
Statistical analysis
Taking total cholesterol as the main variable, a sample size of twenty-three subjects per group was calculated in order to obtain a statistical power of 80 % such that the study will detect a treatment difference at the 0·05 significance level, if the true difference between the treatments is 60 mg/l. This is based on the assumption that the within-patient standard deviation of the response variable is 10.
Data are presented as means with their standard errors, unless specified otherwise. Before the statistical analysis, the normality of distribution and the homogeneity of variance were verified using the Kolmogorov–Smirnov and Levene tests, respectively. The general linear model of variance for repeated measures was used to assess differences in all the parameters studied in response to the interventions. To exclude the carry-over effect for the two periods, we compared the differences in the parameters obtained from the group that started with the C+M intervention with those in the parameters obtained from the group that started with the M intervention. In order to assess the effects of belonging to the normocholesterolaemic or hypercholesterolaemic group, the group was considered as an inter-individual factor. Differences within either the normocholesterolaemic or hypercholesterolaemic group were studied using the Bonferroni test. Statistical significance was set at P< 0·05. The statistical analysis was carried out using the SPSS statistical package (version 19.0; SPSS, Inc., IBM Company) and Statgraphics Centurion XVII (Stat Point Technologies, Inc.).
Results
Cocoa product fibre and polyphenol analysis
The percentage of TDF and the content of the major constituents of the SDF and IDF fractions (NS, UA and Klason lignin) in the studied cocoa product were as follows: the percentage of the SDF fraction, which accounted for approximately 5 % of the TDF, was 1·68 (sem 0·10) % DM, being formed by NS (0·69 (SEM 0·04) % DM) and UA (0·99 (SEM 0·09) % DM). Quantitatively, IDF was the main DF component of cocoa, accounting for 95 % of TDF. Close to 60 % of the IDF fraction corresponded to NSP, which was made of NS (19·06 (sem 1·60) % DM) and UA (1·26 (sem 0·07) % DM), with the remaining being Klason lignin (11·90 (sem 0·28) % DM). Soluble polyphenols in cocoa analysed spectrophotometrically were 13·88 (SEM 0·24)mg/g DM and attending to HPLC-DAD analysis (n 6) cocoa contained 1·47 (sem 0·2) mg/g DM of total flavanols, 0·31 (sem 0·08) mg/g DM of epicatechin, 0·60 (sem 0·11) mg/g DM of catechin and 0·55 (sem 0·04) mg/g DM of procyanidin B2.
Subjects
Of the fifty volunteers who were enrolled into the study, six withdrew due to personal, health or professional reasons. Of the remaining forty-four volunteers, twenty-four were women with an average age of 25·75 (sd 6·29) years and a BMI of 22·2 (sd 2·42) kg/m2, and twenty were men with an average age of 32 (sd 10·04) years and a BMI of 25·15 (sd 3·94) kg/m2. The baseline characteristics of the forty-four subjects who completed the study are given in Table 1 and are separated into normocholesterolaemic (n 24) and moderately hypercholesterolaemic (n 20).
Table 1 Baseline characteristics of the participants in the study (Mean values and standard deviations)
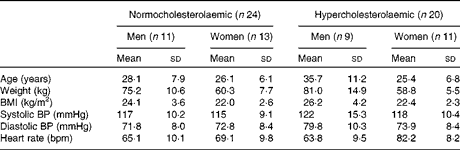
BP, blood pressure; bpm, beats per min.
Dietary control and compliance
Based on the reports of the volunteers and the number of servings returned after the interventions, dietary compliance was high. The 72 h intake reports were pre-filtered to exclude those that were not reliable. We considered reports unreliable if estimations of daily energy intake were 70 % below the light-activity energy intake recommendations or 130 % above the normal-activity energy intake recommendations for age and sex group( Reference Moreiras, Carbajal and Cabrera 39 ), as well as unjustified differences of over 4184 kJ (1000 kcal) between the baseline and final reports. The 72 h detailed food intake reports (Table 2) showed that DF (P< 0·001) and protein (P< 0·001) intakes were statistically higher after the C+M intervention stage than after the other stage. In contrast, the intakes of energy, polyphenols, carbohydrates, lipids, cholesterol and fatty acids (monosaturated, polysaturated and saturated) increased without reaching the level of statistical significance. For all the indicated parameters, both the groups exhibited the same behaviour after the consumption of cocoa, and no significant cocoa × group interaction was observed.
