Inflammatory bowel diseases (IBD), such as Crohn's disease and ulcerative colitis, are increasing in prevalence in southern Europe, Asia and much of the developing world, while their occurrence is stable in high-incidence areas such as northern Europe and North America. It has been estimated that 1·4 million individuals in the USA and 2·2 million individuals in Europe alone suffer from these diseases(Reference Loftus1).
The aetiology of IBD is still unknown, although genetic background and modern lifestyle seem to be important factors(Reference Xavier and Podolsky2). Diet also plays a role in the development or clinical course of IBD, with meat and alcoholic beverages increasing the likelihood of relapse in ulcerative colitis patients(Reference Jowett, Seal and Pearce3). On the other hand, elemental diet (liquid nutrition where N is derived from free amino acids, sugars from simple digestible carbohydrates and fat from TAG) has been shown to have beneficial effects(Reference Yamamoto, Nakahigashi and Umegae4). However, elemental diets are unpalatable and can only be used for limited periods of time due to patient non-compliance and side-effects(Reference Shah5). In practice, there are no effective and acceptable long-term dietary recommendations for IBD.
Phytochemicals, such as polyphenols from fruits and vegetables (i.e. proanthocyanidins), modulate cellular signalling processes, exert anti-inflammatory and antioxidant activity and modify intestinal microflora(Reference Biesalski6–Reference Dolara, Luceri and De Filippo8). Apples contain an exceptional selection of such bioactive phytochemicals. However, most of the studies on apples have focused on single compounds, underestimating the effects related to the combination of a complex phytochemical mixture. On this basis, we wanted to verify whether consumption of foods such as apples might assist in controlling intestinal inflammation in rats.
A variety of experimental models are available for studies on chronic intestinal inflammation in vivo. One of these is the intermittent administration of dextrane sulfate sodium, which damages epithelial cell junctions and makes the mucosa permeable to intestinal bacteria(Reference Gaudio, Taddei and Vetuschi9); however, the dextrane sulfate sodium model is not clearly correlated with spontaneous human IBD.
HLA-B27 transgenic rats, used in the present study, derive from F344 rats and express human β2-microglobulin and the histocompatibility gene HLA-B27; they spontaneously develop immune-mediated intestinal inflammation, resembling histologically human IBD(Reference Hammer, Maika and Richardson10). The microbial intestinal environment influences colitis in HLA-B27 rats and intestinal inflammation is not observed in germ-free environments(Reference Taurog, Richardson and Croft11). In this rat model colitis is induced by administering Bacteroides vulgatus, while Escherichia coli is inactive(Reference Rath, Wilson and Sartor12). Additionally, in HLA-B27 rats, the colitis is mediated by CD4+ but not by CD8+ cells, suggesting a complex host–microbiota interaction in which genetic host factors play a crucial role(Reference Hoentjen, Tonkonogy and Qian13).
The aim of the present study was to verify whether the addition to the diet of two apple varieties, Marie Ménard and Golden Delicious, with different polyphenol composition, could reduce intestinal inflammation in HLA-B27 rats and consequently ameliorate colitis. This topic was addressed through the characterisation of classical inflammation markers, faecal microbiota content and colon mucosa whole-genome expression profiles.
Materials and methods
Apple powder production and characterisation
Cider apple fruits (Malus domestica Borkh.) from the Marie Ménard variety were harvested at maturity during the 2002 season in the experimental orchard of the Centre Technique des Productions Cidricoles (Sées, Orne, France). Golden Delicious apples were bought from a local wholesale grocery in the same location.
The apple powder used in the feeding experiments contained complete apples (skin and pulp) and was prepared from apples frozen in liquid N2 after slicing, and freeze-dried. After freeze-drying, samples were blended in a food processor and portioned in special hermetically sealed bags to avoid storage degradation. All samples were stored at 20°C until analysis or diet preparation.
