Hypercholesterolaemia, specifically elevated LDL-cholesterol concentrations, represent a potent risk factor for the development of CVD(1). Increasingly, individuals are integrating specific cholesterol-lowering foods and supplements into a routine of diet modification and exercise as primary prevention of hypercholesterolaemia(Reference Varady, Ebine and Vanstone2). The unique nutritive composition of the red algae Porphyridium cruentum (PC), including water-soluble sulphated polysaccharides and long-chain PUFA, has been proposed to possess lipid-lowering activity(Reference Rebolloso Fuentes, Acién Fernández and Sánchez Pérez3).
Dietary consumption of red algae is common in Asian countries, while North American and European food industries utilise polysaccharide components of red algae from the Rhodophyta family, to which PC belongs, as stabilisers, fillers or colourants(Reference Dufosse, Galaup and Yaron4). The PC biomass has already been shown to exhibit antioxidant properties within in vitro cell culture models(Reference Tannin-Spitz, Bergman and van-Moppes5). These properties appear to be related to the combination of glycoprotein and sulphated carbohydrate content as well as their unique polymeric structure. Sulphated carbohydrates have also been shown in increase colonic mucous secretion in rats(Reference Shimotoyodome, Meguro and Hase6), a physiological function that may increase faecal output and decrease intestinal transit time. Furthermore, a number of micro-organisms in the human colon are known to ferment indigestible polysaccharides of algal origin(Reference Salyers, Vercellotti and West7). The by-products of this fermentation can impact the colonic micro-environment, changing in redox state and pH(Reference Salyers, Vercellotti and West7). Additionally, PC total fatty acid content has a high proportion of the long-chain PUFA, arachidonic acid (20 : 4n-6) and EPA (20 : 5n-3)(Reference Rebolloso Fuentes, Acién Fernández and Sánchez Pérez3, Reference Guil-Guerrero, Belarbi and Rebolloso-Fuentes8, Reference Vazhappilly and Chen9), both known to affect circulating TAG concentrations, albeit in opposite fashions(Reference Holub10, Reference Holub11). Accordingly, we expect that changes in circulating TAG could result when PC is used as a dietary supplement on regular basis.
In a feeding study in chickens exploring the potential of PC biomass to reduce egg yolk cholesterol concentrations, the authors observed a decrease in circulating cholesterol concentrations(Reference Ginzberg, Cohen and Sod-Moriah12). Similarly, a feeding study conducted in rats showed a link between consumption of PC and decreased circulating cholesterol concentrations(Reference Dvir, Chayoth and Sod-Moriah13). Isolated sulphated polysaccharide fraction from the PC had the most pronounced effect on plasma cholesterol concentrations and neutral sterols excreted in faeces(Reference Dvir, Chayoth and Sod-Moriah13). However, consumption of the whole-cell biomass from PC v. the isolated polysaccharides resulted in the largest effect on excretion of bile salts(Reference Dvir, Chayoth and Sod-Moriah13). These data indicate a discrepancy, as to what aspect of cholesterol trafficking PC consumption actually targets, given that the various fractions of the biomass appear to behave differently. Therefore, establishing how PC affects cholesterol biosynthesis becomes important in determining the mechanism behind the hypocholesterolaemic effect. Moreover, cholesterol management in hamsters is more similar to the human situation as both human subjects and hamsters carry excess cholesterol in LDL particles, unlike other rodents that carry very high concentrations of cholesterol in HDL particles.
While the data presented by Dvir et al. (Reference Dvir, Chayoth and Sod-Moriah13) suggest increased cholesterol and bile salt excretion as the major consequence of consuming the algae, the actual mechanism behind the cholesterol reductions has yet to be explored. Bioactive components of this algal biomass are likely to be absorbed and could disrupt normal cholesterol biosynthesis. Therefore, the purpose of the present study was to determine whether the cholesterol-lowering effect of PC consumption is dose dependent and how cholesterol synthesis rate is affected by the algal consumption in a diet-induced hypercholesterolaemic hamster model.