Table 2 Reported energy and dietary component intakes* (Mean values with their standard errors)

M, milk; C+M, cocoa+milk; C, cocoa.
a,bMean values within a row with unlike superscript letters were significantly different within either the normocholesterolaemic or hypercholesterolaemic group according to the Bonferroni test.
* Volunteers completed a 72 h food intake report, and energy intake and dietary composition were calculated using the program DIAL (Department of Nutrition and Bromatology I, Pharmacy Faculty, UCM), and polyphenol intake was assessed using www.phenol-explorer.eu taking into account the Folin–Ciocalteu method.
† P values were assessed using the general linear model of variance for repeated measures.
Biochemical parameters
Biochemical results are given in Table 3. After the interventions, total cholesterol, LDL-cholesterol and TAG levels did not show statistically significant differences; in contrast, HDL-C levels were significantly increased (P< 0·001). The concentrations of glucose (P= 0·029) and creatinine (P= 0·005) were significantly decreased. Alanine aminotransferase and aspartate aminotransferase enzyme levels were significantly increased (P= 0·001 and 0·001, respectively), although both the parameters remained within their respective SEQC reference ranges of normality (0–41 and 0–38 U/l). The levels of urea were significantly increased (P= 0·001) in contrast to those of creatinine, which were decreased (P= 0·005) after the cocoa intervention, with both the parameters remaining within their respective SEQC reference ranges (100–500 and 5·0–13·0 mg/l).
Table 3 Effects of the consumption of the cocoa product rich in dietary fibre on biochemical parameters (Mean values with their standard errors)

M, milk; C+M, cocoa+milk; C, cocoa; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
a,bMean values within a row with unlike superscript letters were significantly different within either the normocholesterolaemic or hypercholesterolaemic group according to the Bonferroni test.
* P values were assessed using the general linear model of variance for repeated measures.
Inflammatory and adhesion molecule biomarkers
The values of inflammatory and adhesion molecule levels showed a high variability (Table 4). The values of IL-1β, IL-6 and IL-8 levels observed were within the normal physiological range described by Kokkonen et al. ( Reference Kokkonen, Söderstrom and Rocklöv 40 ), whereas those of IL-10 levels were above the corresponding reference range (2·4–6·6 pg/ml) and those of TNF-α levels were below the reference range (14·2–61·7 pg/ml). The values of IL-1β, IL-6, IL-8 and TNF-α levels were slightly decreased at the end of the intervention, particularly in the hypercholesterolaemic group, without reaching the level of statistical significance, in contrast to that of IL-10 levels, which was significantly lower (P= 0·001). The value of monocyte chemoattractant protein-1 levels was within the normal range (32–147 ng/ml) and did not vary after the interventions.
Table 4 Effects of the consumption of the cocoa product rich in dietary fibre on cytokine and cell adhesion molecule levels (Mean values with their standard errors)
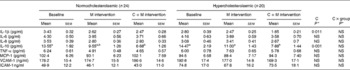
M, milk; C+M, cocoa+milk; C, cocoa; MCP-1, monocyte chemoattractant protein-1; VCAM-1, vascular cell adhesion molecule 1; ICAM-1, intracellular cell adhesion molecule 1.
a,bMean values within a row with unlike superscript letters were significantly different within either the normocholesterolaemic or hypercholesterolaemic group according to the Bonferroni test.
* P values were assessed using the general linear model of variance for repeated measures.