Polyphenols were measured by HPLC after thioacidolysis as previously described(Reference Guyot, Marnet and Sanoner14). The average degree of polymerisation was measured by calculating the molar ratio of all the flavan-3-ol units (thioether adducts plus terminal units) to ( − )-epicatechin and (+)-catechin corresponding to terminal units. The HPLC apparatus was a Waters (Milford, MA, USA) system 717 plus autosampler equipped with a cooling module set at 4°C, a 600 E multisolvent system, a 996 photodiode array detector, and a Millennium 2010 Manager system. The column was a Purospher RP18 endcapped, 5 μm (Merck, Darmstadt, Germany). The solvent system was a gradient of solvent A (aqueous acetic acid, 2·5 % v/v) and solvent B (acetonitrile): initial composition 3 % B; linear gradient to 9 % B from 0 to 5 min; linear gradient to 16 % B from 5 to 15 min; linear gradient to 50 % B from 15 to 45 min followed by washing and reconditioning the column.
N was analysed by the Kjeldahl method. Proteins were calculated as N × 6·25(Reference Hach, Brayton and Kopelove15).
Cell walls were quantified as alcohol-insoluble solids from the freeze-dried apple powders, as described elsewhere(Reference Renard16). Starch was quantified by an enzymic-colorimetric method(Reference Batey17) as described previously(Reference Ella Missang, Renard and Baron18). Sugars (glucose, fructose and sucrose) and l-malic acid were determined by enzymic assays by Boehringer Mannheim/R-Biopharm (R-Biopharm AG, Darmstadt, Germany) according to the producer's specifications.
Animals and diets
The experimental protocol, designed in conformity with the recommendations of the European Economic Community (86/609/CEE) for the care and the use of laboratory animals, in agreement with the Good Laboratory Practices, was approved by the animal care committee of the University of Florence (Italy).
Male HLA-B27 transgenic rats (Taconic Laboratory, Germantown, NY, USA), aged 6–8 weeks, were maintained in specific pathogen-free condition (but not germ-free conditions) and divided into three groups: controls (n 6); rats fed lyophilised Golden Delicious apples (n 7); rats fed lyophilised Marie Ménard apples (n 7). Dietary components (Piccioni, Gessate, Milan, Italy) were based on the American Institute of Nutrition (AIN)-76 diet, modified to contain high fat (23 % maize oil, w/w) and low cellulose (2 %, w/w)(Reference Caderni, Luceri and Lancioni19). The apple-supplemented diets contained 7·6 % of lyophilised apple pulp and were modified to compensate for sucrose and fibres in the apple pulp, deriving from Golden Delicious apples or Marie Ménard apples (Tables 1 and 2).
Table 1 Composition of lyophilised Marie Ménard and Golden Delicious apples (mg/g)
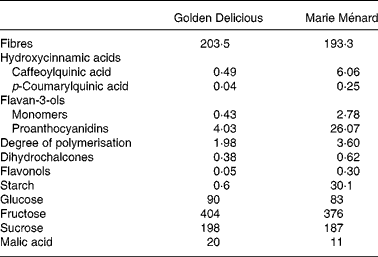
Table 2 Composition of experimental diets (g/kg)
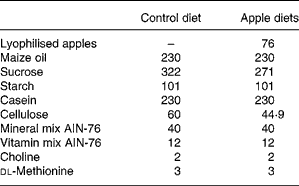
AIN, American Institute of Nutrition.
After 12 weeks of treatment the animals were killed. For each animal, the last part of the distal colon was fixed in buffered formalin for histological analysis. The remaining part of the distal colon was scraped with a glass slide to isolate the mucosal layer and stored at − 80°C for DNA extraction and biochemical determinations and in RNAlater (Qiagen, Milan, Italy) for RNA analyses.
Histological grading of colitis
The extent of tissue damage was determined by assigning a numerical score to colon tissue sections obtained from each rat. Full-thickness sections of fixed colons were stained with haematoxylin–eosin and scored blindly by a pathologist according to established criteria for characterisation of inflammation (0 = normal, 1 = local neutrophil infiltration, 2 = loss of glands due to inflammation, 3 = moderate ulceration or foci with the loss of more than five glands, 4 = extensive ulcerations). Mucosal injury was also assessed (0 = normal, 1 = distortion and/or destruction of the lower third of the glands, 2 = distortion and/or destruction of the lower two-thirds of the glands with remaining surface epithelium, 3 = loss of entire glands). A ‘colitis score’ of each rat was calculated as the sum of the two sub-scores. Results were expressed as average colitis score in each experimental group.