Methods and materials
Study design and animals
Sixty golden Syrian hamsters (Mesocricetus auratus; n 15) were randomised to receive either control hypercholesterolaemia-inducing diet alone, formulated in our laboratory (Table 1), or the same diet with added PC biomass. The PC was prepared for incorporation into the diet Solazyme, Inc. (San Francisco, CA, USA) by first disrupting cell integrity by microfluidisation followed by lyophilisation. The PC was then added to the diet at an increasing percentage (w/w; 2·5, 5 and 10 %) at the expense of maize starch. Table 2 illustrates the approximate increased daily intake of selected nutrients resulting from the added PC in the base diet.
Table 1 Hypercholesterolaemia-inducing diet composition
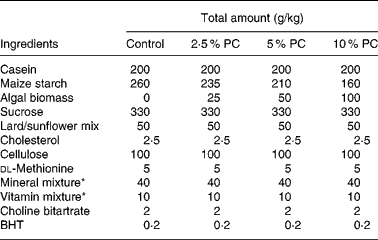
PC, Porphyridium cruentum biomass; BHT, butylated hydroxytoluene.
* Vitamin mix AIN-76A (Harlan Teklad, Madison, WI, USA) and mineral mix AIN-93M (modified for hamsters, Harlan Teklad).
Table 2 Nutrient composition of Porphyridium cruentum (PC) and approximate daily intake for specific nutrients in test diets from algal biomass (intake above control diets) based on mean daily food intake
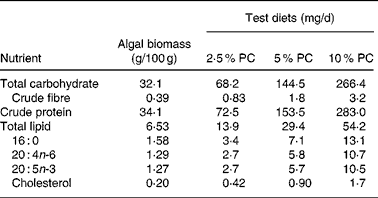
Intakes are based on mean food intakes for each treatment group and previously reported compositional data(Reference Rebolloso Fuentes, Acién Fernández and Sánchez Pérez3, Reference Volkman24).
All animals were then fed ad libitum a standardised hypercholesterolaemic diet(Reference Kassis, Marinangeli and Jain14, Reference Ntanios and Jones15) for 28 d with body weight and food consumption measured every 3 d. On day 25, energy expenditure, expressed as oxygen consumption per gram body weight, was measured by indirect calorimetry using a respiratory gas exchange system for rodents (MM-100 CWE, Inc., Pennsylvania, PA, USA). On day 28, each hamster received an intraperitoneal injection of deuterated water (0·5 ml 2H2O, 99·9 atom percent excess: CDN Isotopes, Pointe-Claire, Que., Canada) to assess fractional cholesterol synthesis(Reference Ntanios and Jones16). Exactly 2 h after injection, animals were anaesthetised with inhaled isoflurane and blood sampled by cardiac puncture. Animals were then euthanised with an overdose of sodium pentobarbital; body composition was determined immediately by dual emission X-ray absorptiometry. Liver, red blood cell (RBC) and faecal cholesterol were assayed in the 2·5 % PC group and their controls, but not in the higher intakes of PC. The study protocol was approved by the University of Manitoba Animal Care Committee in accordance to the Canadian Council on Animal Care Guidelines.
Blood chemistry
Blood was collected in heparinised tubes and separated into plasma and packed RBC by centrifugation at 1500 g. Plasma glucose, TAG, total cholesterol and HDL-cholesterol were measured using the Vitros Chemistry System 350 (Ortho-Clinical Diagnostics, Johnson & Johnson; New Orleans, LA, USA). Non-HDL-cholesterol is the difference between the measured total cholesterol and HDL-cholesterol and would include the sum of VLDL-, IDL- and LDL-cholesterol in the blood.
