Strenuous exercise causes skeletal muscle microtrauma, which is characterised by ultrastructural damage of sarcomeres and cell membranes and results in a loss of force-generating capacity and muscle soreness( Reference Peake, Nosaka and Suzuki 1 ). This exercise-induced muscle damage (EIMD) activates an inflammatory response cascade leading to the sequential infiltration of neutrophils and macrophages into the injured muscle tissue, the production of pro-inflammatory cytokines and reactive oxygen species (ROS) at the injury site and elsewhere, as well as a systemic release of leucocytes and cytokines and oxidative stress( Reference Peake, Nosaka and Suzuki 1 , Reference Fehrenbach and Schneider 2 ). Muscle damage and inflammation also characterise a number of catabolic diseases such as muscular dystrophies, cancer cachexia and sepsis, although with diverse mechanisms (e.g. different profile of released cytokines)( Reference Fehrenbach and Schneider 2 ). Therefore, eccentric exercise appears to be a valid model to study the cellular and molecular pathways regulating the inflammatory process associated with physical trauma.
During the inflammatory phase, a rapid invasion of ROS-generating immune cells (i.e. neutrophils and macrophages) is crucial for the efficient clearance of cellular debris, as well as the subsequent repair and regeneration of muscle fibres. However, ROS produced during this phase by NADPH oxidase (respiratory burst) may also lead to marked perturbations of muscle redox status( Reference Peake, Nosaka and Suzuki 1 , Reference Nikolaidis, Jamurtas and Paschalis 3 , Reference Powers, Ji and Kavazis 4 ). Furthermore, damaged myofibres and cytokines released from phagocytes activate ROS-generating enzymes such as xanthine oxidase, cyclo-oxygenase-2 and NADPH oxidase( Reference Ji 5 ). ROS may cause secondary muscle damage of adjacent intact muscle fibres( Reference Peake, Nosaka and Suzuki 1 ), thereby exacerbating the loss of muscle’s force-generating capacity( Reference Powers, Ji and Kavazis 4 ). It appears that these perturbations of redox status are involved in the regulation of transcriptional pathways such as those of NF-κB, mitogen-activated protein kinases and protein kinase B (Akt)/mammalian target of rapamycin( Reference Powers, Ji and Kavazis 4 , Reference Aoi, Naito and Takanami 6 – Reference Michailidis, Karagounis and Terzis 8 ). These cascades mediate the activation and recruitment of immune cells, adhesion molecules, satellite cells and synthesis of antioxidant enzymes( Reference Powers, Ji and Kavazis 4 , Reference Aoi, Naito and Takanami 6 – Reference Michailidis, Karagounis and Terzis 8 ).
Increasing evidence suggests that elevated ROS production may down-regulate anabolic signalling and protein synthesis( Reference Powers, Morton and Ahn 9 ) and promote oxidative protein modifications and proteolysis via the ubiquitin–proteasome system (UPS) – the main proteolytic system in skeletal muscle( Reference Powers, Morton and Ahn 9 – Reference Sandri 12 ). The 20S proteasome is the ATP-independent proteolytic core, composed by 7 α- and 7 β-subunits, three of which ( β 1, β 2 and β 5) are responsible for the specific proteolytic activities of the complex( Reference Sandri 12 – Reference Chondrogianni, Petropoulos and Grimm 14 ). Furthermore, the immunoproteasome is also active in muscle under pro-inflammatory conditions. UPS activation in muscle, under pro-inflammatory conditions, is mediated by cytokines (i.e. TNFa and IL-6) and NF-κB signalling( Reference Li, Malhotra and Kumar 15 , Reference Puig-Vilanova, Rodriguez and Lloreta 16 ). However, following EIMD, both proteasome- and immunoproteasome-mediated proteolysis promote degradation of damaged and/or unfolding proteins and facilitate myogenesis and recovery of muscle’s functional capacity( Reference Bell, Al-Khalaf and Megeney 17 , Reference Taillandier, Combaret and Pouch 18 ). Indeed, marked elevation in mRNA and protein expression level of specific E3 ubiquitin ligases persists up to 48 h following a single bout of eccentric exercise( Reference Murton, Constantin and Greenhaff 19 ).
Protein supplementation has been postulated to affect skeletal muscle’s remodelling process, by regulating the inflammatory response( Reference Nicastro, da Luz and Chaves 20 ). Whey protein supplementation during recovery from eccentric exercise has been shown to prevent strength decline( Reference Cooke, Rybalka and Stathis 21 – Reference Buckley, Thomson and Coates 22 ) and to accelerate satellite cell proliferation( Reference Farup, Rahbek and Knudsen 23 ). Moreover, branched-chain amino acids (BCAA) may also promote the attenuation of EIMD( Reference Howatson, Hoad and Goodall 24 ) and the delayed onset of muscle soreness (DOMS) following eccentric exercise( Reference Jackman, Witard and Jeukendrup 25 ). However, protein is also a rich source of sulphur-containing amino acids that act as precursors for GSH synthesis, thereby protecting cells from redox status disturbances( Reference Cruzat, Krause and Newsholme 26 ). Although protein supplementation has been extensively examined in terms of stimulating muscle protein synthesis (MPS) following exercise, its ability to regulate both the inflammatory response and UPS following aseptic muscle injury remains unclear. Thus, we investigated the effects of milk protein concentrate supplementation on muscle damage, inflammatory responses, proteasome activity and muscle strength following muscle-damaging eccentric exercise.