Faecal water content
Fresh faecal samples were harvested when the rats were killed and frozen at − 80°C until analyses. To determine the degree of diarrhoea, faecal samples were dried in a dehumidified oven at 50°C until stable in weight and the water content was expressed as percentage of the fresh faecal weight.
Faecal microbiota analysis
To assess the effect of the treatments on intestinal microbiota composition, PCR–denaturing gradient gel electrophoresis (DGGE) and real-time PCR were performed on faecal DNA extracted according to a previously described method(Reference Zoetendal, Heilig and Klaassens20). PCR amplification of total bacteria, Lactobacillus, Bifidobacterium and Bacteroides was carried out by targeting 16S rRNA gene sequences, using primers and PCR conditions as described previously(Reference Heilig, Zoetendal and Vaughan21–Reference Muyzer, de Waal and Uitterlinden24). DGGE analysis of PCR products was performed using the DCode System (Bio-Rad Laboratories, Hercules, CA, USA). Polyacrylamide denaturating gels were poured from the top using a gradient maker and a pump (Econopump; Bio-Rad, La Jolla, CA, USA); gradients of 30–60 %, 35–55 %, 40–50 % and 30–50 % were used for separation of specific amplicons for total bacteria, Lactobacillus, Bifidobacterium and Bacteroides, respectively. Electrophoresis was performed for 16 h at 85 V in a 0·5 × TAE (Tris–acetate–EDTA) buffer at 60°C; gels were stained with AgNO3(Reference Sanguinetti, Neto and Simpson25). DGGE profiles were analysed using the BioNumerics software, version 2.50 (Applied Maths, St-Martens-Latem, Belgium) and the resulting matrices were analysed by principal components analysis (CANOCO for Windows 4.5; Canoco, Wageningen, The Netherlands)(Reference Leps and Smilauer26).
Total bacteria, Lactobacillus, Bacteroides, Clostridium cluster XIVa and Clostridium cluster IV were quantified as copy number/g by real-time PCR using specific primers and PCR conditions targeting 16S rRNA(Reference Requena, Burton and Matsuki27, Reference Matsuki, Watanabe and Fujimoto28). SYBR green PCR amplifications were performed in an iCycler iQ5 Real-Time Detection System (BioRad) associated with the iCycler Optical System Interface software (version 2; BioRad). The reaction mixture contained 2 × SYBR green PCR Mix (BioRad), 0·5 μm of each primer, and either 5 μl of ten times-diluted template DNA or water. For quantification of Bifidobacterium the recA gene was used as the target for real-time PCR(Reference Masco, Vanhoutte and Temmerman29).
Determination of myeloperoxidase activity
Myeloperoxidase activity was measured according to the method of Stucchi et al. (Reference Stucchi, Shofer and Leeman30).
Oxidation damage
DNA oxidative damage in the colon mucosa was assessed using two methods: the comet assay, on samples treated with a bacterial repair enzyme (formamidopyrimidine glycosylase; gift of A. R. Collins, University of Oslo, Norway)(Reference Giovannelli, Decorosi and Dolara31), and the HPLC determination of 8-hydroxy-2′-deoxyguanosine levels(Reference Lodovici, Casalini and Cariaggi32).
Proliferative activity
Colon mucosa proliferation was evaluated by counting nuclei immunoreactive to the proliferating cell nuclear antigen in at least ten longitudinal crypt sections at 400 × (Reference Femia, Luceri and Dolara33). Proliferative activity was expressed as the number of labelled cells/the number of cells in the crypt × 100.
Microarray analysis
Total RNA was extracted using the RNeasy Midi kit (Qiagen, Milan, Italy). Equal amounts of RNA extracted from the colon mucosa of control rats (n 6) were pooled and used as a common reference. RNA from six rats for each experimental group (labelled with Cy5) were compared with the reference RNA (labelled with Cy3) (CyDye Mono-Reactive Dye Pack; Amersham, Cologno Monzese, Milan, Italy), using the indirect labelling method described by DeRisi (http://derisilab.ucsf.edu). The twelve hybridisations were performed on the Agilent Whole Rat Genome 4 × 44 K microarrays (Agilent Technologies, Palo Alto, CA, USA), according to the Agilent protocol; images were scanned using the Agilent DNA microarray scanner (G2505B; Agilent Technologies). Image analysis and initial quality control were performed using the Agilent Feature Extraction Software v9.5 and TIBCO SpotFire Decisionsite 9.0 (TIBCO Spotfire, Somerville, MA, USA).