Erythrocyte, liver and faecal cholesterol concentrations
Approximately 0·5 g of liver, RBC and dried faeces were saponified with freshly prepared KOH–methanol at 100°C for 1 h. The non-saponifiable sterol fraction was extracted with petroleum diethyl ether and dried under N2 gas. Before analysis, internal standard α-cholestane was added to each sample. The sterol fractions of liver and RBC were analysed for cholesterol concentrations using an Agilent 6890N GC fitted with an Agilent 5975 GC/MS captured total ion current monitor. Sterol fractions of the faecal samples were dissolved in chloroform and analysed using an Agilent 6890N gas chromatograph fitted with a flame ionisation detector(Reference Jones, Raeini-Sarjaz and Ntanios17). A SAC-5 capillary column (30 m × 0·25 mm × 0·25 μm, Supelco, Bellefonte, CA, USA) was used for all of the sterol analyses.
Fractional cholesterol synthesis
Approximately 0·5 g RBC were saponified with freshly prepared KOH–methanol at 100°C for 1 h and the sterol fraction was extracted with petroleum diethyl ether(Reference Ntanios and Jones16). GC–thermal conversion–isotope ratio MS (Delta V Plus, Thermo Electron Corporation, Bremen, Germany) was then used to determine the 2H/1H ratio v. Vienna standard mean ocean water. The cholesterol precursor pool is taken as the mean plasma water 2H enrichment, which was determined by thermal conversion elemental analyser-isotope ratio mass spectrometer from plasma prepared by membrane filtration and centrifugation removing proteins >5 kDa. Cholesterol fractional synthesis rate (FSR) rates were calculated using the following equation(Reference Di Buono, Jones and Beaumier18):
where δ is 2H enrichment of cholesterol or plasma water above baseline and time refers to the 2 h 2H incorporation period. The factor 0·478 is the fraction of hydrogen atoms per cholesterol molecule possibly labelled by a 2H(Reference Di Buono, Jones and Beaumier18).
Statistical analysis
All outcomes were assessed by the generalised linear model univariate ANOVA using SPSS version 11 (SPSS, Inc., Chicago, IL, USA) including food intake as a covariate and study experiment as a fixed factor with P < 0·05 considered significant. The variance was not homogeneous for cholesterol and non-HDL-cholesterol concentrations; therefore, these variables were log transformed for statistical testing but back transformed for the purpose of reporting in this paper. All data are reported as mean values with their standard errors.
Results
Weight gain and food intake
All hamsters were fed ad libitum, gaining weight similarly, with no difference observed in the rate of weight gain between either group over time or when expressed as percentage of weight gain from start (Table 3). Food intake did not differ from control for any treatment. However, the 5 % PC group mean daily food consumption (9·0 (sem 0·2) g/d) was higher (P < 0·05) than that of the 10 % PC group (8·3 (sem 0·2) g/d).
Table 3 Weight gain, body composition, food intake and oxygen consumption for Syrian golden hamsters consuming increasing concentrations of red algae (Porphyridium cruentum (PC)) biomass
(Mean values with their standard errors)
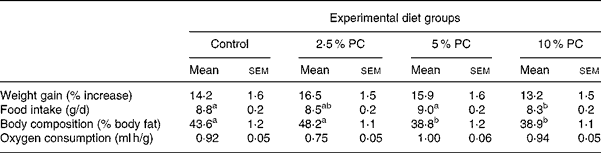
a,b Mean values with unlike superscript letters were significantly different (P ≤ 0·05).
Body composition and energy expenditure
Body composition, reported as percentage of body fat, was lower (Table 3; P < 0·001) in both the 5 % body mass and 10 % PC groups compared with controls animals. Those animals receiving 2·5 % PC were not different from control animals. Energy expenditure, reported as VO2 per gram body weight per hour, did not differ from control in either treatment group.
