Non-alcoholic fatty liver disease (NAFLD) is the most common cause of elevated plasma levels of hepatic enzymes in Western industrialised countries, its worldwide prevalence being 6–35 %. In Europe, the median prevalence is 26 %, with wide variations in different populations or in subjects at higher risk, such as obese or type 2 diabetes, reaching peaks of 50–70 %(Reference Araújo, Rosso and Bedogni1–Reference Younossi, Koenig and Abdelatif3).
The NAFLD natural history is associated with a higher cerebro- and cardiovascular risk, not only in diabetics but also in healthy subjects(Reference Stefan, Häring and Cusi4); in view of its high prevalence, NAFLD treatment and prevention is of great interest from a public health point of view. At present, the main guidelines recommend lifestyle changes, including exercise and correct diet to promote weight loss, while no conclusive evidence is available of drug treatments capable of correcting elevated aminotransferase levels and preventing liver steatosis progression in subjects with NAFLD(Reference Chalasani, Younossi and Lavine5).
The multifactorial pathogenesis of NAFLD suggested the use of different nutraceutical elements, and clinical research is ongoing on that direction(Reference Cicero, Colletti and Bellentani6). The most used substances, alone or in combination, are silymarin from Cardo marianum, vitamin E and other antioxidants, or n-3 PUFA. A recent clinical trial showed an improvement in hepatic function among NAFLD subjects treated with a combination of silymarin, phosphatidylcholine and vitamin E(Reference Loguercio, Andreone and Brisc7). More studies are, however, needed because until now no health claim has been approved by the European Food Safety Authority (EFSA) for improving hepatic function by nutraceuticals. Choline-containing foods may only be labelled as ‘maintenance of normal liver function (ID 1501)’(8).
The aim of the present study was to evaluate the effect of a mixture of different natural ingredients, namely, DHA, phosphatidylcholine, silymarin, choline, curcumin and d-α-tocopherol (vitamin E), on the elevated plasma levels of hepatic enzymes in subjects with NAFLD.
Active ingredients were selected based on the clinical evidence of their hepatoprotective activities(Reference Cicero, Colletti and Bellentani6) and on the in vitro evidence of a synergistic beneficial effect of the nutraceutical mixture on lipid accumulation and liver oxidative stress damage(Reference Stellavato, Pirozzi and de Novellis9).
Other parameters of liver function, the metabolic syndrome and inflammation were evaluated as secondary endpoints of the study. Moreover, considering that NAFLD is associated with alterations of the haemostatic system, which might contribute to the increased cardiovascular and thrombotic risk seen in this condition(Reference Tripodi, Fracanzani and Primignani10–Reference Potze, Siddiqui and Boyett12), the effect of the nutraceutical mixture on coagulation–fibrinolysis balance was investigated in depth, using global functional assays and specific markers of hypercoagulability.
Methods
This article conforms to the Consolidated Standards of Reporting Trials consortium(Reference Schulz, Altman and Moher13).
Trial design
The present study was a double-blind, randomised, multicentre controlled trial of two parallel groups. The trial, coordinated by the IRCCS Neuromed, was performed at three different sites: one at the Ospedale ‘Veneziale’ of Isernia, Italy, and two at the University Hospital, Azienda Ospedaliero-Universitaria Policlinico of Bari, Italy. It was conducted according to the guidelines laid down in the Declaration of Helsinki, and its protocol was approved by the Ethical Committee of the IRCCS Neuromed, followed by each recruitment site’s Ethical Committee, namely, the Azienda Sanitaria Regionale del Molise and the Azienda Ospedaliero-Universitaria Policlinico of Bari. All participating subjects signed a written informed consent previously approved by the ethical committees. The study was registered in clinicaltrial.gov with identifier NCT 02369536 (https://clinicaltrials.gov/ct2/show/NCT02369536).
Participants
Subjects of either sex, 18–80 years old, with NAFLD were recruited in the study. They did not manifest nor had previously manifested any liver-related clinical symptoms but simply had altered laboratory and instrumental tests as compared with normal ranges.