The value of VCAM-1 levels in the normocholesterolaemic group was close to the higher limit of the normal physiological range (46–166 ng/ml), and in the hypercholesterolaemic group, the value of the observed levels was above the upper limit. In contrast, the values of ICAM-1 levels in both groups were within the physiological range (39–79 ng/ml)( Reference Kokkonen, Söderstrom and Rocklöv 40 ), with that of the hypercholesterolaemic group being near the upper limit. The consumption of cocoa led to a slight decrease in the value of ICAM-1 levels in the normocholesterolaemic group and in that of VCAM-1 levels in the hypercholesterolaemic group (Table 4).
Blood pressure parameters
Based on the systolic and diastolic blood pressure results (Table 5), the subjects were normotensive (systolic blood pressure < 140 mmHg and diastolic blood pressure ≥ 80 mmHg) at the start and end of the interventions, since no changes were observed. All blood pressure values throughout the study were higher in the hypercholesterolaemic group than in the normocholesterolaemic group. No changes were observed regarding heart rate.
Table 5 Effects of the consumption of the cocoa product rich in dietary fibre on oxidation and antioxidant biomarker levels, blood pressure and heart rate (Mean values with their standard errors)

M, milk; C+M, cocoa+milk; C, cocoa; bpm, beats per min; TE, Trolox equivalents; MDA, malondialdehyde; FRAP, ferric reducing/antioxidant power; ORAC, oxygen radical absorbance capacity; ABTS, free radical 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)-scavenging capacity.
* P values were assessed using the general linear model of variance for repeated measures.
Oxidation and antioxidant biomarkers
The levels of MDA and carbonyl group biomarkers did not show statistical changes (Table 5).
The ferric reducing power in serum evaluated by the ferric reducing/antioxidant power method was slightly increased at the end of the intervention periods, without reaching the level of statistical significance. Similarly, oxygen radical absorbance capacity and ABTS values in serum showed no significant differences after the M and C+M interventions (Table 5).
Anthropometric measurements
The consumption of cocoa did not induce an increase in body weight or changes in the other anthropometric parameters measured except for a significant decrease (P< 0·001) in the tricipital skin folds (Table 6). However, the percentage of body fat calculated from the skin-fold data did not show statistical differences and nor did any other anthropometric parameters.
Table 6 Effects of the consumption of the cocoa product rich in dietary fibre on anthropometric parameters (Mean values with their standard errors)
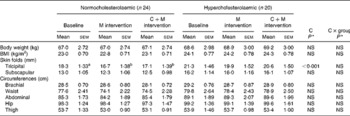
M, milk; C+M, cocoa+milk; C, cocoa.
a,bMean values within a row with unlike superscript letters were significantly different within either the normocholesterolaemic or hypercholesterolaemic group according to the Bonferroni test.
* P values were assessed using the general linear model of variance for repeated measures.
Physical activity
The ocupation of all the volunteers involved low physical activity levels; therefore, their energy expenditure was calculated by taking into account only leisure time, and no statistical differences were observed throughout the study. The normocholesterolaemic group showed a mean energy expenditure of 993·96 (sem 92·43) at baseline, 922·93 (sem 124·14) at the M intervention stage and 833·60 (sem 104·72) at the C+M intervention stage. The hypercholesterolaemic group exhibited a mean energy expenditure of 742·36 (sem 101·25) at baseline, 664·18 (sem 135·99) at the M intervention stage and 631·63 (sem 114·71) at the C+M intervention stage.
Discussion
The present study shows that regular consumption of a soluble cocoa product rich in DF as part of a typical Mediterranean-Spanish diet increases the intake of DF to the recommended levels and improves cardiovascular health by increasing HDL-C levels and decreasing glucose, IL-1β and IL-10 levels without leading to weight gain or other anthropometric changes.
Soluble cocoa powder is widely consumed in many different countries including Spain( Reference Cooper, Donovan and Waterhouse 41 ) by different population groups, particularly as part of breakfast or between main meals. The consumption rate of two cocoa beverages per day reproduces real conditions in the Spanish population and may be considered moderate (http://www.cacaoychocolate.com/consumoen.html). The servings used correspond to the quantity of the product that can reasonably be expected to be consumed. In fact, the optimal health effects of cocoa on coronary artery disease prevention have been associated with a moderate intake, while healthy outcomes disappear at a high intake( Reference Di Castelnuovo, di Giuseppe and Lacoviello 42 ).