Blood glucose and lipid chemistry
Plasma glucose concentrations on day 28 were not different across treatments (Table 4). Plasma TAG were higher (P < 0·02) in the 5 % PC animals compared with control, but not those animals consuming 10 % PC. Total plasma cholesterol was lower (P < 0·001) in all treatment groups compared with control animals and the response was dose dependent with 14, 38 and 53 % reductions for 2·5, 5 and 10 % PC treatments, respectively. Similarly, both HDL (45 and 52 % reductions) and non-HDL (28 and 45 % reductions)-cholesterol concentrations were lower (P < 0·001) in groups consuming 5 and 10 % PC, respectively, compared with control animals.
Table 4 Blood glucose and lipid chemistry for Syrian golden hamsters consuming increasing concentrations of red algae (Porphyridium cruentum (PC)) biomass
(Mean values with their standard errors)
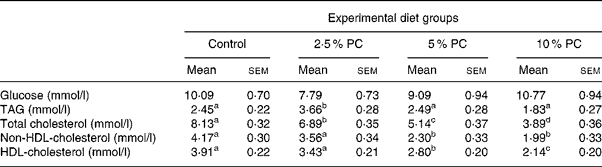
a,b,c Mean values with unlike superscript letters were significantly different (P ≤ 0·05).
Erythrocyte, liver and faecal cholesterol concentrations
There was no difference between the 2·5 % PC or controls for RBC, liver or faecal cholesterol content (Table 5).
Table 5 Sterol concentrations from red blood cells (RBC), liver and faeces of hamsters consuming 2·5 % (w/w) of red algae (Porphyridium cruentum (PC)) biomass
(Mean values with their standard errors)
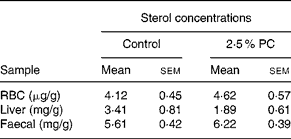
RBC cholesterol n 13–14, liver cholesterol n 8–10, faecal sterols n 8.
Fractional cholesterol synthesis
Higher (P < 0·005) cholesterol FSR was observed in the 10 % PC animals v. the 5 % PC animals, with only a marginally higher FSR (P = 0·08) compared with the control animals. There were no differences between the 2·5 or 5 % supplemented group and controls (Fig. 1).
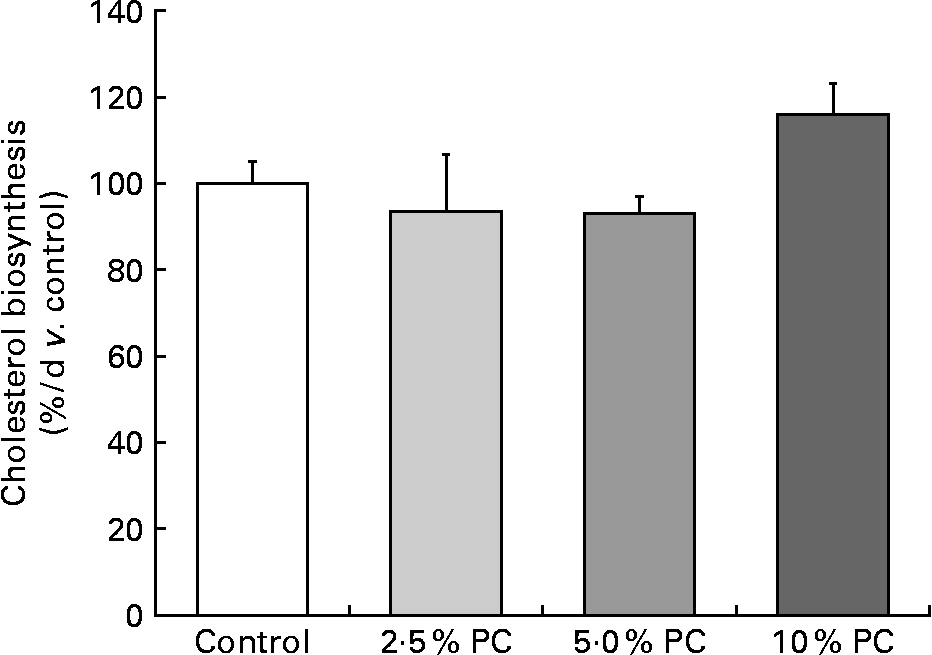
Fig. 1 Cholesterol fractional synthesis (% v. control) from male Syrian golden hamsters with increasing doses of dietary Porphyridium cruentum biomass. Values are mean values with their standard errors, n 13–15. PC, Porphyridium cruentum biomass.