Methods
Participants
All participants (n 11) were informed of the purpose of the study, the experimental procedures involved and all the risks, discomforts and benefits involved, before obtaining written consent. The study was approved by the Institutional Review Board of the University of Thessaly and procedures were in accordance with the 1975 Declaration of Helsinki, as revised in 2000. Participant’s characteristics are shown in Table 1. Participants were recreationally active (VO2max>45 ml/kg per min) and had been engaged in systematic resistance exercise training of ≥3 times/week for the past 12 months. For inclusion in the study, the criteria for participants were as follows: (1) non-smokers; (2) abstained from any vigorous physical activity during the experimental period; (3) had no consumption of alcohol, caffeine, any type of nutritional supplements and medication before (at least 6 months) and throughout the experimental period; (4) had no allergies or intolerance to milk protein; and (5) had no recent history of muscle lesions, lower limb trauma and metabolic diseases.
Table 1 Participants’ characteristics in each trialFootnote * (Mean values and standard deviations)
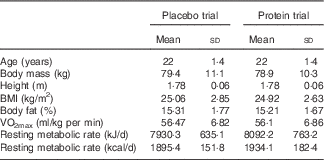
* Participants demonstrated comparable anthropometric profile and physical conditioning status at baseline (n 11). One-way (time) repeated measures ANOVA was used. VO2max, maximal O2 uptake.
Experimental design
A two-trial, cross-over, double-blind, repeated measures design was used in this investigation. Before each trial, participants’ body weight and height, RMR and body composition (dual-energy X-ray absorptiometry scan) were measured and they were then provided with accelerometers to measure their habitual physical activity and diet recalls in order to record their dietary intake, over a 7-d period. Thereafter, diet recalls were analysed and participants entered a 3-week adaptive period to ensure that all of them received a standard protein intake of 1 g protein/kg per d, which was considered to be the mean and population-safe protein intake( Reference Elango, Humayun and Ball 27 , Reference Humayun, Elango and Ball 28 ).
Participants were randomly assigned to consume either milk protein concentrate (PRO) or placebo (PLA), immediately after an acute bout of eccentric exercise on an isokinetic dynamometer and daily for 8 consecutive days post exercise (Fig. 1). A 6-week washout period was adapted between trials. Before each trial, muscle force-generating capacity was tested following familiarisation with the equipment while VO2 max was measured during a graded exercise test to exhaustion using an automated online gas exchange analyser as previously described( Reference Chatzinikolaou, Christoforidis and Avloniti 29 ). Participants were asked to refrain from resistance exercise or any other strenuous physical activity for at least 2 weeks before each trial and throughout the experimental period. On each exercise day of each trial, participants arrived at the laboratory after an overnight fast, and baseline blood samples and muscle biopsies were collected. Subsequently, they performed an acute bout of unilateral eccentric contractions on an isokinetic dynamometer. Muscle biopsies and blood samples were also collected at 2 and 8 d after exercise, whereas muscle strength was evaluated at 6 h post exercise and daily for 8 consecutive days. All measurements were performed at the same time of day, in both trials, to prevent the effect of circadian variations.
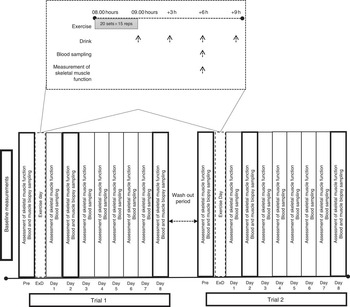
Fig. 1 Experimental design. ExD, exercise day.
Exercise protocol
The exercise protocol applied in the present study has been described to effectively induce myofibrillar disruption, as indicated by electron microscopy and immunohistochemistry( Reference Raastad, Owe and Paulsen 30 ), and has been efficiently used in a previous study of our group to induce aseptic inflammation( Reference Michailidis, Karagounis and Terzis 8 ). In brief, participants performed 300 eccentric unilateral contractions (twenty sets, fifteen repetitions/set, 30-s rest between sets) of knee extensors on an Isoforce isokinetic dynamometer (TUR Gmbh) at a speed of 30°/s.
Supplementation
On exercise day, participants consumed a protein supplement consisting of 80 % protein (80 % casein and 20 % whey), 1·5 % fat and 5 % carbohydrates or a matching PLA (containing no protein) as repeated ‘pulsed’ drinks: immediately post exercise and then one every 3 h on three occasions (+3, +6, +9 h). According to previous reports( Reference Areta, Burke and Ross 31 , Reference Moore, Areta and Coffey 32 ), distributing 80 g of protein in 4×20 g doses every 3 h is superior to either pulse (8×10 g every 1.5 h) or bolus (2×40 g every 6 h) feeding in stimulating MPS and maximising whole-body protein balance over a 12-h post-exercise period. On each day of the remaining days, participants consumed a single drink (protein or PLA) with breakfast. A single dose of 20 g of protein supplemented on non-exercise days is considered the minimum amount of protein required in order to increase protein intake on non-training days( Reference Naclerio, Larumbe-Zabala and Ashrafi 33 , Reference Volek, Volk and Gomez 34 ). Each PLA supplement contained 20 g of maltodextrin to keep the two supplements isoenergetic. All drinks were prepared in 250 ml of water and flavoured with bannana, to make the contents indistinguishable and non-transparent.
Muscle performance
To assess muscle performance, maximal knee extensor eccentric peak torque was measured on the same isokinetic dynamometer at 60°/s as described( Reference Raastad, Owe and Paulsen 30 ). The evaluation was preceded by a familiarisation period (CV for repeated measures was 4·1 %). Participants rated their DOMS on a visual analogue scale (1–10) during palpation of the muscle belly and the distal region of relaxed vastus medialis, vastus lateralis and rectus femoris, following three repetitions of a full squat( Reference Theodorou, Nikolaidis and Paschalis 35 ).
