Red meat refers to3 all mammalian muscle meat, including, beef, veal, pork, lamb, mutton, horse and goat, which may be minced or frozen, and are usually consumed cooked(1,Reference Bouvard, Loomis and Guyton2) . Processed meat refers to any meat (including red meat and poultry, offal or meat by-products such as blood) that has been transformed through one or several of the following processes: salting, curing, fermentation, smoking or other processes to enhance flavour or improve preservation(1,Reference Bouvard, Loomis and Guyton2) . The history of meat consumption in humans may date back to the end of the last ice age, 10 000 to 12 000 years ago(Reference Smil3,Reference Potter John4) . Currently, meat is a common part of the daily diet, especially in Western countries. In developed countries such as the USA, Australia and New Zealand, people consume approximately 110–120 kg of meat/year(Reference Potter John4). Recently, a global survey of animal source food consumption across 185 countries found that between 1990 and 2018, mean unprocessed red meat and processed meat intake per person increased globally by 88·1 % and 152·8 %, respectively, and the increase of unprocessed red meat intake in China was largest by 312·5 % (equivalent to an additional 5·89 servings/week) due to increased pork consumption(Reference Miller, Reedy and Cudhea5). According to the FAO of the United Nations, world meat production is projected to double by 2050, most of which is expected in developing countries(6).
Until now, the association between red/processed meat consumption and human health has been widely documented in epidemiological studies and systematic reviews. High red/processed meat consumption was associated with a range of harmful outcomes, especially in chronic non-communicated disease, including CVD, type 2 diabetes mellitus (T2DM)(Reference Neuenschwander, Ballon and Weber7) and many types of tumors(Reference Chao, Thun Michael and Connell Cari8,Reference Ferro, Rosato and Rota9) . Recently, Huang et al. conducted an umbrella review to summarise the associations of red/processed meat intake and several cancer outcomes(Reference Huang, Cao and Chen10). However, there were few publications that overly evaluated the existing evidence of red/processed meat consumption and non-cancer-related outcomes. Considering the large consumption of red/processed meat, we conducted an umbrella review to systematically collect and evaluate data on red/processed meat consumption and non-cancer-related outcomes and provide comprehensive evidence(Reference Papatheodorou11).
Methods
Umbrella review
Umbrella review is a useful tool to help us systematically understand the knowledge of specific topics, which can provide a comprehensive overview of evidence of existing systematic reviews and meta-analyses about a topic area(Reference Aromataris, Fernandez and Godfrey12,Reference Yi, Wu and Zhuang13) . We carried out an umbrella review of red meat and processed meat consumption on diverse health-related outcomes by retrieving comprehensive evidence. The umbrella review has been registered in PROSPERO (CRD 42021218568).
Literature research
We searched PubMed, Embase, Web of Science and the Cochrane Library for related studies from inception to February 2022. The search strategy was as follows: (red meat OR processed meat OR beef OR veal OR pork* OR lamb OR mutton OR ham OR sausage* OR bacon OR frankfurter*) AND (systematic review* OR meta-analys*), and the terms were truncated for all fields. The references included in each eligible meta-analysis were also searched by hand. The search strategies are shown in Supplementary Table 1. The processes of literature retrieval were performed by two authors independently following predefined eligibility criteria. Any discrepancies were resolved by consensus or involved in the third one.
Eligibility criteria
Whether a meta-analysis was eligible for our umbrella review or not depended on the following criteria: (1) Systematic reviews with meta-analysis (quantitative analysis) of observational studies or interventional studies evaluated the associations of red and/or processed meat with non-cancer-related outcomes in human beings; (2) the pooled relative risk, hazard risk and odds risk for observational studies and the mean difference and weight mean difference for meta-analyses of interventional studies were reported and (3) published in English language. Meta-analysis with total red meat (refers to no processed red meat and processed red meat), red meat (refers to no processed red meat) and processed meat intake (refers to processed red meat or white meat such as chicken) was included, while meta-analysis with mixed types of meat consumption (for example, red meat and white meat were not discussed separately) was excluded. Meta-analyses on biological indicators such as blood lipids rather than health-related outcomes were excluded. Systematic reviews without quantitative analysis were excluded as well. Any disagreements were resolved by discussion.