For microarray data analysis, the text files were imported into R 2.5.1 using the Bioconductor ‘limma’ package 2.8.1 for statistical analysis(Reference Smyth, Gentleman, Carey and Huber34, Reference Smyth, Yang and Speed35). Values for control spots and spots that did not meet the quality criteria were flagged. Quality criteria for inclusion included having a spot of at least thirty-five pixels, a median:mean ratio of at least 0·9 and a non-saturated spot intensity for both channels. Quality criteria for inclusion included the first two criteria for both channels, a non-saturated spot intensity and a signal well above background for at least one channel (background-corrected spot intensity larger than 2·6 times the sd of the measured background). The background-corrected intensities of all microarrays were normalised using the locally weighted scatterplot smoothing (LOESS) algorithm(Reference Bolstad, Irizarry and Astrand36, Reference Yang, Dudoit and Luu37). A linear model was then fitted to the intensities for each reporter, and treated rats were compared with controls using an unpaired moderated t test using the ‘limma’ functions lmfit and eBayes(Reference Smyth38). Benjamini–Hochberg-adjusted P values were used to correct for multiple testing. All Agilent reporters were re-annotated to ensure reporter specificity(Reference Gaj, van Erk and van Haaften39) and to optimise recognition by the pathway procedures. All non-specific reporters were included in the analysis step but were flagged to allow for correct interpretation.
Biological pathway analysis was performed using the GenMAPP/MAPPFinder software tandem(Reference Luceri, Giovannelli and Pitozzi40) (http://www.genmapp.org; University of California at San Francisco, San Francisco, CA, USA). For GenMAPP, the ‘Rn_Std_20070817’ gene database and the ‘Rn_20080619’ pathway set were used. MAPPFinder analyses for pathways enrichment were performed for both diets and for up- and down-regulated genes separately. MAPPFinder selection criteria were set at P < 0·05 (Benjamini–Hochberg-adjusted) and>1·1-fold change in the relevant direction. Only pathways showing a permute P < 0·05 and a positive (enrichment) z-score were selected.
RT-PCR
For first-strand cDNA synthesis, 1 μg of total RNA from each sample was reverse-transcribed. Primers were designed on the basis of the rat GenBank sequences for cyclo-oxygenase-2 (COX2), inducible NO synthase, IL1β, TNFα and interferon γ. β-Actin was co-amplified as the reference(Reference Luceri, Caderni and Sanna41). Primer sequences are available on request.
Statistical analyses
Statistical analyses were conducted using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA). Differences on faecal water content, myeloperoxidase activity, oxidative DNA damage, cell proliferation and RT-PCR measurements were analysed by one-way ANOVA followed by post hoc Tukey's multiple-range test, whereas the non-parametric Mann–Whitney test was used to analyse real-time PCR for Bacteroides fragilis spp. data. Results are presented as mean values with their standard errors. Significance was assigned at P < 0·05.
Results
During the feeding period, food intake and body-weight gain were similar among the three experimental groups; at the time of killing, no animals presented macroscopic intestinal bleeding. However, histological examination showed signs of inflammation and mild mucosal injury in all rats. The colitis score was significantly lower in rats treated with Marie Ménard apples compared with controls (P < 0·05). Values were as follows: 1·2 (sem 0·8) in the control diet group, 1·0 (sem 0·6) in rats fed lyophilised powder from Golden Delicious apples and 0·43 (sem 0·4) in rats fed 7·6 % lyophilised powder from Marie Ménard apples.
Compared with controls, the severity of diarrhoea, evaluated as percentage of faecal water, was significantly reduced in rats fed lyophilised Marie Ménard apples, whereas rats fed lyophilised Golden Delicious apples had some improvements that did not reach statistical significance (Table 3).
Table 3 Effect of treatments with different lyophilised apples on diarrhoea (expressed as % faecal water content), myeloperoxidase activity, oxidative DNA damage (expressed as % of damaged DNA migrating in the cell tail after digestion of DNA with formamidopyrimidine glycosylase (FPG) or as 8-oxodG:dG×10−6 ratio) and cell proliferation (expressed as labelling index)
(Mean values with their standard errors)

* Mean value was significantly different from that of the control group (P < 0·05).