Inclusion criteria were ultrasound alterations typical of mild or moderate hepatic steatosis(Reference Saverymuttu, Joseph and Maxwell14) and serum levels higher than normal (i.e. above the upper limit of each laboratory) for at least one of the three liver enzymes, namely, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and γ-glutamyl transpeptidase (γ-GT).
Exclusion criteria were alcohol abuse history, use of drugs associated with hepatic steatosis development, malnutrition, alcoholic liver disease, chronic hepatic disease of different aetiologies (autoimmune, primary biliary cholangitis, primary sclerosing cholangitis, hereditary haemochromatosis, Wilson’s disease, α-1 antitrypsin deficiency, coeliac disease), severe renal, cardiac or respiratory failure, malignant tumours, intolerance to the components of the active ingredients of the formulation, women who were pregnant or had planned for pregnancy in the following 3 months or were breast-feeding, and subjects who refused to sign informed consent.
According to the current guidelines(Reference Leoni, Tovoli and Napoli15), alcohol abuse was considered a weekly intake > 210 g by men and > 140 g by women, corresponding to >2–3 and >1–2 small glasses of wine/d, respectively.
Interventions
The subjects eligible for the study were randomised (see below) to receive the active or the control treatment, in two capsules once a day, for 3 months.
During the recruitment visit, all subjects received appropriate recommendations about dietary and physical activity lifestyle, according to each site protocols. Recommendations mainly focused on physical activity and healthy diet(Reference Leoni, Tovoli and Napoli15), in particular through adhesion to Mediterranean diet(Reference Abenavoli, Di Renzo and Boccuto16).
The study product was a mixture of active ingredients, formulated as soft gel capsules, each composed of fish oil containing 70 % DHA (250 mg), phosphatidylcholine concentrated in sunflower oil (150 mg), silymarin (75 mg), choline bitartrate (35 mg), curcumin (35 mg) and d-α-tocopherol (10 mg), for a total of 830 mg. The total content of choline is 21·5 mg per capsule (43 mg/d), an amount relatively low as compared with the minimal amount recommended by the health claim of EFSA (82·5 mg/d), corresponding to 15 % of the median daily intake of an adult subject. The comparator capsules (control) contained the formulation excipients and the same amount of choline present in the active mixture (in the form of bitartrate salt). This ‘restrictive’ choice was made to exclude the possibility of any positive effect of the mixture that could be attributed to choline, which has an EFSA-recognised health claim for maintenance of normal liver function. Both the active and the control capsules were prepared and provided by Bouty S.p.A.
Clinical evaluation
At the first visit (T0), clinical and pharmacological anamnesis and anthropometric and clinical measures were collected for each subject. The main risk factors for CVD were recorded: diabetes (blood glucose >6·94 mmol/l), hypertension (systolic blood pressure SBP > 140 and/or diastolic DBP > 90 mmHg or antihypertensive treatment), hypercholesterolaemia (serum cholesterol ≥ 5·17 mmol/l or anticholesterol treatment), hypertriacylglycerolaemia (serum TAG ≥ 1·69 mmol/l), sedentary and smoking habits (ex-smokers for more than 1 year were considered as non-smokers), alcohol consumption. BMI was calculated as kg/m2. Waist (umbilical) circumference was measured at a level midway between the lower rib margin and iliac crest. NAFLD fibrosis score (NFS) was calculated to evaluate the hepatic fibrosis state of each subject(Reference Angulo, Hui and Marchesini17). A weekly food diary was administered to participants in order to detect changes in eating habits during the study.
The clinical visit was repeated at the end of treatment (T1). Fasting venous blood for haematological and chemical analyses (see below) was collected at T0 and T1; serum and citrated plasma were prepared and stored at –20°C until assay.