Data extraction
Two researchers extracted the data independently. The following information in each eligible meta-analysis was recorded: health-related outcomes, mane of first author, publication year, types of meat (red/processed), number and designs (randomised controlled trial/cohort/case–control/cross-section) of studies included in each meta-analysis, number of total participants in each meta-analysis, population, dose–response analysis, period of follow-up, effects model (random/fixed), metric, the pooled estimates and 95 % confidential interval (95 % CI), heterogeneity of each outcome and publication bias. When a meta-analysis reported more than one outcome, or a meta-analysis included various meats, we extracted them one by one. If two or more meta-analyses investigated the same health outcome, the one with the highest quality was included.
Assessment of methodological quality and quality of evidence of included meta-analysis
We used the AMSTAR 2 (Revised AMSTAR: A Measurement Tool to Access Systematic Reviews) to assess the methodological quality of each involved meta-analysis, which was a critical appraisal tool for systematic review of observational or interventional study consisting of sixteen items including seven critical domains and grades the methodological quality of each meta-analysis as ‘high’, ‘moderate’, ‘low’ and ‘critically low’ based on detailed and specific explanations of bias(Reference Shea, Reeves and Wells14,15) . The Nutri-GRADE (Grading of Recommendations Assessment, Development and Evaluation, GRADE) system was used to assess the quality of evidence for the included meta-analysis, which was modified from GRADE by Lukas et al. (Reference Schwingshackl, Knüppel and Schwedhelm16) to rate the certainty of meta-evidence from nutritional studies(Reference Tobias, Wittenbecher and Hu17). It assorts the quality of meta-evidence from cohort studies as ‘high’ (8–10 points), ‘moderate’ (6–7·99 points), ‘low’ (4–5·99) and ‘very low’ (0–3·99) according to eight items including risk of bias, precision, heterogeneity, directions, publication bias, funding bias, effect size and dose–response(Reference Schwingshackl, Knüppel and Schwedhelm16).
Data analysis
The summary estimates and 95 % CI of each related outcome were extracted and calculated by fixed or random effects methods. We extracted the I 2 metric and Egger’s test or Begg’s test to measure the heterogeneity and publication bias if they were available. A P < 0·1 for Egger’s regression test was regarded as statistically significant publication bias. If the total estimate effects were not reported, we chose the outcomes derived from cohort rather than case–control or cross-sectional studies because of the strengths of the study design. We did not reanalyse other data or primary studies included in the meta-analysis.
Results
Characteristics of the meta-analyses
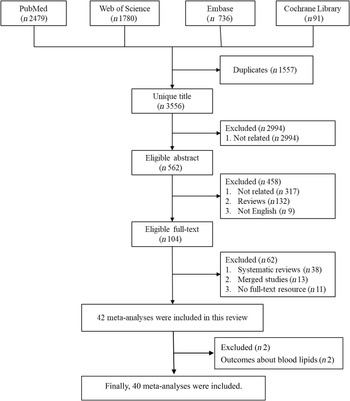
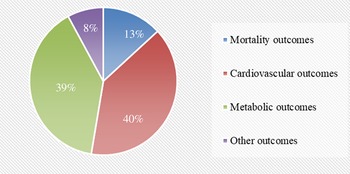
We searched PubMed, Embase, Web of Science and the Cochrane Library to identify data investigating the association of red and/or processed meat consumption and non-cancer-related outcomes in humans. The search yielded forty-two meta-analyses related to the topic. The other two meta-analyses(Reference O’Connor, Kim and Campbell18,Reference Guasch-Ferre, Satija and Blondin19) of randomised controlled trials were excluded in the main text because the outcome indicators were blood lipids (online Supplementary Table 2). Finally, forty meta-analyses of observational studies with fifty-four unique outcomes were included in the umbrella review. The processes and results of systematic selection are shown Fig. 1. Thirteen meta-analyses exploring the associations of meat consumption and health outcome were excluded because all of them failed to specify the kinds of meat. The associations between red/processed meat and all non-cancer-related outcomes are available in Supplementary Table 2. The map of outcomes associated with red/processed meat consumption is shown in Fig. 2.

Fig. 1. The flow chart of selection process.