Diet records
In the beginning of the study, participants were taught (by a trained dietitian) how to estimate food servings and sizes and how to record their regular dietary intake. Then, participants completed 7-d diet recalls to evaluate their protein and energy intake. On the basis of diet analysis, participants were provided with a dietary plan providing 1·0 (sd 0·02) g protein/kg/day over a 3-week period (adaptive period). During the first trial (8-d testing period), as well as during the washout period and the second trial (8-d testing period), participants completed again 7-d diet recalls to record their daily intake to verify that they followed the same diet practice as they did in the preliminary adaptive period. Analysis of diet recalls collected throughout the study indicated a similar diet composition (total energy: 2581·7 (sd 105·4) %, carbohydrate intake: 56·3 (sd 3·1) %, fat intake: 28·1 (sd 3·1) %, protein intake: 15·6 (sd 1·5) %) of participants during all phases of the study (adaptive period, trial 1, washout period, trial 2). Science Fit Diet 200 A (Science Technologies) was used for the analysis of diet records.
Blood sampling and assays
Resting blood samples were collected at 07.00 hours on the exercise day, after an overnight fast. All samples were collected via a 20-gauge disposable needle equipped with a Vacutainer tube holder (Becton Dickinson) from an antecubital arm vein, while subjects were in a seated position. For serum separation, blood samples were allowed to clot at room temperature first and then centrifuged (1500 g , 4°C, 15 min). The supernatant was dispensed in multiple aliquots (into Eppendorf tubes) that were stored at −80°C for later analysis of creatine kinase activity (CK). A blood portion was collected into tubes containing EDTA and centrifuged (1370 g , 4°C, 10 min). Plasma was collected into Eppendorf tubes and stored at −80°C, and it was used for the measurement of insulin and glucose. The remaining plasma erythrocytes were then lysed and the resultant lysate was collected as described( Reference Barbas, Fatouros and Douroudos 36 ) and used for analysis of protein carbonyls( Reference Kraemer, Solomon-Hill and Volk 37 ). Another blood portion (2 ml) was immediately collected in EDTA tubes and were used for the determination of leucocyte count using an automated haematology analyzer (BC-5500; Shenzhen Mindray). CK and glucose concentrations were measured using a Clinical Chemistry Analyser Z1145 (Zafiropoulos Diagnostica) using commercially available kits (Zafiropoulos). Insulin was measured with a commercially available insulin (Human) ELISA kit (AbnovaR corporation). Protein carbonyls (PC) were assayed spectrophotometrically (Hitachi UV/VIS; Hitachi Instruments Inc.) at 375 nm, as described( Reference Theodorou, Nikolaidis and Paschalis 35 ). Plasma and serum samples were stored in multiple aliquots at −80°C and thawed only once before analysis. All assays were performed in duplicate. Inter- and intra-assay coefficients of all assays ranged from 1·8 to 8·2 % and from 2·9 to 8·6 %, respectively.
Muscle biopsy sampling
Muscle samples were obtained using the standard Bergström needles, with suction, from the middle portion of vastus lateralis of the exercising leg under local anaesthesia (xylocaine 1 %)( Reference Terzis, Stratakos and Manta 38 ). The pre-exercise sample was obtained 25 cm from mid-patella, 30 min before exercise. Subsequent biopsies (at 2 and 8 d post exercise) were obtained from the same muscle, 5 cm distal to each previous biopsy site. A different limb (randomly selected) was used for eccentric exercise and biopsy sampling in each trial. Adipose tissue and blood were carefully removed from muscle samples that were rapidly frozen in N2 and stored at −80°C for further analysis of protein phosphorylation, proteasome activity and expression of its catalytic subunits.
Proteasome peptidase assay
This method allows the direct quantification of proteasome activities and is performed as previously described( Reference Chondrogianni, Stratford and Trougakos 39 , Reference Myeku, Metcalfe and Huang 40 ). In brief, tissue samples were homogenised in proteasome activity lysis buffer (1 m Tris-HCl, pH 7·6, 100 mm ATP, 3 m KCl, 0·1 m EDTA, 1 m dithiothreitol, 0·2 % NP-40, 10 % glycerol, 10 μg/ml aprotinin and 10 mm phenylmethylsulfonyl fluoride (PMSF)). Chymotrypsin-like (CT-L), caspase-like (C-L) and trypsin-like (T-L) activities were assayed with hydrolysis of the fluorogenic peptide LLVY-AMC, LLE-AMC and LSTR-AMC, respectively (Enzo Life Sciences), for 30 min at 37 °C. Specific activity was determined in the presence of 20 μm MG132-specific proteasome inhibitor. Fluorescence was measured in the Safire2 Multi-detection Microplate Reader (Tecana).
Immunoblot analysis
Immunoblot analysis was used to detect protein expression levels of proteasome (β5, β2 and β1) and immunoproteasome (β5i, β2i and β1i) subunits. A portion of the tissue samples that were homogenised during the proteasome peptidase assay was boiled in non-reducing Laemmli buffer (NRLB) for 10 min, as previously described( Reference Chondrogianni, Stratford and Trougakos 39 ). SDS-PAGE and immunoblotting were then contacted with specific antibodies against 20 S, 19 S, proteasome complexes and immunoproteasome. GAPDH was used as a loading control. Each immunoblot was repeated at least twice.
Antibodies
Polyclonal NF-κB p65Ser536 was purchased from Cell Signaling Technology Inc. GAPDH was purchased from Santa Cruz Biotecnology Inc. Anti-rabbit (IgG, horseradish peroxidase linked) secondary antibody was also purchased from Signaling Technology Inc. Antibodies against β2, β1, β5, β2i, β1i and β5i proteasome subunits were purchased from Enzo Life Sciences and antibody against HSP70 was purchased from Santa Cruz Biotechnology.