Outcomes
Primary endpoints were serum levels of the hepatic enzymes AST, ALT and γ-GT; secondary endpoints were liver function markers (direct and indirect bilirubin), metabolic syndrome parameters (cholesterol, TAG, glucose and insulin), C-reactive protein (CRP) and changes in plasma coagulation–fibrinolysis assays (see below).
All measurements were done at completion of the study and were centralised in two laboratories. Biochemical analyses, including re-evaluation of hepatic enzymes and assay of metabolic variables and CRP were performed in the Neuromed Laboratory using standard photometric methods. Insulin was measured by chemiluminescence test and insulin-resistance (homeostatic model assessment of insulin resistance) was calculated(Reference Wallace, Levy and Matthews18). Plasma coagulation and fibrinolysis assays were performed in the Laboratory for Haemostasis and Thrombosis, Department of Biomedical Sciences and Human Oncology, University ‘Aldo Moro’ of Bari. Thrombin generation, which reflects the overall plasma clotting potential, was studied by the calibrated automated thrombinography method as described(Reference Semeraro, Incampo and Ammollo19). Plasma clot lysis, which reflects the overall fibrinolytic capacity, was evaluated by a turbidimetric assay(Reference Semeraro, Colucci and Caironi20). Thrombin activatable fibrinolysis inhibitor (TAFI) was measured by a commercial functional assay (Stachrom TAFI; Diagnostica Stago). The following plasma biomarkers were assayed by commercially available ELISA kits: prothrombin fragment 1 + 2 (F1 + 2, a marker of in vivo thrombin generation) by Enzygnost F1 + 2 ELISA (Dade Behring); d-dimer, a specific fragment derived from the lysis of cross-linked fibrin, by HemosIL AcuStar d-dimer; Instrumentation Laboratory; tissue plasminogen activator (t-PA) and plasminogen activator inhibitor-1 (PAI-1) by Imubind Plasma t-PA ELISA and Imubind Plasma PAI-1 ELISA, respectively (Sekisui Diagnostics GmbH); activated TAFI (TAFIa/ai, a combination of TAFIa and its inactive derivative TAFIai) by a specific ELISA (Asserachrom TAFIa/ai; Diagnostica Stago); plasmin-α2–antiplasmin complex (PAP, a marker of in vivo plasmin generation) by Technozyme PAP (Technoclone GmbH).
All measurements were performed by trained personnel blinded to participant identifying information.
Compliance to treatments was achieved by asking the participants to return all unused medications and empty boxes at the end of the trial. They were also asked about the days of missing treatment.
Sample size
Sixty subjects per group were estimated to allow to detect differences between the two groups equal to 54 % of sd of the mean of the investigated biomarkers with 80 % potency and α = 0·05. Concerning the primary endpoints, our sample was sized to detect differences greater than 8·3 IU/l for AST, 13·5 IU/l for ALT and 16·2 IU/l for γ-GT (20, 20 and 22 % of the means, respectively).
Randomisation
The random allocation sequence to treatment was computer based per blocks of four or six subjects stratified for each recruiting centre; it was generated by the principal investigator (PI)’s statistician and forwarded to each recruitment centre.
The doctor responsible for each recruitment centre enrolled participants and assigned them to interventions, according to the allocation sequence received by the PI of the trial.
Blinding
Participants, doctors, technicians and statisticians who participated or evaluated the study outcomes were blinded to the supplements. Active and control capsules were identical for organoleptic properties and coded as A and B by the producer. Only after completion of statistical analyses of the trial results, the company who provided the capsules opened the blinding.
Statistical methods
Data were analysed according to the intention-to-treat approach. Results are presented as mean and sd or median and interquartile range, depending on distribution, as assessed by the D’Agostino-Pearson test. Differences between basal values in control and active groups and between T1 v. T0 % changes in the two groups were assessed by ANOVA and by Fisher’s exact test. Non-normally distributed data were log transformed before analysis.
Additional analyses for composite endpoints were performed using multivariate ANOVA (MANOVA), and for ‘intra-subject’ (T1 v. T0) changes paired data analysis was used. Statistical analyses were performed using the SAS/STAT software, version 9.4 of the SAS System for Windows©2009.