Discussion
The present primary objective was to determine whether the consumption of whole-cell biomass from PC would lower blood lipids in a dose-dependent manner in the present dietary-induced hypercholesterolaemic hamster model. Indeed, total plasma cholesterol was reduced at all three PC concentrations, 14, 38 and 53 %, respectively. There were also separate dose-dependent reductions in the level of HDL- and non-HDL-cholesterol for the 5 and 10 % PC consumption levels. Decreases in non-HDL- and HDL-cholesterol have been noted for functional foods and nutraceuticals such as plant sterols and fibres and appear to be due to efflux of cholesterol from the periphery to the liver in response to decreasing hepatic cholesterol level, not changes in LDL production(Reference Ntanios and Jones15, Reference Rideout, Harding and Jones19). This decrease in HDL-cholesterol, while not desirable, does not outweigh the beneficial aspect of lowering total and non-HDL-cholesterol, which is primarily LDL-cholesterol. The present findings support those of other researchers who have demonstrated a similar response in rats consuming both PC biomass and a purified polysaccharide derived from PC(Reference Dvir, Chayoth and Sod-Moriah13). However, the rat does not model the human hypercholesterolaemic condition as well as the hamster. Similarly, a 28 % reduction in blood total cholesterol concentrations was reported in chickens consuming 10 % Porphyridium sp. for 10 d compared with controls receiving standard diet(Reference Ginzberg, Cohen and Sod-Moriah12).
The present second objective was to address whether PC dietary supplementation reduced cholesterol synthesis rate as the mechanism behind its ability to lower blood cholesterol. The present data for cholesterol FSR indicate that the decrease in circulating cholesterol concentrations were not due to reduced synthesis, as is the case with cholesterol lowering by statins(Reference Maron, Fazio and Linton20). In fact, cholesterol FSR was observed to be higher in the 10 % PC-supplemented group, most likely a compensatory increase given the lower circulating concentrations that indirectly indicate lower hepatic intracellular cholesterol concentrations. Furthermore, it has been shown recently that the regulatory controls on the processing of SREBP-2 favouring cholesterol biosynthesis are affected by very small changes in endoplasmic reticulum cholesterol concentrations(Reference Radhakrishnan, Goldstein and McDonald21). Therefore, because cholesterol FSR was not decreased and actually increased at the highest levels of PC intake, we believe that the mechanism behind PC lowering of blood cholesterol is either related to a fibre-like situation of cholesterol excretion or the possibility that a particular component of the algae is affecting cholesterol transporters in favour of efflux from the liver and gastrointestinal tissues into the lumen and excreted in the faeces.
A limitation of the present study was the lack of isotopes to determine cholesterol absorption and full faecal collections to determine excretion. However, faeces and livers from a subset of hamsters (2·5 % PC and controls) were collected and cholesterol content in RBC, liver and faeces was assessed. While numerically the data suggest that there is lower hepatic and higher faecal sterol concentrations, these data did not meet statistical significance. Furthermore, expressing the faecal cholesterol concentration data as mg/g v. total faecal cholesterol is a far less powerful measure since the high cholesterol content of the diet and total faecal output are not accounted for properly here. Therefore, our opinion regarding cholesterol excretion as the mechanism responsible for cholesterol lowering is speculative at this point.