Measurement of intracellular-signalling-related proteins
Changes in the phosphorylation of NF-κB p65Ser536 and protein expression of HSP70 were analysed by immunoblotting. Briefly, muscle samples (approximately 40–50 mg) were homogenised in activity lysis buffer and then centrifuged at 13 000 rpm at 4°C for 10 min and the supernatant was collected. Total protein concentration was measured (Bradford Protein Assay; Bio-Rad) to prepare working samples of equal concentration in NRLB. Equal amounts of protein (20 μg) were loaded onto 8 % or gradient precast gels (Mini-PROTEAN TGX Gels; Bio-Rad) and separated by SDS-PAGE electrophoresis. Immediately after, proteins were transferred to nitrocellulose membrane, blocked with 5 % non-fat milk/Tris-buffered saline for 1 h and incubated with primary antibody overnight at 4°C. Membranes were washed with Tris-buffered saline-Tween (TBS-T) and incubated with secondary antibody for 1 h at room temperature. Subsequently, membranes were washed again with TBS-T, exposed, visualised by chemiluminescence and quantified by densitometry. Phosphorylation status of NF-κB and HSP70 expression was then expressed relative to a corresponding GAPDH from the equivalent sample lysate.
Statistics
On the basis of a preliminary power analysis, a sample size of ten to twelve subjects was suggested in order to detect differences between repeated measurements with a power of 80 % at a significance level of 5 % (α error). Accordingly, fourteen healthy, trained male volunteers were recruited in the present study, but data from eleven volunteers have been analysed (for three participants, biopsy samples were not collected at all time points or the quantity of muscle tissue was not adequate for analysis).
All data are presented as means and standard deviations. A two-way (trial (PRO and PLA)×time (before exercise, and 2 and 8 d post exercise)) repeated measures ANOVA with planned contrasts on different time points was used to determine the effects of treatment and time on dependent variables. Bonferonni test was used for post hoc analysis, when a significant main effect was detected. Significance was set at P<0·05. Effect sizes (ES) were also calculated on results of CT-L proteasome activity. We also conducted bivariate Pearson’s correlation analysis to estimate the relation of changes in skeletal muscle performance (isometric peak torque) and DOMS with proteasome function (activity and expression level of different β-subunits). Correlation coefficients of r<0·2, 0·2<r<0·7 and r>0·7 were characterised as small, moderate and high, respectively. The level of statistical significance was set at P<0·05. All analyses were performed using the SPSS 20.0 software (IBM SPSS Statistics).
Results
Participants’ physical characteristics and dietary profile
Participants’ characteristics are presented in Table 1. The dietary profile was similar among trials, with the exception of protein intake (Table 2). Baseline values of muscle performance variables were indistinguishable between trials (Fig. 2), indicating that the 6-week washout period was efficient in eliminating the systemic inflammatory responses induced by the first trial.
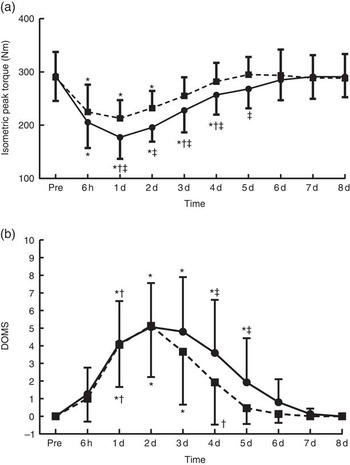
Fig. 2 Changes of muscle performance in response to protein and placebo administration. (a) Isometric peak torque and (b) delayed onset of muscle soreness (DOMS) at baseline (Pre) and throughout recovery in the two trials. Values are means and standard deviations. ![]() , Placebo;
, Placebo; ![]() , protein. * Significantly different from baseline (P<0·05). † Significantly different from the previous time point (P<0·05). ‡ Significant difference between placebo and protein (P<0·05).
, protein. * Significantly different from baseline (P<0·05). † Significantly different from the previous time point (P<0·05). ‡ Significant difference between placebo and protein (P<0·05).
Table 2 Daily total dietary energy intake and macronutrient consumption during the course of placebo (PLA) and protein (PRO) trials (protein supplement not included) (Mean values and standard deviations)
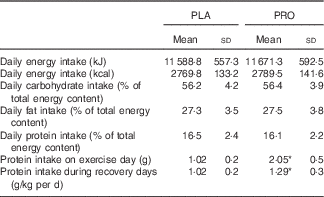
* Significant difference between trials (P<0·05).
Skeletal muscle performance
With PLA, peak torque (Fig. 2(a)) decreased at 6 h post exercise and remained below baseline until day 4 (−39 to −12 %, P<0·05), reaching its lowest value at 1 d of recovery. With PRO, peak torque remained below baseline until day 2 (−26·5 to −20 %, P<0·005) and recovered thereafter. Supplementation with PLA resulted in greater decline of isometric peak torque during the first 5 d of recovery compared with PRO (day 1: P=0·02, ES: −0·91; 95 % CI −1·79, −0·03; day 2: P=0·001, ES: −1·18; 95 % CI −2·09, −0·27; day 3: P=0·006, ES: −0·69; 95 % CI −1,55, 0·17; day 4: P=0·003, ES: −0·67; 95 % CI −1·52, 0·19; day 5: P=0·001, ES: −0·74; 95 % CI −1·61, 0·12). The online Supplementary Fig. S1 demonstrates percent changes between baseline and two dimensional in muscle strength of all participants. DOMS (Fig. 2(b)) in both trials increased at 6 h post exercise until day 7 (6 h: 4·1-fold in PLA v. 3·8-fold in PRO, P=0·000; day 1: 6·8-fold in PLA v. 5·6-fold in PRO, P=0·000; day 2:9·3-fold in PLA v. 8.1-fold in PRO, P=0·000; day 3: 8-fold in PLA v. 6.9-fold in PRO, P=0·000; day 4: 6·2-fold in PLA v. 5.2-fold in PRO, P=0·000; day 5: 4·5-fold in PLA v. 3.8-fold in PRO, P=0·000; day 6: 2·7-fold in PLA v. 2.2-fold in PRO, P=0·000; day 7: 1·4-fold in PLA v. 1.1-fold in PRO, P=0·002) and recovered on day 8. The rise in DOMS was of a lower magnitude in PRO compared with PLA on day 1 through day 5 of recovery (day 1: P=0·000, ES: 1·54; 95 % CI 0·58, 2·49; day 2: P=0·000, ES: 1·79; 95 % CI 0·80, 2·77; day 3: P=0·000, ES: 1·17; 95 % CI 0·26, 2·07; day 4: P=0·002, ES: 1·13; 95 % CI 0·23, 2·03; day 5: P=0·046, ES: 0·62; 95 % CI −0·23, 1·48).