Results
Participant flow
A total of 126 participants from three centres were eligible for the study, which was assessed by the willingness to participate in the trial and to sign the informed consent. They were randomly allocated to the active (n 62) or the control (n 64) supplementation groups. The subjects lost at follow-up were seven in the active group and six in the control group, for the following reasons: bone fracture (1), relocation to another city (1), discontinued supplementation due to dermatitis (1), unmotivated personal decision (4) in the active group; discontinued supplementation for faecal colour abnormality (green faeces, 1) and unmotivated personal decision (5) in the control group. The final number of subjects analysed was fifty-five and fifty-eight, in the active and control groups, respectively.
Recruitment
Subject recruitment started on 18 August 2015, and ended on 19 July 2016. Follow-up visits occurred from 19 November 2015 to 13 October 2016. The recruitment had to be completed by July 2016 in order to meet the project deadline.
Baseline data
General and clinical characteristics were similar in the active (n 55) and control (n 58) supplementation groups (Table 1). Overall, a relatively high proportion of study participants were carriers of risk factors for CVD, such as diabetes, hypertension, or hypercholesterolaemia, hypertriacylglycerolaemia, or had unhealthy lifestyles, smokers being more than 25 % and people with sedentary habits being about 45 %; average BMI was 30 kg/m2, with about 50 % of subjects obese (Table 2). Similarly, no difference was found between the two groups with regard to anthropometric variables, medications (Table 2), blood pressure and complete blood cell counts (data not shown). The NFS was on average low and similar for the two treatment groups. Only a minority (7 %) of participants yielded an NFS compatible with severe fibrosis or cirrhosis. None of the subjects had signs of advanced liver disease.
Table 1. General and clinical characteristics of subjects included in the study
(Mean values and standard deviations; numbers of subjects and percentages)
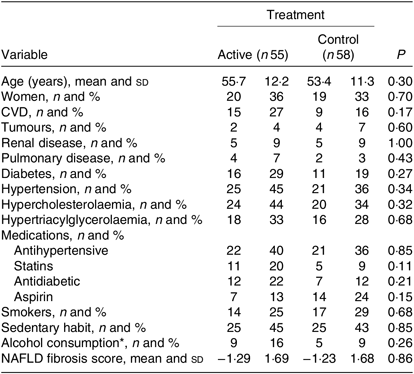
NAFLD, non-alcoholic fatty liver disease.
* Less than 210 g/week for men and 140 g/week for women.
Table 2. Hepatic enzymes, metabolic and anthropometric variables at baseline and after the 3-month supplementation with active mixture or control*
(Mean values and standard deviations or medians and interquartile ranges (IQR))
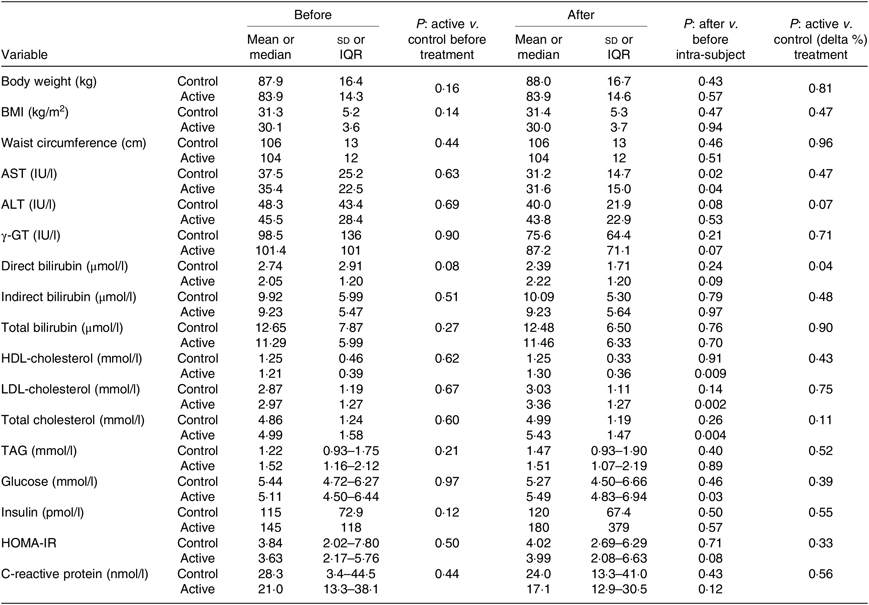
AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GT, γ-glutamyl transpeptidase; HOMA-IR, homeostatic model assessment of insulin resistance.