More experiments are needed to clearly identify exact mechanisms responsible for the decrease in circulating concentrations. As mentioned previously, PC and algae in general contains high concentrations of sulphated carbohydrates, known to increase colonic mucous secretion in rats(Reference Shimotoyodome, Meguro and Hase6). This increase in mucin production could increase faecal output and decrease intestinal transit time, thereby interfering with the recovery of cholesterol and bile acids secreted during the digestion process, much like the process associate with the hypocholesterolaemic effect of soluble fibre.
Alternatively, the composition of the algal biomass itself may shift the balance between absorption and efflux of cholesterol from both the liver and the gastrointestinal tract towards efflux and subsequent excretion. Cholesterol absorption is recognised not as a passive process but the result of a balance between the expression and the function of the known intestinal transporters, Niemann-Pick C1 Like 1 protein(Reference Altmann, Davis and Zhu22) and the ATP-binding cassette transporters (ABCG5/G8)(Reference Lee, Lu and Hazard23). An increase in the expression of intestinal and hepatic ABCG5/G8, target genes of the sterol-sensing nuclear hormone receptors known as liver X receptors (α and β), is a reasonable possibility, given this particular species of algae produces mammalian sterols(Reference Volkman24, Reference Durmaz, Monteiro and Koru25). Some well-known and strong agonists of the liver X receptor are oxysterols and other cholesterol precursors as well as the phytosterol, stigmasterol, each of which make up the total sterol content of PC(Reference Volkman24, Reference Durmaz, Monteiro and Koru25). Furthermore, the most potent of the endogenously produced oxysterols is 24(S),25-epoxycholesterol, which is produced at low concentrations in both human subjects and hamsters. Because human and hamster cells seem to produce higher concentrations of 27-hydroxycholesterol, a weaker agonist of the liver X receptor, they do not efficiently dispose of cholesterol through faecal excretion or storage in HDL-cholesterol when challenged with cholesterol loading, implicating this metabolism in the atherosclerotic process(Reference Repa and Mangelsdorf26).
Overall, the data from this experiment confirm that PC as a functional component of foods reduces circulating cholesterol concentrations in a dose-dependent fashion. The present data also confirm that the mechanism by which the PC is acting to reduce the circulating cholesterol concentrations is not via reduced biosynthesis rates. While speculative, we suggest that the unique composition of PC biomass may increase faecal sterol excretion by firstly interfering with cholesterol and bile acid absorption in much the same way as soluble fibre and possibly by increasing the expression of the sterol transporter, i.e. G5/G8, in both the liver and the gastrointestinal tract collectively increasing sterol excretion.
Acknowledgements
S. V. H. wrote the original draft of this manuscript and contributed to the study design and carried out animal trial, isotope analysis and all data analysis. H. L. Z. carried out GC-flame ionisation detector analysis and assisted in both animal trials. C. P. F. M. analysed animals using dual emission X-ray absorptiometry and assisted with the animal trial. Both A. G. D. and H. F. D. are employed by Solazyme, Inc., contributed to study design and produced the PC used in this analysis. H. F. D. is listed as a co-inventor on a published patent application for this particular algal biomass and its cholesterol lower effects. D. J. assisted in carrying out animal trial and sample analysis. P. J. H. J. is the principal investigator of the present study and contributed to the study design and directed all aspects of the study. All authors contributed to the review and editing of this manuscript. The authors have no other conflicts of interest to report. We thank Khatima Khalloufi, Kathleen Gannon and Melinda Mintarno for their assistance with both the animal trials and sample analysis, and Stephanie Jew for data handling and editorial assistance in the preparation of this manuscript. We thank Drs Todd Rideout and Amira Kassis for the editorial review of this manuscript and providing helpful comments. Funding was partially by an unrestricted research and development grant from Solazyme, Inc. and the Natural Science and Engineering Research Council of Canada Discovery grant (U of M Project #30 893). P. J. H. J. holds a Canada Research Chair in Nutrition and Functional Foods.