Muscle damage and inflammatory responses
CK (Fig. 3(a)) increased throughout recovery in both trials (P=0·000–0·001). PRO exhibited a rise of lower magnitude than PLA at 1 d (P=0·001; ES 0·25; 95 % CI −0·59, 1·08) and 2 d (P=0·001; ES 0·2; 95 % CI −0·64, 1·04). PC (Fig. 3(b)) remained above baseline throughout recovery in PLA, demonstrating a peak on day 2 (3–24 %, P=0·000–0·002). PC in PRO remained elevated until day 2 (7–18 %, P=0·000), demonstrating a peak on day 2 and recovered thereafter. PC rise was greater in PLA than that in PRO at 1 d (P=0·002), 2 d (P=0·034; ES: 0·62; 95 % CI −0·2, 1·44) and 8d (P=0·037; ES: 0·36; 95 % CI −0·45, 1·16). In both trials, leucocyte counts (Fig. 3(c)) increased (P<0·05) 6 h post exercise, and were maintained above baseline values at 1 d of recovery and were normalised thereafter. However, there were no significant differences at any time point between PLA and PRO.

Fig. 3 Changes of muscle damage and inflammatory markers in response to protein and placebo administration. Changes in (a) creatine kinase activity (CK), (b) protein carbonyls (PC) and (c) leucocyte count during the two trials. Values are means and standard deviations represented by vertical bars.![]() , Placebo;
, Placebo; ![]() , protein. * Significantly different from baseline (P<0·05). † Significantly different from the previous time point (P<0·05). ‡ Significant difference between placebo and protein (P<0·05).
, protein. * Significantly different from baseline (P<0·05). † Significantly different from the previous time point (P<0·05). ‡ Significant difference between placebo and protein (P<0·05).
Proteasome activity and protein levels
Proteasome’s CT-L activity declined in PLA on day 2 (30 %, P=0·009) but not in PRO (Fig. 4(a)). Furthermore, the two trials differed in CT-L proteasome activity at 2 d (P=0·09, ES:−0·75; 95 % CI −1·61, 0·12). The online Supplementary Fig. S2 demonstrates individual percentage changes (eleven participants) in CT-L activity between baseline and 2 d. No changes were observed in T-L (Fig. 4(b)) and C-L (Fig. 4(c)) activities, in both trials. None of the β-subunit (catalytic proteasomal β-subunits β1, β2 (n 9) and β5 and the corresponding β1i, β2i (n 8) and β5i subunits of immunoproteasome) protein expression levels measured demonstrated any alterations following exercise in both trials (Fig. 5). As a quality control, blood levels of glucose and insulin were measured because increased insulin may lead to Akt phosphorylation and subsequent proteasome inhibition through the forkhead box O (FoXO) transcription factors( Reference Sala and Zorzano 41 ). Glucose and insulin remained unchanged throughout recovery in both trials (online Supplementary Fig. S3).
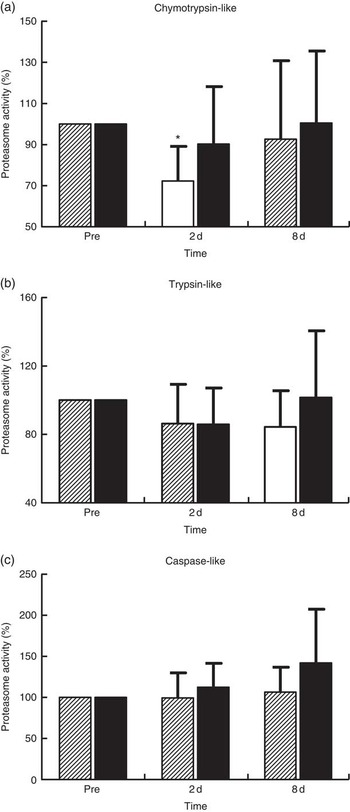
Fig. 4 Changes of proteasome activities in response to protein and placebo administration. Percentage change of (a) chymotrypsin-like activity, (b) trypsin-like activity and (c) caspase-like activity during the two trials. Values are means and standard deviations represented by vertical bars. ![]() , Placebo;
, Placebo; ![]() , protein. * Significantly different from baseline (P<0·05).
, protein. * Significantly different from baseline (P<0·05).
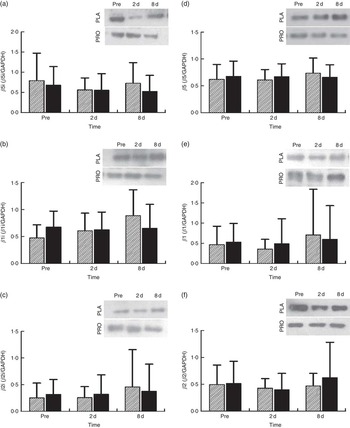
Fig. 5 Changes in the expression of proteasome subunits in response to protein (PRO) and placebo (PLA) administration. Changes in PRO levels of (a) β5i, (b) β1i, (c) β2i, (d) β5, (e) β1 and (f) β2 proteasome subunits during the two trials. Values are means and standard deviations represented by vertical bars. ![]() , PLA;
, PLA; ![]() , PRO.
, PRO.