* Number of subjects = 58 and 55, for control and active treatment groups, respectively.
Outcomes
Table 2 reports the results on primary and secondary endpoints of the trial. No differences were observed in the baseline levels of the hepatic enzymes, AST, ALT and γ-GT between the two treatment groups, confirming a successful randomisation. After treatment, both groups displayed a decrease in the three enzymes, which reached statistical significance only for AST levels, whose mean reduction amounted to 16·8 and 10·7 % for active and control groups, respectively (P < 0·04 and < 0·02). Posttreatment levels did not differ between the two groups. The lack of difference was also seen in an ancillary analysis, when the three enzymes were considered as a composite endpoint, both by MANOVA and by intra-subject analyses.
The results of the secondary endpoints of the study, that is, other liver function, metabolic and inflammatory variables, did not show any difference within and between treatments, except for a slight but significant increase at T1 of HDL, LDL and total cholesterol by 7·1, 13·0 and 8·8 %, respectively, and glucose by 5 % in the active group.
The anthropometric measures (body weight, BMI and waist circumference) also remained unchanged in both control and active mixture supplementations, suggesting that neither group made noticeable changes in lifestyle.
Table 3 reports the circulating levels of the main markers of in vivo activation of coagulation and fibrinolysis (F1 + 2, PAP, d-dimer and TAFIa/ai), the circulating levels of factors known to influence fibrinolytic capacity (t-PA, PAI-1 and TAFI) and the results of the clot lysis assay, a global test that measures the fibrinolytic resistance of plasma clots exposed to exogenous t-PA. Thrombin generation parameters, which reflect the pro-coagulant capacity of plasma, are reported in Table 4. The latter parameters were also evaluated in the presence of thrombomodulin to assess the generation of thrombin in the presence of a functioning protein C system in order to better mimic the physiological conditions. On the whole, the baseline levels of these variables, if compared with values of healthy subjects obtained in the same laboratory using the very same experimental conditions(Reference Ammollo, Semeraro and Milella21,Reference Incampo, Carrieri and Galasso22) , suggest an increased pro-coagulant potential in NAFLD subjects. In particular, the main thrombin generation parameters, that is, endogenous thrombin potential and peak thrombin activity, were visibly higher compared with a control group investigated in the same period using the same reagents(Reference Ammollo, Semeraro and Milella21). Consistent with the majority of hepatic and inflammatory variables, the baseline coagulation–fibrinolytic data were similar in the two groups and were not affected by either treatment.
Table 3. Haemostatic variables at baseline and after the 3-month supplementation with active mixture or control*
(Mean values and standard deviations)
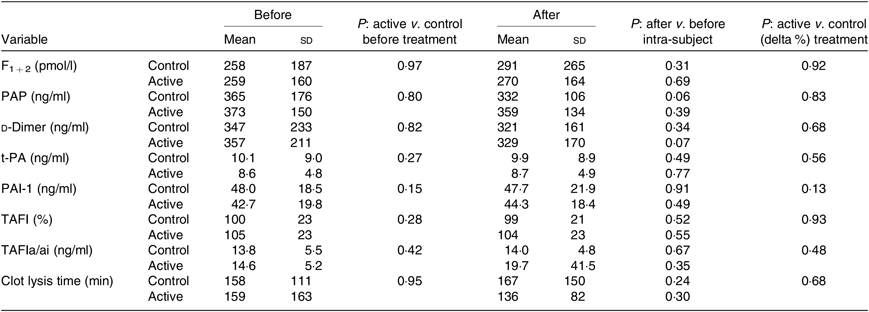
PAP, plasmin-α2–antiplasmin complex; t-PA, tissue plasminogen activator; PAI-1, plasminogen activator inhibitor-1; TAFI, thrombin activatable fibrinolysis inhibitor; TAFIa, activated TAFI; TAFIa/ai, a combination of TAFIa and its inactive derivative TAFIai.