Fig. 2. Map of outcomes associated with red/processed meat consumption.
Mortality
Red meat consumption was related to an 11 % accession in CVD mortality risk (95 % CI (1·09, 1·14))(Reference Zeraatkar, Han and Guyatt20). Processed meat consumption was associated with a higher risk of all-cause mortality (1·09; 95 % CI (1·04, 1·10))(Reference Zeraatkar, Han and Guyatt20), CVD mortality (1·11; 95 % CI (1·03, 1·19))(Reference Zeraatkar, Han and Guyatt20) and cancer mortality (1·08; 95 % CI (1·06, 1·11))(Reference Wang, Lin and Ouyang21). Dose–response analysis showed that one serving/d consumption of processed meat was related to a 15 % higher risk of all-cause mortality (95 % CI (1·11, 1·19))(Reference Wang, Lin and Ouyang21). The associations of red/processed meat consumption and mortality are shown in Table 1.
Table 1. Associations between red/processed meat consumption and mortality

MA, meta-analysis; RMT, red meat; PMT, processed meat; RR, risk ratio; HR, hazard ratio; NR, not report; VS, verse.
Cardiovascular outcomes
High red meat intake was related to an increased risk of ischaemic heart disease (1·09; 95 % CI (1·06, 1·12)(Reference Papier, Knuppel and Syam22), CHD (1·16; 95 % CI (1·08, 1·24)(Reference Bechthold, Boeing and Schwedhelm23), stroke (1·16; 95 % CI (1·08, 1·25)(Reference Bechthold, Boeing and Schwedhelm23), hypertension (1·15; 95 % CI (1·02, 1·28)(Reference Schwingshackl, Schwedhelm and Hoffmann24) and heart failure (1·12; 95 % CI (1·04, 1·21))(Reference Bechthold, Boeing and Schwedhelm23). In the dose–response analysis, each additional daily 100 g red meat was positively associated with a 15 % increased risk of CHD, 14 % of hypertension(Reference Schwingshackl, Schwedhelm and Hoffmann24), 12 % of stroke and 8 % of heart failure(Reference Bechthold, Boeing and Schwedhelm23).
Processed meat consumption was associated with an increased risk of CVD, with an estimated relative risk of 1·23; 95 % CI (1·07, 1·41)(Reference Cui, Liu and Zhu25) for heat failure, 1·18; 95 % CI (1·12, 1·25)(Reference Papier, Knuppel and Syam22) for ICH, 1·16; 95 % CI (1·07, 1·26)(Reference Bechthold, Boeing and Schwedhelm23) for stroke and 1·12; 95 % CI (1·02, 1·23)(Reference Zhang and Zhang26) for hypertension. Dose–response analysis revealed that more than 50 g processed meat intake/d was associated with a higher risk of CHD (1·27; 95 % CI (1·09, 1·49)(Reference Bechthold, Boeing and Schwedhelm23), stroke (1·17; 1·02, 1·34)(Reference Bechthold, Boeing and Schwedhelm23), heart failure (1·12; 95 % CI (1·05, 1·19)(Reference Bechthold, Boeing and Schwedhelm23) and hypertension (1·12; 95 % CI (1·00, 1·26))(Reference Schwingshackl, Schwedhelm and Hoffmann24). The associations between red/processed meat consumption and CVD are shown in Table 2.
Table 2. Associations between red/processed meat consumption and cardiovascular outcomes

MA, meta-analysis; IHD, ischaemic heart disease; HF, heart failure; RR, risk ratio; HR, hazard ratio; NR, not report; VS, verse.
Metabolic outcomes
High red meat consumption was related to a higher risk of metabolic syndrome (1·32; 95 % CI (1·14, 1·54))(Reference Guo, Ding and Liang27), abdominal obesity (1·18; 95 % CI (1·06, 1·32))(Reference Schlesinger, Neuenschwander and Schwedhelm28), T2DM (1·15; 95 % CI (1·08, 1·23))(Reference Zhang, Fu and Moore29) and non-alcoholic fatty liver disease (1·12, 95 % CI (1·04, 1·21))(Reference He, Li and Guo30). Dose–response showed that a 100 g/d increase in red meat was associated with a 17 % increased risk of T2DM (95 % CI (1·08, 1·26))(Reference Schwingshackl, Hoffmann and Lampousi31), a 10 % higher risk of abdominal obesity(Reference Schlesinger, Neuenschwander and Schwedhelm28) and a 14 % increased risk of weight gain(Reference Schlesinger, Neuenschwander and Schwedhelm28).