Intracellular signalling proteins
NF-κB phosphorylation (Fig. 6(a), n 8) in PLA increased by approximately 45 % at 2 d (P=0·036) and by approximately 111 % at 8 d (P=0·05). In PRO, although NF-κB phosphorylation increased by 18 % at 2 d and by 130 % at 8 d, no statistical significance was detected for both time points. The online Supplementary Fig. S4 demonstrates individual percentage changes (eight participants) of NF-κB phosphorylation between baseline and 2 d. Immunoblot analysis for HSP70 (n 8; Fig. 6(b)) was also performed as it is considered to be a potent activator of proteasome. In PLA, although HSP70 increased at 2 d by approximately 162 % and at 8 d by approximately 194 %, only the later change was found to be statistically meaningful (P=0·069). HSP70 remained unaffected in PRO throughout recovery (2 d: 18 %; 8 d: 85 %). The online Supplementary Fig. S5 illustrates individual percentage changes (eight participants) of HSP70 between baseline and 2 d.
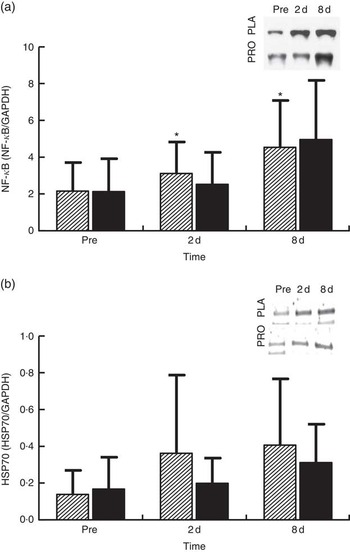
Fig. 6 Changes of molecules regulating proteasome activity in response to protein (PRO) and placebo (PLA) administration. Changes in PRO levels of (a) phosphorylated NF-κB and (b) HSP70 during the two trials. Values are means and standard deviations represented by vertical bars. ![]() , PLA;
, PLA; ![]() , PRO. Representative immunoblots are shown in panels. * Significantly different from baseline (P<0·05).
, PRO. Representative immunoblots are shown in panels. * Significantly different from baseline (P<0·05).
Correlations
Changes in isometric peak torque were not correlated with changes in proteasome activity (CT-L, C-L and T-L) or changes in the protein expression of β-(β5, β2 and β1) and βi-subunits (β5i, β2i and β1i) at 2 and 8 d. Similarly, no significant correlation was observed between DOMS and either proteasome activity or protein expression of β- and βi-subunits throughout recovery.
Discussion
In this study, we investigated the effect of exercise-induced aseptic inflammation and protein ingestion on UPS, muscle damage, inflammatory responses, as well as muscle function. The primary findings of this investigation suggest that milk protein concentrate supplementation may preserve the status of proteasome CT-L activity, as well as accelerate the rate of skeletal muscle strength recovery, under conditions of intense aseptic inflammation.
The extreme model of eccentric exercise applied in this study resulted in severe muscle injury and aseptic inflammation, as indicated by the increased levels of CK, leucocytes, PC and NF-κB phosphorylation. Furthermore, in agreement with previous work, we observed a pronounced decline in force-generating capacity along with a remarkable elevation in DOMS( Reference Michailidis, Karagounis and Terzis 8 , Reference Raastad, Owe and Paulsen 30 ). Although the rise observed in CK exceeded 2000 U/l during the first 2 d of recovery, it was of smaller magnitude than that previously observed in response to the same protocol( Reference Michailidis, Karagounis and Terzis 8 ). This difference may be attributed to the higher fitness status of participants in this investigation (VO2max: 56·4 (sd 6·8) v. 47·6 (sd 4·5) ml/kg per min), indicating a superior level of cardiovascular conditioning, which has been shown to positively affect muscle damage responses( Reference Morton, Kayani and McArdle 42 ).
The main proteolytic sites of UPS are hosted in the β1, β2 and β5 subunits that exhibit C-L, T-L and CT-L activities, respectively( Reference Chondrogianni, Petropoulos and Grimm 14 ). Although changes in the mRNA and protein expression of muscle atrophy F-box (MAFbx) and muscle ring finger-1 (MuRF1) have been extensively examined in response to different types and loads of exercise alone( Reference Rom and Reznick 43 – Reference Nedergaard, Vissing and Overgaard 46 ) or in combination with nutritional supplements( Reference Borgenvik, Apro and Blomstrand 47 – Reference Rahbek, Farup and de Paoli 49 ), alterations in proteasome activities and expression of the β-subunits have been investigated to a lesser extent in response to muscle inflammation. To the best of our knowledge, this is the first study examining the responses of β-subunits, βi-subunits (related to the immunoproteasome) and their activities to aseptic inflammation induced by extremely damaging exercise and in combination with protein supplementation.