* Number of subjects = 58 and 55, for control and active treatment groups, respectively.
Table 4. Thrombin generation parameters at baseline and after the 3-month supplementation with active mixture or control*
(Mean values and standard deviations)
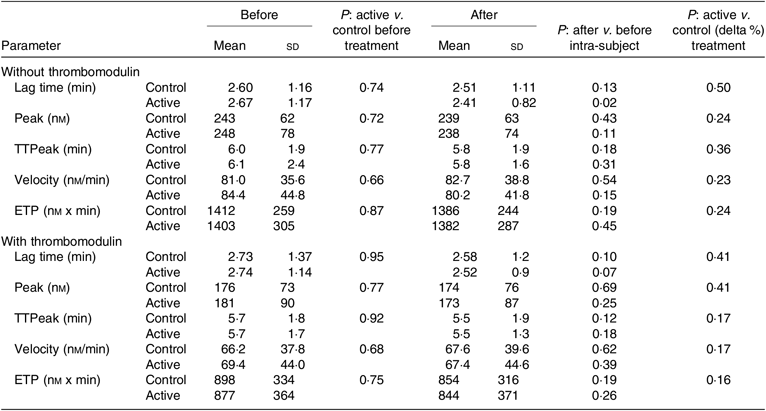
TTPeak, time to peak; ETP, endogenous thrombin potential.
* Number of subjects = 58 and 55 for control and active treatment groups, respectively.
Compliance to the treatment
The distribution of missed days and returned capsules, as a measure of treatment compliance, was similar in the two treatment groups: overall 98 % of the study participants were totally compliant to the therapy and assumed all the capsules; the time lapse between basal and second visit was 100 (sd 12) and 101 (sd 15) (P = 0·46) in the active and control treatment groups, respectively. A subgroup analysis by excluding subjects who were available for the final visit (T1) more than 103 d after enrolment, therefore remaining without supplementation for at least 2 weeks, did not substantially modify the results. They were eleven and fifteen for active and control treatments, respectively.
No relevant changes in dietary habits were recorded.
The reported complaints of adverse effects were dermatitis (in one subject who abandoned the study), sudden increase in body weight (n 1), night pyrosis (n 1), abdominal bloating (n 1) in the active group, abnormal faeces colour (n 1, dropout subject) and dysenteriae (n 1) in the control group.
Discussion
Our study of adult subjects with NAFLD tested a novel multicomponent mixture, selected on the basis of previous knowledge, with the potential advantage to target different mechanisms and to utilise relatively low doses of each compound. The chronic supplementation with the nutraceutical mixture was well tolerated and apparently safe in NAFLD subjects: the adverse effects reported were of relatively low severity and the compliance to treatment was high. Choline was used both in the control and in the active mixture at a dose of 43 mg/d, which is relatively low compared with the minimal dose reported in the EFSA’s health claim, and corresponds to 15 % of the median daily intake of an adult subject. Its presence in the active mixture was expected to facilitate intracellular transport of other compounds, such as silymarin and curcumin. Despite these promising grounds, we did not observe any significant effect of the multicomponent nutraceutical on liver enzymes. In fact, the average reductions in hepatic enzymes after treatment ranged from 23·2 to 3·7 %, independent of the type of supplement received but did not reach statistical significance (except for AST) nor discriminated subjects between active and control supplement groups. Most likely, the observed improvement in AST was due to choline, which was present in both intervention arms. However, the possibility of a ‘spontaneous’ or casual effect cannot be excluded because we did not include a true placebo group.