Three important confounding factors, diet, physical activity and weight gain, were controlled throughout the present study. Regarding diet, this was a study with free-living subjects and the only dietary modification introduced, apart from consuming the cocoa product, was the restriction of the consumption of other cocoa products and the fruit and vegetables mentioned previously. All the methods used to estimate food intake present limitations; nevertheless, in order to obtain the most accurate data from the 72 h dietary records, the volunteers were trained to fill them out and meetings were held to reinforce dietary instructions and assist the volunteers to complete the records as precisely as possible. The intake of polyphenols (Table 2) increased after the consumption of cocoa and milk in both the groups due to the phenolic content of cocoa, which is particularly rich in monomeric (epicatechin and catechin) and oligomeric (procyanidins) flavonols( Reference Flammer, Hermann and Sudano 15 , Reference Gómez-Juaristi, González-Torres and Bravo 43 ), although the increase was not statistically significant probably owing to the restriction of the consumption of fruit and vegetables containing high amounts of antioxidant compounds.
The intake of carbohydrates in both the study groups at the beginning and end of the study (Table 2) was below the recommended range according to the study of Moreiras et al. ( Reference Moreiras, Carbajal and Cabrera 39 ). Contrarily, lipid and protein intake levels in the normocholesterolaemic group were within the recommended range at baseline, in contrast to those in the hypercholesterolaemic group, which were slightly above the respective recommended nutrient ranges. The levels of both macronutrients increased, although not significantly in the case of carbohydrates after regular intake of cocoa and milk, which may be explained by the sucrose (34·4 %) and DF (33·9 %) content in the cocoa product and protein in milk. In accordance, the values of serum urea levels were significantly higher (P= 0·001) after the consumption of cocoa compared with baseline values (Table 3), although they remained within the SEQC reference range (10–50 mg/ml), in contrast to those of serum creatinine levels, which were decreased (P= 0·005), but remained within the SEQC reference range (5·0–13·0 mg/l). There is no gold standard method for evaluating the intake of DF. However, the assessment of the intake of DF using dietary food records is a well-accepted alternative, particularly if the influence of smoking, alcohol intake, sex and education is considered( Reference Loening-Baucke, Miele and Staiano 44 ). These factors were controlled, as the volunteers who participated in the present study were non-smokers, consumed very low amounts of alcohol and had a similar medium-high education level. In both the groups, the intake of DF at baseline and after the M intervention was within the range estimated in the Spanish population (16·3–18·4 g/d)( Reference Saura-Calixto and Goñi 45 ) and below that in European countries (18·5 g/d)( Reference Tabernero, Serrano and Saura-Calixto 46 ). The consumption of cocoa significantly increased the intake of DF (P< 0·001) and the recommended levels (25 and 28 g/d in women and men, respectively)( Reference Kassis, Santosa and Jones 47 ) would have been reached had cocoa been included in the normal diet of the volunteers without any restrictions.