† Mean value was significantly different from that of the rats fed lyophilised Golden Delicious apples.
To determine the dietary impact of the different diets on intestinal microbiota, total faecal bacteria and predominant intestinal bacterial populations were analysed at the end of the treatments by 16S rRNA gene-based real-time PCR and DGGE. Real-time PCR indicated variations in the number of bacteria and population sizes of Bifidobacterium, Lactobacillus, Clostridium cluster XIVa and Clostridium cluster IV which were not significantly different among groups (data not shown). However, animals fed lyophilised Marie Ménard apples had significantly lower numbers of bacteria belonging to the B. fragilis group compared with those fed lyophilised Golden Delicious apples (P = 0·011) and controls (P = 0·051) (Fig. 1(a)). The real-time PCR data were confirmed by principal components analysis of the DGGE profiles (Fig. 1(b)), showing that Marie Ménard apple-fed rats had a distinct faecal Bacteroides population compared with the other two groups.

Fig. 1 (a) Real-time PCR for Bacteroides fragilis spp. from faeces of rats fed different diets, at the end of the treatment period. (![]() ), Individual values; (■), mean values, with standard errors represented by vertical bars. * Mean value was significantly different from that of the Golden Delicious apple-fed group (P = 0·011; Mann–Whitney test). † Mean value was marginally different from that of the control group (P = 0·051; Mann–Whitney test). (b) Principal components analysis of the B. fragilis-group-specific denaturing gradient gel electrophoresis profiles in the three different dietary groups: controls (○); Marie Ménard apple-fed rats (
), Individual values; (■), mean values, with standard errors represented by vertical bars. * Mean value was significantly different from that of the Golden Delicious apple-fed group (P = 0·011; Mann–Whitney test). † Mean value was marginally different from that of the control group (P = 0·051; Mann–Whitney test). (b) Principal components analysis of the B. fragilis-group-specific denaturing gradient gel electrophoresis profiles in the three different dietary groups: controls (○); Marie Ménard apple-fed rats (![]() ); Golden Delicious apple-fed rats (⋄). Each symbol represents an individual faecal sample. → , First three principal components. The Eigen value of the x axis is 0·43; the Eigen value of the y axis is 0·231.
); Golden Delicious apple-fed rats (⋄). Each symbol represents an individual faecal sample. → , First three principal components. The Eigen value of the x axis is 0·43; the Eigen value of the y axis is 0·231.
The myeloperoxidase activity in the colon mucosa, a marker of inflammatory cell infiltration, was significantly reduced in rats fed lyophilised Marie Ménard apples, compared with control rats and rats fed Golden Delicious apples (Table 3). Feeding lyophilised Golden Delicious apples reduced colon proliferative activity significantly (P < 0·05), and Marie Ménard apples had a borderline effect (P = 0·1), compared with the control diet (Table 3).
Oxidative DNA damage in the colon mucosa was not altered by the administration of either apple variety, measured with single cell electrophoresis (comet assay) or as the 8-hydroxy-2′-deoxyguanosine:2-deoxyguanosine ratio in DNA (Table 3).
Gene expression profiling revealed a large number of differentially expressed genes in rats fed lyophilised apples, relative to the control group (8492 and 11 230 in rats fed Golden Delicious and Marie Ménard apples, respectively). Most differentially expressed genes (6945) were shared between the two apple treatments, whereas 1547 were exclusively modulated by Golden Delicious and a larger number (4285) by Marie Ménard apples. In the animals fed lyophilised apples, analysis of gene expression data with GenMapp/MAPP Finder programs identified several differentially expressed pathways (Table 4) relative to control: specifically, PG pathways and mitogen-activated protein kinase (MAPK) signalling were down-regulated. The TNFα–NF-κB pathway was down-regulated only in rats fed lyophilised Marie Ménard apples. The list of enriched pathways and the graphical representations showing the effect of both diets on gene expression in each pathway are available online at http://www.bigcat.unimaas.nl/public/data/GenMAPP/Apples
Table 4 Results of the functional analysis performed using GenMAPP/MAPPFinder software*

GPCRDB, G Protein-Coupled Protein Receptor Database; MAPK, mitogen-activated protein kinase; KEGG, Kyoto Encyclopedia of Genes and Genomes; HSP, heat shock protein; BiGCaT, Bioinformatics Group; GPCRs, G protein-coupled protein receptors; EGFR1, epidermal growth factor receptor-1.