Although the UPS proteolytic pathway is upregulated under inflammatory conditions( Reference Bowen, Schuler and Adams 50 , Reference Onesti and Guttridge 51 ), it seems that it contributes to the remodelling of skeletal muscle following muscle injury induced by exercise( Reference Taillandier, Combaret and Pouch 18 ). In previous studies, both mRNA and protein levels of ubiquitin ligases (MuRF1 and MAFbx/Atrogin-1) and the 20S proteasome have been repeatedly shown to be upregulated following eccentric exercise for as long as 24–48 h( Reference Rom and Reznick 43 , Reference Reid 45 , Reference Kostek, Chen and Cuthbertson 52 ). Moreover, increased CT-L activity has been observed at 14 d following downhill running( Reference Feasson, Stockholm and Freyssenet 53 ), further supporting the fundamental role of UPS in skeletal muscle remodelling( Reference Taillandier, Combaret and Pouch 18 , Reference Murton, Constantin and Greenhaff 19 ). In contrast, we observed a 30 % decrease in CT-L activity at 2 d post exercise that was not accompanied by any change in protein levels of 20S proteasome and immunoproteasome β-subunits and βi-subunits, respectively. It is characteristic that all participants demonstrated a decline of CT-L activity of proteasome at 2 d, suggesting that there was limited inter-individual variability on this outcome. Interestingly, our data corroborate previous findings, showing decreased CT-L activity (by 21 %) in response to extreme exercise such as a 200-km run( Reference Kim, Jamart and Deldicque 54 ). The authors assumed that an inhibitory mechanism results in the decline of proteasomal activity in response to increased load of oxidative stress( Reference Kim, Jamart and Deldicque 54 ). This assumption may also be valid in our case, as the 300 eccentric contractions protocol performed in our present study resulted in pronounced oxidative stress (40–95 %) and inflammatory responses that persisted throughout the recovery phase, especially in PLA, a response also reported previously( Reference Michailidis, Karagounis and Terzis 8 ). Likewise, a 200-km run, another form of excessive exercise resulting in EIMD, induced a marked increase in metallothionein 1F, metalothionein 1H and NADPH oxidase by approximately 519, 666 and 162 %, respectively( Reference Kim, Jamart and Deldicque 54 ). Therefore, reduced proteasomal activity has been observed only in response to cases of extreme exercise models (e.g., ultra-marathons, high-volume eccentric exercise) that predispose skeletal muscle to excessive ROS production and redox status disturbances. In agreement with this theory, an excessive amount of protein carbonylation in SH-SY5Y cells has been shown to cause a substantial decrease in proteasome S6 ATPase activity and 26S proteasome-mediated degradation( Reference Ishii, Sakurai and Usami 55 ).
On the other hand, protein supplementation prevented the decline of proteasome activity. Preservation of UPS activity, and as such clearance of oxidised proteins, might have also contributed to the accelerated recovery of the skeletal muscle following damaging exercise. Interestingly, individual changes of CT-L proteasomal activity at 2 d are very similar, with eight out of eleven subjects exhibiting either maintained or enhanced CT-L proteasomal activity and only three participants exhibiting reduced CT-L proteasomal activity in PRO. To explain this response, we measured changes in HSP70 expression and NF-κB phosphorylation. The molecular chaperone HSP70 protein is considered to directly activate the 26S proteasome activity, thereby preventing the accumulation of unfolding proteins and aggregates( Reference Reeg, Jung and Castro 56 , Reference Um, Im and Lee 57 ). HSP70 increased markedly at 2 and 8 d post exercise, but this rise was more pronounced in PLA compared with PRO. Indeed, it has been reported that HSP70 exhibits an intensity-dependent response to exercise( Reference Liu, Lormes and Baur 58 ). Furthermore, its expression has been shown to increase under conditions of elevated oxidative stress (as in this case) to promote degradation of oxidised proteins through the 20S proteasome following its dissociation with 26S( Reference Reeg, Jung and Castro 56 , Reference Grune, Catalgol and Licht 59 ). Interestingly, HSP70 has been shown to mediate the dissociation and re-association of the 26S proteasome in response to oxidative damage induced by an acute rise of ROS( Reference Grune, Catalgol and Licht 59 ). Therefore, the lower HSP70 response in PRO compared with that observed in PLA may have contributed to the lack of an adverse exercise effect on proteasome activity in PRO. Although the two trials demonstrated HSP70 changes of considerable difference in magnitude, no statistically meaningful differences were detected. However, when individual responses are observed, it is obvious that the vast majority of participants (six out of eight exhibited a reduced response in PRO, one had a similar response in the two trials and one had a more pronounced response in PRO) demonstrated a reduced HSP70 response in PRO compared with PLA.
The NF-κB pathway is one of the main redox-sensitive signalling pathways that regulates inflammatory and oxidative stress responses in human skeletal muscle( Reference Peake, Nosaka and Suzuki 1 , Reference Ji 5 , Reference Tidball and Villalta 60 ). It may be activated in response to elevated pro-inflammatory mediators and ROS, but it also controls the expression of genes including cytokines such as TNF-α, IL-6 and IL-1β, suggesting a vicious cycle between NF-κB activation, inflammatory and oxidative stress responses( Reference Li, Malhotra and Kumar 15 , Reference Draganidis, Karagounis and Athanailidis 61 ). The increased phosphorylation of NF-κB observed following eccentric exercise was mitigated by PRO (although it was not found to be statistically meaningful, NF-κB phosphorylation was lower in PRO compared with PLA by approximately 26 % at 2 d and approximately 23 % at 8 d). When individual responses are taken into account at 2 d, it appears that 50 % of participants showed an attenuated NF-κB response in PRO and 50 % did not. Similar perturbations of NF-κB signalling following aseptic muscle injury have been observed with ingestion of thiol-based and other antioxidants( Reference Michailidis, Karagounis and Terzis 8 , Reference Gomez-Cabrera, Martinez and Santangelo 62 ).
Because insulin levels may also interfere with UPS through the IGF1-Akt-FoXO signalling pathway( Reference Schiaffino, Dyar and Ciciliot 11 , Reference Greenhaff, Karagounis and Peirce 48 ), we measured glucose and insulin levels during recovery. Both glucose and insulin remained unaltered throughout recovery, suggesting that they had no impact on proteasome activity. This also indicates that the reduction observed in CT-L activity at 2 d was clearly induced by the exercise stimulus per se.