Processed meat intake was associated with a higher risk of metabolic syndrome (1·48; 95 % CI (1·11, 1·97)(Reference Guo, Ding and Liang27), T2DM (1·27; 95 % CI (1·15, 1·40)(Reference Zhang, Fu and Moore29), obesity (1·82; 95 % CI (1·69, 1·97)(Reference Daneshzad, Askari and Moradi32) and abdominal obesity (8·8; 95 % CI (1·20, 64·28))(Reference Schlesinger, Neuenschwander and Schwedhelm28). Dose–response analysis found that the risk of T2DM increased by 37 %, 95 % CI (1·22, 1·55))(Reference Schwingshackl, Hoffmann and Lampousi31) for each 50 g/d increment in processed meat consumption. The associations between red/processed meat consumption and metabolic outcomes are shown in Table 3.
Table 3. Associations between red/processed meat consumption and metabolic and other outcomes

MA, meta-analysis; BE, Barrett’s oesophagus; WG, weight gain; T2DM, type 2 diabetes mellitus; MS, metabolic syndrome; COPD, chronic obstructive pulmonary disease; IBD, Inflammatory bowel disease; WC, waist circumference; AO, abdominal obesity; NAFLD, non-alcoholic fatty liver disease; RR, risk ratio; HR, hazard ratio; MD, mean difference; NR, not report.
* Number of cases.
Other outcomes
The highest red meat intake was associated with a higher risk of inflammatory bowel disease (2·37; 95 % CI (1·40, 3·99))(Reference Ge, Han and Liu33). In addition, there was a significant association between processed red meat consumption and the risk of COPD (hazard risk: 1·40; 95 % CI (1·21, 1·62))(Reference Salari-Moghaddam, Milajerdi and Larijani34). Linear dose–response analysis showed that each 50 g/week increase in processed red meat intake was associated with an 8 % higher risk of COPD (1·08; 95 % CI (1·03, 1·13))(Reference Salari-Moghaddam, Milajerdi and Larijani34) (Table 3).
Heterogeneity
In all the included studies, approximately 23·3 % of the meta-analyses had lower heterogeneity, with I 2 < 25 %; approximately 28·4 % of the meta-analyses had moderate heterogeneity, with I 2 between 25 % and 75 %; and 23·3 % of meta-analyses had high heterogeneity, with I 2 > 75 %. In addition, approximately 5 % of the results were derived from a single study; therefore, heterogeneity does not apply. However, 20 % of studies did not report heterogeneity, and we could not reanalyse because of the unavailability of information. The heterogeneity of each meta-analysis may be influenced by geographical and demographic factors, the difference in parades, the measurement of meat consumption, the volume of meat consumption and the time of follow-up and the evaluation of the results (online Supplementary Table 2).
Publication bias
Funnel plots, Egger’s test and Begg’s test were used in this umbrella. Approximately 58·3 % of studies reported that there were no publication biases. Two meta-analyses found significant evidence for publication biases in studies of meat consumption and metabolic syndrome(Reference Guo, Ding and Liang27) (P = 0·07) and waist circumference(Reference Rouhani, Salehi-Abargouei and Surkan35) (P = 0·052). The other meta-analysis did not report the outcomes of publication bias due to the insufficient number of studies.
The methodological quality of included meta-analyses
The results of the methodological quality assessment are shown in Table 4 (AMSTAR-2). The retrieved meta-analyses were rated as four levels: 45·0 % were rated as ‘high’, approximately 2·5 % were rated as ‘moderate’, approximately 32·0 % were rated as ‘low’ and 20·0 % were classified as ‘critically low’. The reason was that most studies failed to report the funding sources of the single article included in each meta-analysis (Item 10 of AMSTA-2).