After the consumption of the cocoa product, no changes in the total lipid levels or in the intake of SFA were observed. In accordance with that study of Cooper et al. ( Reference Cooper, Donovan and Waterhouse 41 ), the fatty acid composition of cocoa had neutral effects on blood lipid and lipoprotein levels, except on HDL-C levels, which were significantly higher (P= 0·008). The HDL-C level-increasing effect of cocoa had already been described in 1994( Reference Kris-Etherton and Mustad 48 ) in healthy subjects who consumed a diet containing a moderate amount of milk chocolate (46·2 g/d) v. a group who consumed an isoenergetic high-carbohydrate snack. Similarly, the HDL-C effect has recently been described in high-risk CVD patients who regularly consumed 40 g of cocoa powder with milk for 4 weeks( Reference Khan, Monagas and Andres-Lacueva 49 ), as well as in other studies performed in patients with hypercholesterolaemia( 8 ) and in healthy subjects( Reference Davison, Coates and Buckley 6 ). Interestingly, according to the meta-analysis carried out by Hooper et al. ( Reference Hooper, Kroon and Rimm 50 ), the beneficial effects on HDL-C levels are greater in longer-term trials. Different mechanisms have been postulated for the rise in HDL-C levels induced by cocoa polyphenols such as the increase in the expression and secretion of apoA1, the synthesis of apoA1 and apoA2, the efflux cholesterol promoter and ATP-binding cassette transporter A1, and the activity of phospholipid transfer proteins as well as decreases in the levels of cholesteryl ester transfer protein and the possible formation of micelles in the intestine, thus modifying fat absorption( Reference Khan, Monagas and Andres-Lacueva 49 ). In addition, the DF present in cocoa more than likely contributed to the up-regulation of HDL-cholesterol levels in accordance with previous studies( Reference Hermann, Spieker and Ruschitzka 14 , Reference Flammer, Hermann and Sudano 15 ). To a certain extent, the present results are in contrast with those reported by Tokede et al. ( Reference Tokede, Gaziano and Djoussé 51 ), who concluded that the consumption of dark chocolate/cocoa products induces beneficial effects on LDL levels and neutral effects on TAG and HDL levels in short- and intermediate-term interventions, although dark chocolate seems to be a more effective matrix for delivering flavanols than cocoa beverages.
Other mechanisms may underlie the cardiovascular benefits of DF, such as the reduction of postprandial glucose concentrations, as fibre-rich foods can delay glucose absorption from the small intestine( Reference Giacco, Parillo and Rivellese 52 ) and improve insulin sensitivity( Reference van der Du, Boshuizen and Forouhi 53 ). In fact, long-term, prospective observational studies have suggested that diets containing larger quantities of whole grains and DF are associated with a reduced risk of type 2 diabetes( Reference Schulze, Hoffmann and Manson 54 ). However, in the meta-analysis mentioned previously( Reference Hooper, Kroon and Rimm 50 ), significant reductions in fasting serum insulin concentrations were described after cocoa interventions, but no effects were observed on fasting glucose levels. In contrast, in the present study, serum glucose levels were significantly decreased (P= 0·038), which is in line with the hypoglycaemic results described in moderately hypercholesterolaemic subjects after the consumption of a fibre-rich cocoa product for 8 weeks( 8 ).
Inflammation and endothelial dysfunction are important contributors to the development of atherosclerosis. IL-6 and TNF-α levels may be stronger predictors of incident cardiovascular events than CRP levels, as any dietary influence would first influence IL-6 and TNF-α levels( Reference Ma, Hebert and Li 24 ). In the present study, no significant changes in the levels of pro-inflammatory cytokines were observed after the consumption of cocoa and milk, although they were slightly decreased, in contrast to that observed in in vitro studies that indicate that cocoa exerts anti-inflammatory effects via TNF-α( Reference Selmi, Cocchi and Lanfredini 1 ). IL-10 directly inhibits the release of inflammatory cytokines by monocytes and neutrophils and can dampen the inflammatory response by inhibiting Th1-cell cytokine production. The anti-inflammatory and anti-atherogenic properties of IL-10 have been demonstrated using several models of atherosclerosis in mice. In human subjects, the expression of IL-10 has been demonstrated in both coronary arteries and atherosclerotic plaques, and higher serum levels of IL-10 have been shown in atherosclerosis patients compared with controls, suggesting that the levels of IL-10, as an anti-inflammatory molecule, may be elevated in response to the pro-inflammatory environment of atherosclerosis( Reference Lakoski, Liu and Brosnihan 55 ). In agreement with this, in the present study, the value of IL-10 levels was higher in the hypercholesterolaemic subjects v. the normocholesterolaemic subjects. Surprisingly, the values of IL-10 levels were significantly decreased after the C+M intervention in both the groups, being 50 % lower than their respective baseline ranges. IL-10 is involved in the inflammatory response by the down-regulation of the synthesis of other cytokines, including that of IL-1β( Reference Heiskanena, Kähönen and Hurme 56 ), which is in accordance with the statistically significant reduction of IL-1β levels (P= 0·011) observed in the present study. In vitro studies have shown that smaller flavanol fractions (monomer through tetramers) induce an anti-inflammatory response by suppressing IL-1β mRNA expression and protein secretion( Reference Mao, Powell and van de Water 57 ). Nevertheless, no changes were observed in IL-8 and monocyte chemoattractant protein-1 levels throughout the study.