* Pathways significantly enriched (z-score>1·97) are listed. The different columns show the number of genes differentially expressed compared with controls, the number of genes analysed and the number of genes listed in each map (http://www.genmapp.org; University of California at San Francisco, San Francisco, CA, USA).
RT-PCR showed a significant reduction in COX2 (P < 0·05) and inducible NO synthase-2 gene expression (P < 0·01) in the colon mucosa of rats fed lyophilised Marie Ménard apples compared with controls (Fig. 2). The interferon γ gene expression tended to be lower in rats fed Marie Ménard apples (P = 0·057) (Fig. 2), whereas TNFα and IL1β expression was similar in the three experimental groups.
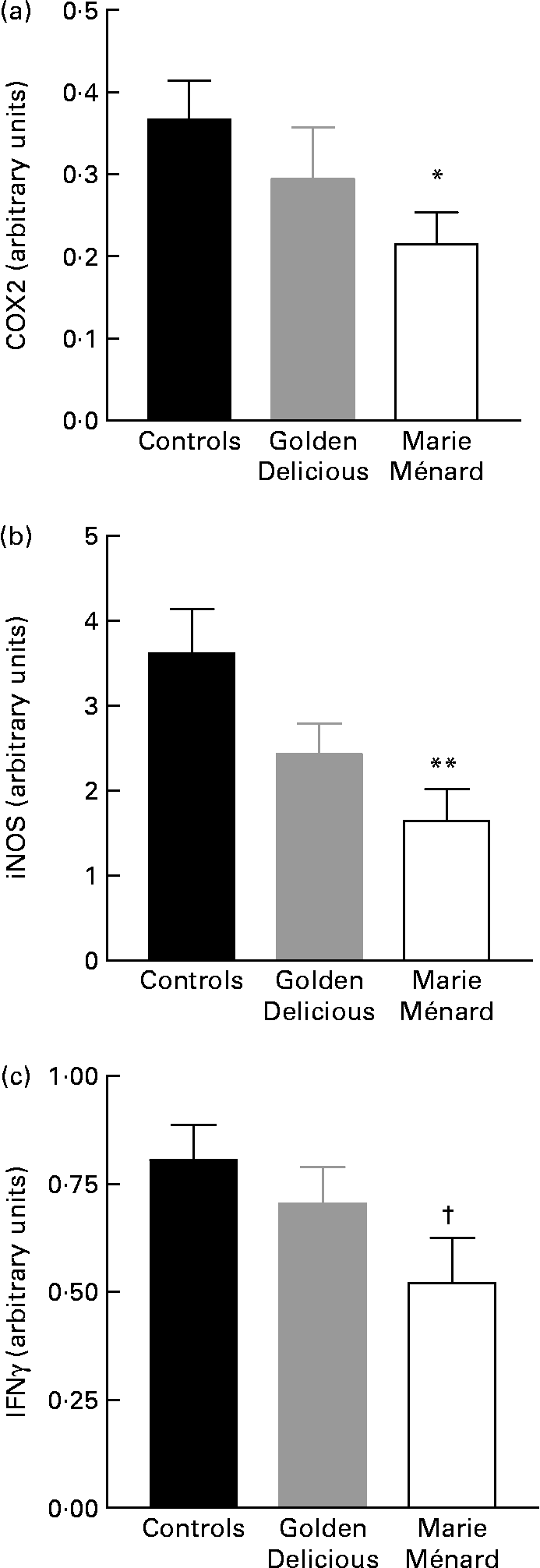
Fig. 2 Effect of lyophilised apple dietary treatments on the expression levels of selected pro-inflammatory genes: (a) cyclo-oxygenase-2 (COX2); (b) inducible NO synthase (iNOS); (c) interferon γ (IFNγ). Values are means for six control rats, seven Golden Delicious apple-fed rats or seven Marie Ménard apple-fed rats, with standard errors represented by vertical bars. Mean value was significantly different from that of the control group: * P < 0·05, ** P < 0·01. † Mean value was marginally different from that of the control group (P = 0·057).