Table 4. Results of AMSTAR-2 and Nutri-GRADE

AMSTAR, a measurement tool to access systematic reviews; Nutri-GRADE, the grading of recommendations assessment, development, and evaluation for nutrition research; ICH, ischaemic heart disease; NAFLD, non-alcoholic fatty liver disease; COPD, chronic obstructive pulmonary disease; IBD, Inflammatory bowel disease.
The quality of the meta-evidence
The results of quality of the meta-evidence are shown in Table 4 (Nutri-GRADE), and the detail scores of items in Nutri-GRADE are shown in Supplementary Table 3. Approximately 47·5 % were graded as ‘moderate’, 17·5 % were graded as ‘low’ and 35·0 % were graded as ‘very low’. None of the associations was stratified as ‘high’. The main reason was that many of those outcomes came from sub-group analysis with a limited number of studies and resulted in 0 points in the items 3 and 5. In addition, many meta-analyses failed to conduct dose–response analysis, and the effect size was limited.
Discussion
Main findings of the umbrella review
A total of forty meta-analyses of observational studies with forty unique health-related outcomes were included in our umbrella review. Red and processed meat consumption likely did more harm than benefits for a variety of non-cancer-related outcomes in this umbrella review. Red meat, especially processed meat consumption, was associated with an increased risk of all-cause mortality, CVD and metabolic outcomes.
Red/processed meat consumption was associated with an increased risk of all-cause mortality and cause-specific mortality in our umbrella review, which is consistent with the risk of CVD and cancer. Recently, a cohort study(Reference Zhong, Van Horn and Greenland36) with 29 682 participants found that red meat and processed meat consumption was significantly associated with all-cause mortality (adjusted hazard risk, 1·03 (95 % CI (1·01, 1·05); adjusted hazard risk, 1·03 (95 % CI (1·02, 1·05), respectively). This may be because the major cause of all-cause mortality is likely to be a combination of CVD and cancer aetiology(Reference Kwok, Gulati and Michos37). The carcinogenic effects of red and processed meat have been well investigated in both epidemiological and laboratory studies(Reference Huang, Cao and Chen10,Reference Gamage, Dissabandara and Lam38–Reference Soladoye, Shand and Dugan41) . The International Agency for Research on Cancer classified the consumption of processed meat as ‘carcinogenic to humans’ (Group 1) and red meat as ‘probably carcinogenic to humans’ (Group 2A) in 2015(Reference Bouvard, Loomis and Guyton42).
High red/processed meat consumption was related to an increased risk of CVD, including CHD/(ischaemic heart disease) stroke, hypertension and heart failure. Red and processed meat consumption was associated with several non-communicable diseases, including hypertension, diabetes and vascular depression(Reference Willett, Rockström and Loken43). Recently, many prospective cohort studies have shown consistent results(Reference Al-Shaar, Satija and Wang44,Reference Key, Appleby and Bradbury45) . Several mechanisms may contribute to the adverse effect of red/processed meat intake on CVD risk: (1) Red meat is high in saturated fat and cholesterol, and consumption of red meat has been linked to higher levels of LDL-cholesterol in the blood(Reference Guasch-Ferré, Satija and Blondin46). If the concentration of LDL-cholesterol in the blood increases, it will be deposited in the arterial wall of the blood vessels in the heart and brain and gradually form atherosclerotic plaques, which will block the corresponding blood vessels, and cause serious diseases such as stroke and peripheral arterial disease; (2) heme Fe, which is abundant in red meat, has been established and was associated with an increased risk of CVD. A study showed that each 1 mg/d increase in heme Fe intake was associated with a 7 % increase in the risk of CVD (95 % CI (1·01, 1·14))(Reference Fang, An and Wang47). Excess heme iron might catalyse several cellular reactions, thus increasing the levels of oxidative stress(Reference Rajpathak, Crandall and Wylie-Rosett48), and leading to enhanced lipid peroxidation, protein modification and DNA damage(Reference Rajpathak, Crandall and Wylie-Rosett48,Reference Hori, Mizoue and Kasai49) ; (3) high salt and Na were the conceived factors for hypertension(Reference He, Li and Macgregor50), and the high sodium and salt content of processed meat may increase blood pressure, which was associated with a higher risk of CVD. Studies have shown that a reduction in salt intake will likely lower population BP and, thereby, reduce cardiovascular disease(Reference He, Li and Macgregor51). The largest differences between processed and unprocessed meat are sodium and nitrates, which are 400 % and 50 % higher/g of meat, respectively(Reference Micha, Michas and Mozaffarian52); and (4) in addition, L-carnitine(Reference Koeth, Wang and Levison53), sialic acid N-glycolylneuraminic acid(Reference Kawanishi, Dhar and Do54) in red meat and preservatives in processed red meat, such as nitrates and nitrate by-products, contribute to the risk of CVD such as atherosclerosis, endothelial dysfunction and insulin resistance(Reference Al-Shaar, Satija and Wang44,Reference Förstermann55) .