Soluble adhesion molecules are early biomarkers of alterations in vascular function that indirectly indicate vascular inflammation and endothelial cell activation. Dissimilar effects of flavanol-rich foods on cell adhesion molecule levels have been described. A low-dose intake of white and red wine decreases the serum concentrations of ICAM-1; however, only red wine decreases the serum concentrations of VCAM-1( Reference Sacanella, Vázquez-Agell and Mena 58 ). In contrast, black tea lowers soluble P-selectin levels without affecting soluble ICAM-1 and VCAM-1 levels( Reference Hodgson, Puddey and Mori 59 ). Hypercholesterolaemic postmenopausal women who consumed a high-flavanol cocoa beverage (446 mg of total flavanols) for 6 weeks had significantly lower levels of sVCAM-1 compared with those consuming the low-flavanol cocoa beverage (43 mg of total flavanols)( Reference Wang-Polagruto, Villablanca and Polagruto 22 ), with epicatechin and certain B-type dimers, as well as their related metabolites, being the candidates for the effects of high-flavanol cocoa beverage, as they inhibit the activation of the oxidative stress-sensitive nuclear transcription factor NF-κB, a known promoter of VCAM-1 expression( Reference Wang-Polagruto, Villablanca and Polagruto 22 ). In the present study, VCAM-1 levels were slightly decreased; this small effect can be greatly attributed to cocoa polyphenols and their metabolites, as other flavanol-rich foods had been restricted during the study. In the hypercholesterolaemic group, ICAM-1 levels remained similar in contrast to that observed in a previous study( Reference Monagas, Khan and Andrés-Lacueva 60 ) in high-risk CVD subjects in whom ICAM-1 levels were significantly decreased after consuming 40 g of cocoa powder with milk (495 mg of total polyphenols). Once again, the lack of effects on ICAM-1 levels in the present study may be attributed to the flavanol-rich food restrictions.
Observational data on the relationship between cocoa intake and cardiovascular health suggest that the consumption of cocoa is associated with lower blood pressure. When subgrouping by epicatechin dose, greater effects were observed at doses >50 mg/d( Reference Hooper, Kroon and Rimm 50 ). Accordingly, regular consumption of two servings of the cocoa product rich in fibre provided 9·3 mg/d of epicatechin (M Gomez-Juaristi, unpublished results) and did not induce hypotensive effects in the normocholesterolaemic or hypercholesterolaemic group. In addition, the participants of the present study were normotensive at baseline, and thus the present results are in agreement with those reported by Ried et al. ( Reference Ried, Sullivan and Fakler 61 ), who observed a significant reduction of blood pressure in hypertensive subjects but not in normotensive subjects after the consumption of cocoa, and with those of a previous study carried out in our group in moderately hypercholesterolaemic subjects whose diastolic blood pressure was near the lower limit of the range of high blood pressure at baseline( Reference Sarriá, Mateos and Sierra-Cinos 20 ).
There was no significant effect on lipid and protein oxidation biomarker levels and on serum ferric reducing power, free radical-scavenging activity in vivo or oxygen radical absorbance capacity. As the contribution of fruit and vegetables to the antioxidant status of the volunteers had been reduced, it seems that plasma cocoa polyphenols did not reach a concentration high enough or were not in a chemical form to show antioxidant activity, which is in line with the results reported by Turner et al. ( Reference Turner, Baron and Wolffram 62 ) and Rimbach et al. ( Reference Rimbach, Weinberg and de Pascual-Teresa 63 ) However, it should not be disregarded that the study was not powered to evaluate changes in antioxidant parameters and also that when chocolate is mixed with milk the absorption of antioxidants is reduced( Reference Serafini, Bugianesi and Maiani 64 ).