In our present study, protein supplementation resulted in the attenuation of markers of muscle damage as evidenced by CK and DOMS values during the post-exercise recovery. Specifically, the elevation of both plasma CK and reported DOMS was greater in the PLA group compared with the PRO group. This effect might be explained by the high BCAA content of milk protein( Reference Bendtsen, Lorenzen and Bendsen 63 ), which has been shown to attenuate DOMS following damaging exercise when consumed in adequate amounts( Reference Howatson, Hoad and Goodall 24 , Reference Shimomura, Inaguma and Watanabe 64 ). However, a number of studies reported no effect in response to BCAA supplementation( Reference Nosaka, Sacco and Mawatari 65 , Reference White, Wilson and Austin 66 ). This discrepancy among studies may be attributed to differences in the length of the supplementation period, as well as the amount of BCAA administered. For instance, in the study by Nosaka et al.( Reference Nosaka, Sacco and Mawatari 65 ), it was clearly shown that in contrast to acute supplementation (before and after exercise) long-term supplementation (before and after exercise and at eight more occasions) with amino acids during exercise recovery results in lower plasma CK and muscle soreness.
Soreness develops because of sensitisation of nociceptors at the level of the perimysium and epimysium by various inflammatory stimuli( Reference Shimomura, Inaguma and Watanabe 64 , Reference Proske and Morgan 67 ). In accordance with the above, in our present study, we observed an increase in CK, PC, NF-κB in PLA, whereas PRO resulted in attenuated inflammatory responses. Therefore, it is likely that protein supplementation reduces DOMS via the attenuation of specific inflammatory markers. An alternative mechanism explaining this protein effect on DOMS may be related to feeding per se, but a mechanism relating these two is lacking( Reference Howatson, Hoad and Goodall 24 , Reference Jackman, Witard and Jeukendrup 25 ).
In line with others, protein suppressed the exercise-induced decline in force-generating capacity and resulted in a faster strength recovery compared with PLA( Reference Cooke, Rybalka and Stathis 21 , Reference Buckley, Thomson and Coates 22 ). Although the molecular mechanisms are still obscure, recent evidence suggests that amino acids may promote muscle repair and remodelling by attenuating the excessive inflammatory responses following injury( Reference Kato, Miura and Nakano 68 – Reference Pereira, Baptista and Carlassara 70 ). In fact, administration of a leucine-enriched protein supplement (70 g protein/15 g leucine) following high-intensity endurance exercise activated a pro-inflammatory transcriptome at 30 min and promoted an anti-inflammatory and pro-myogenic transcriptome at 4 h, characterised by decreased leucocyte migration( Reference Rowlands, Nelson and Raymond 69 ). The transition from a pro- to an anti-inflammatory phenotype, mediated by the progressive shift of M1 macrophages to M2, is pivotal for muscle regeneration and growth( Reference Tidball and Villalta 60 ). In this study, PRO attenuated exercise-induced inflammatory response, as evidenced by the reduced PC and CK, as well as the unaltered phosphorylation levels of NF-κB at 2 and 8d. Thus, PRO may have resulted in a faster transition to an anti-inflammatory phenotype and consequently an accelerated recovery of muscle function. NF-κB activation may inhibit myogenesis and increase the expression of many pro-inflammatory cytokines (e.g. TNF-α, IL-6 and IL-1β) that are able to amplify the inflammatory response( Reference Tidball and Villalta 60 ). A recent animal study reported decreased IL-6 expression and enhanced muscle performance after damaging exercise following supplementation with leucine-enriched essential amino acids( Reference Kato, Miura and Nakano 68 ). Moreover, during phagocytosis, infiltrating neutrophils generate ROS through the NADPH oxidase, a phenomenon known as oxidative burst, which induces secondary damage in skeletal muscle( Reference Kyriakides, Austen and Wang 71 ). The lower PC levels observed in PRO may suggest, among others, reduced ROS generation that indicates reduced oxidative burst and as such smaller secondary muscle damage. This mechanism may also explain the attenuated reduction of force-generating capacity in PRO during recovery, as previously shown with thiol-based antioxidant supplementation in humans( Reference Michailidis, Karagounis and Terzis 8 , Reference Cobley, McGlory and Morton 72 ).
Proteasome activity though was not correlated with peak isometric torque and DOMS, suggesting that neither the former nor the latter is related directly to UPS. In contrast to these findings, the study of Pereira et al. ( Reference Pereira, Baptista and Carlassara 70 ) provided evidence that leucine supplementation in rats, after induction of muscle damage through cryolesion, improved muscle function via suppression of protein ubiquitination and decreased activation of FOX3a at days 3 and 10 of recovery. The discrepancy with that study may be partly explained by the fact that Pereira et al. ( Reference Pereira, Baptista and Carlassara 70 ) measured protein ubiquitination and expression of FOXO family transcription factors that lead to activation of E3 ligases, whereas we evaluated the direct proteasome activity and expression of specific catalytic subunits. Moreover, transcription factors other than FOXO1 and FOXO3a are probably responsible for the activation of E3 ubiquitin ligases in humans (e.g. MuRF1 and MAFbx/Atrogin-1) as compared with animals( Reference Stefanetti, Lamon and Rahbek 73 ).
Conclusions
This is the first investigation showing that under severe aseptic inflammation proteasomic activity may be severely impaired. Protein supplementation may not only prevent the attenuation of proteasome activity possibly via the expression of HSP70, reduced oxidative stress and altered NF-κΒ phosphorylation but also results in reduced muscle discomfort and an acceleration of skeletal muscle strength recovery. These results suggest that PRO may provide a viable nutritional intervention to enhance muscle remodelling and maintain muscle function under conditions of increased inflammation induced by exercise.
Acknowledgements
The authors acknowledge the subjects for their participation and commitment to the study.
The authors received departmental funding for this study.
D. D., N. C., A. C., G. T., L. G. K., A. Z. J. and I. G. F. contributed to the research design. D. D., N. C., A. C., G. T., A. S., A. A., M. P., A. Z. J. and I. G. F. contributed to study implementation. D. D., N. C., A. C., A. S., A. A., M. L., M. P., C. K. D., L. G. K. and I. G. F. performed the sample analysis and data interpretation. D. D., N. C., L. G. K. and I. G. F. wrote the first version of the manuscript. All authors contributed to the paper, reviewed it and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517001829