Discussion
Several studies have suggested that phytochemicals contained in apples can prevent some important human diseases. In vitro (Reference Veeriah, Hofmann and Glei42) and in vivo experiments(Reference Gossé, Guyot and Roussi43) suggest that apple extracts may have a protective role against colorectal cancer. Also, apple polyphenols can alleviate the symptoms of allergic rhinitis(Reference Enomoto, Nagasako-Akazome and Kanda44) and reduce the risk of type 2 diabetes(Reference Song, Manson and Buring45).
Apples may contain high amounts of polyphenols, depending not only on the cultivar but also on the harvesting, storage and processing steps. The bioavailability of polyphenols differs greatly from one compound to another. A recent review of the ninety-seven published studies focused on the bioavailability of polyphenols(Reference Manach, Williamson and Morand46) reported that the plasma concentrations of total metabolites may range from 0 to 4 μmol/l after ingestion of 50 mg of aglycone equivalents.
Regarding the high-molecular-weight compounds, although it has been reported that oligomers from apples are absorbed from the digestive tract and present in rat plasma(Reference Shoji, Masumoto and Moriichi47), polymeric proanthocyanidins are poorly absorbed in the gastrointestinal tract. However, it is possible that their beneficial effects at the intestinal level may not require efficient absorption through the gut be mediated by direct activity on the intestinal mucosa and/or indirect effect by varying the intestinal microbiota.
We tested the ability of lyophilised samples of two apple cultivars added to the diet to control colitis in an experimental inflammation model (HLA-B27 rats). The rationale for testing Marie Ménard apples, which are now used only for cider production, was based on their particularly high content of proanthocyanidins and hydroxycinnamic acids. It has been reported that proanthocyanidins modulate the expression of inflammatory genes such as COX2 and inducible NO synthase in murine macrophages stimulated with endotoxins(Reference Hou, Masuzaki and Hashimoto48, Reference Terra, Valls and Vitrac49); moreover, rats fed Arabidopsis thaliana seeds containing proanthocyanidins show a down-regulation of genes associated with the inflammatory response in the colon mucosa(Reference Luceri, Giovannelli and Pitozzi40). Hydroxycinnamic acids, such as p-coumaric and ferulic acid, can also control the expression of COX2 in experimental inflammation in rodents(Reference Sudheer, Muthukumaran and Devipriya50, Reference Luceri, Guglielmi and Lodovici51). Recently, we observed that lyophilised Marie Ménard apples administered to Wistar rats for 4 weeks down-regulated genes associated with inflammatory responses in the colon mucosa (C Luceri, unpublished results), suggesting a possible anti-inflammatory activity of this apple variety.
In the present study, rats fed lyophilised Marie Ménard apples had significantly fewer histological signs of colitis, less diarrhoea and a reduced colon mucosa myeloperoxidase activity. These effects were not related to the antioxidant activity of apple components, since DNA oxidative damage did not vary relative to controls by the administration of apples.
By gene expression profiling of the colon mucosa, we identified many down-regulated pathways, most of which were interconnected, such as the PG synthesis cascade, TNFα–NF-κB signalling and the epidermal growth factor receptor (EGFR) pathway. COX2 is a key regulator of inflammation, pivotal to the induction of chemically induced colon cancer(Reference Müller-Decker and Fürstenberger52). Although the role of prostaglandins in intestinal inflammation is not totally clear, several eicosanoids increase in IBD and a correlation exists between their tissue levels and disease activity(Reference Hendel and Nielsen53); moreover 5-aminosalicylic acid is widely used in the treatment of IBD and is believed to act through the inhibition of the activation of transcription factor NF-κB(Reference Bantel, Berg and Vieth54). TNFα is a pleiotropic cytokine that regulates many physiological processes, such as inflammation, proliferation and cell death, and exerts these effects by activating multiple downstream effectors including NF-κB. EGFR signalling has also emerged as an important therapeutic target. EGFR can be activated by several ligands and these interactions result in the activation of several downstream pathways including phosphoinositol-3-kinase–Akt; activated Akt phosphorylates a number of downstream targets and transcription factors such as NF-κB(Reference Talapatra and Thompson55). NF-κB activates the transcription of a wide spectrum of pro-inflammatory genes with consequent production of cytokines, adhesion molecules and enzymes and is involved also in cell proliferation and apoptosis. Inhibitors of NF-κB activation have been shown to be potent anti-inflammatory agents(Reference Roman-Blas and Jimenez56, Reference Peterson and Guttridge57). Apple extracts, at high concentrations (200–2000 nm), inhibit the TNFα signal via NF-κB in human umbilical vein endothelial cells(Reference Yoon and Liu58) and in human breast cancer MCF-7 cells at a dose of 5 mg/ml(Reference Davis, Polagruto and Valacchi59). Therefore, it is possible that some of the effects observed in the present study may be related to the inhibition of NF-κB activation.