With respect to the remaining confounding factors, physical activity and weight gain, energy expenditure at leisure time was relatively high. The assumption that one metabolic equivalent is approximately 4·2 kJ/min (1 kcal/min) may have led to an overestimation of energy expenditure. Nevertheless, that the physical activity level was not different throughout the study makes the possible influence of this factor uniform. Accordingly, none of the anthropometric parameters measured, weight, BMI, subscapular skin folds, or the brachial, waist, abdominal, hip and thigh circumferences, showed changes (Tables 1 and 6). A significant decrease in the tricipital skin folds in both the groups was observed, but this result was not supported by the biometry data. Therefore, the results of the present study are in line with that of Buijsse et al. ( Reference Buijsse, Feskens and Kok 65 ), who reported that energy intake is higher in dark chocolate consumers than in non-consumers; however, the BMI was lower in the former group, although we did not observe a decrease in BMI. This ‘antiobesity’ effect has been attributed to the physiological activity of polyphenols, which has been described in human subjects who consumed tea( Reference Nagao, Komine and Soga 66 ) and rats that consumed cocoa( Reference Matsui, Itoa and Nishimura 67 ). In the latter study, the antiobesity effects of cocoa polyphenols were explained by the modulation of lipid metabolism, especially by decreasing fatty acid synthesis and transport system activity, and the enhancement of part of the thermogenesis mechanism in the liver and white adipose tissue.
The present study has limitations: the lack of blinding of subjects and investigators may have led to certain bias; the number of subjects was relatively small; treatment duration was relatively short to predict the effects of habitual cocoa intake on the cardiovascular parameters studied. Finally, it is not possible to specify to what extent the observed effects are due to DF, polyphenols or other ingredients of cocoa.
Concluding remarks
In recent years, there has been growing interest in cocoa and health. Although many studies have shown that cocoa induces cardioprotective effects, certain related aspects need to be clarified. This was the aim of the present study, in which the effects of regular consumption of a cocoa product rich in fibre (33·9 % TDF, providing 10·17 g of TDF and 416·4 mg of polyphenols/d) within a typical Mediterranean-Spanish diet were assessed. According to the results obtained, it may be concluded that:
-
(1) cocoa is an efficacious alternative to increase the intake of DF to the recommended levels, without leading to weight gain or other anthropometric changes;
-
(2) it increases serum HDL-C levels, without affecting other lipid or lipoprotein parameters;
-
(3) it decreases plasma IL-1β and IL-10 concentrations and slightly lowers other pro-inflammatory molecule concentrations;
-
(4) it reduces fasting serum glucose levels without changing fasting serum antioxidant or blood pressure values;
-
(5) there are no differences in the effects observed between the moderately hypercholesterolaemic and normocholesterolaemic subjects.
In contrast to chocolate, where the long-term side effects and optimal dose need to be questioned, we conclude that regular and moderate consumption of cocoa might be a worthwhile dietary approach for improving cardiovascular health.
Acknowledgements
The authors thank the volunteers who participated in the present study, Laura Barrios for her statistical assistance and Aránzazu Fernández-Espinosa for her technical assistance. They also acknowledge Project Consolider-Ingenio (CSD2007-00063) from the Spanish Ministry of Science and Innovation. S. M.-L. thanks the Spanish National Research Council for her predoctoral fellowship under the JAE-Pre programme funded by the European Social Fund. The present study was funded by Nutrexpa S.L. The authors' contributions were as follows: L. B. was involved in the trial conception, design and data interpretation; S. M.-L. and B. S. conducted the study and carried out the statistical analysis of data; S. M.-L., B. S. and R. M. carried out the assays and interpreted the results; J. L. S.-C. and L. G.-D. carried out the anthropometric measurements and statistically analysed the corresponding data; B. S. wrote the initial draft and all authors critically reviewed it and contributed to the final version of the manuscript. There are no conflicts of interest.