High red/processed meat consumption was associated with T2DM, metabolic syndrome, obesity and other metabolic outcomes in the umbrella review. For more than a decade, epidemiological studies have shown that a Western diet characterised by high consumption of red and processed meats is related to a higher risk of T2DM both in men(Reference van Dam, Rimm and Willett56) and women(Reference Schulze, Manson and Willett57). There are several possible mechanisms: (1) Clinical trials and animal models have shown that the ingredients and metabolites of red/processed meat include saturated fatty acids (SFA), sodium, advanced glycation end products (AGEs), nitrates/nitrites, heme iron, trimethylamine N-oxide (TMAO), branched amino acids (BCAAs) and endocrine disruptor chemicals (EDCs), which play a role in the development of T2DM by increasing insulin resistance and other pathways(Reference Kim, Keogh and Clifton58); (2) Red meat is a major source of heme iron, which is a strong pro-oxidant that leads to increased levels of oxidative stress, which can lead to tissue damage, particularly pancreatic beta cells, and therefore increase the risk of T2DM(Reference Li, Wang and Lu59); (3) Several studies have shown that a high intake of dietary protein has negative effects on glucose homeostasis by facilitating insulin resistance and increasing gluconeogenesis; and (4) saturated fatty acids may contribute to the aetiology of metabolic disorders(Reference Storlien, Hulbert and Else60). The main compounds of red meat such as iron, nitrites and Na, from processed red meat have been proven to be related to the risk of MetS(Reference de Oliveira Otto, Alonso and Lee61,Reference Altamura, Müdder and Schlotterer62) . In addition, Choi et al. found an association between the presence of the minor alleles of rs662799 and high red and processed meat consumption and the incidence of MetS in Korean adults(Reference Kokkinopoulou, McGowan and Brogan63).
Strengths and limitations
The umbrella review systematically summarised the current evidence for red meat and processed meat intake and a series of non-cancer-related outcomes in humans. The AMSTAR-2 and Nutria-GRADE were used to assess the quality of methods and the evidence for each meta-analysis. However, several possible limitations should be considered. The meta-analysis with pooled analysis was included, and systematic reviews without meta-analyses were omitted, which would have impacts on the outcomes. In addition, most of the outcomes came from observational studies, which may limit the power of the association effect for each outcome due to heterogeneity and bias across studies. Besides, this umbrella review emphasised the association of red/processed meat intake and non-cancer-related health outcomes, and the cancer-related outcomes were omitted because they have been well discussed. Last but not least, we are unable to compare the differences in the effects of unprocessed red meat or processed red meat on human health at the same serving size according to existing literature because the amount of red meat consumption is mostly 100 g and that of processed meat is mostly 50 g in all of the included meta-analyses. Obviously, this is a great idea to compare the differences of the two at the same serving size in future studies.
Conclusions
Red and processed meat consumption is positively associated with a higher risk of several non-cancer-related outcomes in this umbrella review. Reduction of red meat, especially processed red meat consumption, should be taken into consideration when developing nutrition-related policies, which will be of great public health importance. However, more additional randomised controlled trials are warranted to clarify the causality.
Acknowledgements
We are highly indebted to Yuying Zhang for providing guidance in discussion.
This work was supported by National Natural Science Foundation of China (grant number 71974135).
X. Z. formulated the research question and wrote the article; K. L. and Y. Z. designed the study and improved help in interpreting the findings; D. L. gave a hand in analysing the data; S. L. and J. Y. gave help in carrying out the study.
There are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114522003415