Other liver function and metabolic parameters were also unaffected by the supplementation. An unexpected finding of our study was a minor (less than 10 %) increase in serum cholesterol (total, LDL and HDL) and glucose levels in the subjects randomised to the active mixture. Within the different compounds present in the mixture tested in the present study, DHA has been shown to increase LDL-cholesterol particle size(Reference Theobald, Chowienczyk and Whittall23) and to down-regulate receptor-mediated LDL-cholesterol clearance, in part via reduced expression of LDL receptors by hepatocytes. These data, however, were not confirmed by other studies(Reference Jacobson, Glickstein and Rowe24).
Liver is the source and the site of clearance of most clotting/fibrinolytic factors (both activators and inhibitors), and a progressive pro-coagulant imbalance has been reported in liver disorders, suggesting a pathogenetic role of the prothrombotic shift in NAFLD-associated risk of CVD and fibrosis progression(Reference Tripodi, Fracanzani and Primignani10–Reference Potze, Siddiqui and Boyett12). For this reason, we performed an in-depth study of coagulation and fibrinolysis, through the use of global tests (i.e. thrombin generation and clot lysis), which gives an overall picture of the prothrombotic potential of plasma, and through the assay of specific factors and activation markers, which reflect the in vivo activation of coagulation and fibrinolysis. On the whole, the data obtained point to a heightened prothrombotic potential in NAFLD. Even though our trial did not include a control group of healthy subjects, the marked difference in the results obtained in normal subjects by the same laboratory, using the same methods and reagents(Reference Ammollo, Semeraro and Milella21,Reference Incampo, Carrieri and Galasso22) , provides an indirect support to this conclusion. Besides this aspect, which needs to be confirmed by a head-to-head comparison, our study shows that none of the variables tested was affected by supplementation with active or control nutraceuticals. It should be stressed that in a recent study(Reference Ammollo, Semeraro and Milella21) we could demonstrate a significant reduction in the prothrombotic potential (e.g. reduced thrombin generation, faster clot lysis) of healthy subjects following a 4-week intake of fresh grape, showing that some of the variables assayed in the present study can be influenced by dietary supplementation. The latter finding, along with the array of variables measured in the present study, allows us to conclude that the nutraceutical formulation tested in this trial has no antithrombotic effect, at least after a 3-month period of treatment.
NAFLD is a condition characterised by a wide spectrum of hepatic changes, which may progress into overt liver disease such as liver fibrosis and cirrhosis but is also associated with comorbidities such as insulin resistance, the metabolic syndrome, thyroid dysfunction, chronic inflammation, hypercoagulable state, chronic kidney disease, hyperuricemia and others, all conditions at increased risk of CVD and mortality. Therefore, although the mechanisms underlying the onset and progression of NAFLD are not clearly defined, several types of nutraceuticals have been proposed and for some of them clinical trials have shown an improvement of liver function tests and possibly of liver histological changes.
Nutraceutical supplementation has been previously tested in NAFLD to prevent or reduce hepatic enzyme imbalance by targeting different potential mechanisms such as insulin resistance, inflammation and oxidative stress. The most studied compounds are vitamins D, E and C, carnitine, n-3-fatty acids such as DHA and EPA, plant-derived polyphenols including the flavonoids silymarin and anthocyanins, curcumin and the stilbene resveratrol. Mediterranean diet has been also proposed as a potentially useful approach for NAFLD patients. The available clinical evidence on the effects and possible mechanisms of action of nutraceuticals has been recently reviewed(Reference Cicero, Colletti and Bellentani6).
The following considerations may be useful for future studies. The study design, a randomised double-blind, controlled trial, was correctly applied and randomisation of patients from different centres was successful. The study was powered to detect a 20 % reduction in the primary endpoints by the active supplement. Thus, the small, though significant, decrease in AST levels following both treatments may be considered casual and of little clinical relevance.