Improvement in colitis in HLA-B27 rats was associated with changes in the faecal microbiota; principally a reduction in B. fragilis group in animals fed Marie Ménard apples. This observation is consistent with previous findings showing that the addition of B. vulgatus to a cocktail of five anaerobic bacteria in germ-free HLA-B27 rats generates serious colitis, whereas administration of the same bacterial cocktail without B. vulgatus has no pro-inflammatory effect(Reference Rath, Wilson and Sartor60). Another study in mice showed that B. fragilis exerts immunomodulatory activity on the intestine, inducing the transition from CD4+ CD45Rbhigh to CD4+ CD45Rblow subsets through polysaccharide A activity(Reference Mazmanian, Round and Kasper61). In our experiments, the anti-inflammatory activity of Marie Ménard apples was associated with reduced B. fragilis group in the faeces. The interaction of Bacteriodes with intestinal inflammatory processes is complex and probably depends on the particular inflammation model studied. However, faecal Bacteriodes variations could influence intestinal inflammation.
The aim of the present study was to test a possible anti-inflammatory effect of the whole apple added to the diet as lyophilised powder. The experimental approach mimics the effect of adding whole apple fruit to the human diet. This approach did not allow identification of single bioactive components. The effects we observed are probably due to a combination of different phytochemicals, including polyphenols, stilbenoids, lignans, triterpenoids and other constituents such as starches or fibres. We do not know at present which specific components of the different apple varieties are responsible for the anti-inflammatory effects. However, we did observe a strong effect in animals fed Marie Ménard apples. Considering that the main differences between the two apple cultivars are related to the proanthocyanidin and hydroxycinnamic acid content, we can infer that these compounds are probably the mediators of the anti-inflammatory effect.
In conclusion, the administration of Marie Ménard apples, now used only in the manufacturing of cider, ameliorates spontaneous colon inflammation in HLA-B27 transgenic rats. If effective also in humans, a simple treatment with lyophilised extracts of specific apple varieties might improve the quality of life of millions of individuals affected by IBD.
Acknowledgements
The authors thank Val-de-Vire Bioactives for their kind gift of apple fibres, G. Le Bail for lyophilised apple preparation, Professor Alessandra Renieri and Dr Filomena Papa for technical support for the scanning of microarray images, and Mary C. Forrest for linguistic revision of the manuscript.
The present study was supported by the European Network of Excellence in Nutrigenomics, NuGO (FOOD-CT-2004-506360) (P. D., C. C., E. B. and C. L.), the European Union programme FLAVO (FOOD-CT-2004-513960) (V. P.), the Ministero dell'Università e della Ricerca Scientifica e Tecnologica, Italy, and the University of Florence, Italy. S. G. was partly funded by the Nutrigenomics Consortium of the Top Institute Food and Nutrition and by the Dutch BSIK Fund.
C. C., C. L. and P. D. conceived of and designed the experiments. The following authors performed the experiments: C. L. and E. B. (diets and animal treatment); C. L. (microarray and RT-PCR); E. B. and M. L. (8-hydroxy-2′-deoxyguanosine); L. G. and V. P. (comet assay); L. M. (histopathology); C. C., R. M. and E. G. Z. (faecal microbiota analysis); G. C., A. P. F. and M. S. (proliferative activity); C. M. G. C. R. and A. B. (apple powder production and characterisation). The statistics were analysed by S. T.; C. C., S. G., L. E. and C. T. E. analysed the bioinformatics. C. C., C. L. and P. D. wrote the paper.
C. C. and C. L. contributed equally to the present study.
The authors state that there are no conflicts of interest.