The dose of each component was selected on the basis of previous clinical studies on single ingredient or on the combination of two components. We purposely chose to reduce the concentration of each component in view of a possible multitarget potentiation, as discussed above. Concerning the duration of the study, the 3-month period was considered appropriate based on our previous experience with dietary supplementation in healthy subjects(Reference Ammollo, Semeraro and Milella21) and on previously published studies(Reference Gajos, Zalewski and Rostoff25,Reference Sanchez, Poggi and Morange26) . On the other hand, a relatively short treatment period was considered suitable to guarantee a good compliance and provide a relatively rapid answer on treatment efficacy. Unfortunately, no measurements of the supplemented compounds or of their biomarkers in body fluids could be performed.
The subjects included in the trial showed an ample inter-individual variability, not only for the hepatic and metabolic markers but also for other clinical conditions and biological variables such as obesity, diabetes, inflammation markers, status of fibrosis, which could differently modulate the response to supplementation. This heterogeneity, while representing a limitation for it may decrease the statistical power of the study, was considered to better reflect real-life situation and thus may represent an advantage as to the applicability of the results to a general population.
What have we learnt from the present study?
First of all, trials may obviously end with either positive or negative results: the latter are as important as the former, as they may clarify some uncertain points on the clinical condition selected and/or its potential treatment.
Second, it may be quite difficult to show any significant decrease in biochemical parameters that are only marginally altered before the treatment. In our previous experience on other nutraceuticals, we had observed a measurable effect of these compounds only in ‘stressed’ conditions, such as challenging healthy volunteers with a fatty meal, that induces an acute increase in inflammatory reactions(Reference Cerletti, Gianfagna and Tamburrelli27). We cannot exclude that the inclusion in the present study of patients with a more severe form of NAFLD could have produced more apparent results.
Third, in a chronic condition such as NAFLD, a longer treatment might have been preferable but could not be performed in our study for organisation reasons.
Fourth, the significant increase in cholesterol and glucose levels should caution against consuming nutraceuticals for which no evidence-based efficacy has been previously shown, because some unexpected, though minor, side effects might occur.
In conclusion, in the present trial we were unable to show any effect of a mixture of dietary natural compounds on different relevant physiopathological markers of NAFLD. The results of our study, as well as our protocol choices, may be useful for future trials, which will have to take into account the number of subjects, the inclusion criteria (to obtain a more homogeneous sample, preferably at higher risk), the nature and the concentration of nutraceuticals, a longer duration of treatment, along with other aspects such as the gut microbiota interference in lipid metabolism.
Acknowledgements
We thank doctors and nurses of the recruiting sites for the valuable support provided to complete the present study.
The present study was supported by the Italian Ministry of University and Research (MIUR, PON01_01226/1 - Decr. N.l/Ric 18-1-2010).
G. d. G., L. I. and N. S. designed the study and revised the manuscript; C. C. prepared the protocol and documents for ethical committee approval and trial registration, supervised all the aspects of the study and wrote the manuscript; M. C. supervised the coagulation–fibrinolysis concepts, tests and writing and supervised trial completion at the Bari sites; M. S., F. S., C. T. A. and F. I. performed the laboratory tests; S. C. and A. D. C. performed the statistical analyses. G. D. B., P. P. and M. B. were responsible for recruitment of subjects for the trial. All authors approved the final version of the manuscript.
All authors declare that they have no conflicts of interest. The Company who provided the capsules tested in the trial had no role in the study design, collection, analysis and interpretation of data, nor in the decision to submit a manuscript for publication and in its writing.
Ethical statement
The study protocol was approved by the ethical committee of the IRCCS Neuromed, the trial Coordinator’s Institution, followed by each recruitment centre’s Ethical Committee, namely, the Azienda Sanitaria Regionale del Molise (ASReM) and the Azienda Ospedaliero-Universitaria Policlinico of Bari.
Informed consent
Prior to the study, all participating subjects signed a written informed consent approved by the ethical committees.







