Lycopene is an acyclic C40 non-polar carotenoid, present in several dietary sources such as tomato, watermelon, guava and apricot. Although not being a provitamin A, lycopene has been shown to have multiple biological activities including decreasing the risk of prostate cancer(Reference Gann, Ma and Giovanucci1), inhibition of cell proliferation, migration and invasion in breast of endometrial and liver carcinoma cells(Reference Blaner, Olson, Sporin, Roberts and Goodman2–Reference Nahum, Zeller and Danilenko7), and prevention of mutagenesis and chromosome instability(Reference Liu, Russell and Wang8, Reference Russell9). In addition, a variety of epidemiological trials indicated that high intakes of lycopene-containing foods (primarily tomato products) or elevated blood lycopene concentrations are associated with a decreased risk of CVD and prostate cancer(Reference Rao10–Reference Etminan, Takkouche and Caamaño-Isorna13).
Lycopene has eleven conjugated double bonds and each of them could be either in an (E) or (Z) configuration. (All-E)lycopene is the predominant isomer in plants, representing about 80–97 % of total lycopene in tomatoes and related products(Reference Shi and Le Maguer14). In human body fluids and tissues such as plasma, prostate, testis and skin, 25–70 % of lycopene is found in various (Z) forms(Reference Alien, Smith and Clinton15–Reference Wu, Schwartz and Platz20). The high concentrations of (Z)-isomers in vivo trigger the hypothesis that they are more bioavailable and/or that (all-E) lycopene is transformed into (Z)-isomers within the human body. Moreover, when a tomato-based meal rich in the (all-E) isomer is consumed daily over a few weeks, plasma lycopene concentration increases and its main part (60 %) is present as (Z)-isomers(Reference Richelle, Bortlik and Liardet21). Recently, we have demonstrated that lycopene isomerisation takes place in the enterocytes and not in the gastrointestinal lumen(Reference Richelle, Sanchez and Tavazzi22).
In terms of bioactivity, Shi & Le Maguer(Reference Shi and Le Maguer14) indicated that the biological potency of (Z)-lycopene isomers is different from that of the (all-E) form. Böhm et al. (Reference Böhm, Puspitasari-Nienaber and Ferruzzi23) reported that some (Z)-isomers have a stronger in vitro antioxidant activity than the (all-E) form. For these reasons, (Z)-lycopene isomers are regarded as offering potentially better health benefits than the (all-E)-isomer.
In the present study, we investigated, in human subjects, the effect of meals enriched in lycopene on the lycopene isomer profile of plasma TAG-rich lipoprotein (TRL) fractions. The main objective was to compare the profile of lycopene isomers appearing in TRL following the ingestion of tomato preparations rich in (Z)-lycopene isomers. Three different tomato preparations rich in Z-lycopene (about 70 % of total lycopene) were produced. One preparation was rich in 5-(Z) (65 %), one in 13-(Z) (42 %) and one in a mixture of 9-(Z) (31 %) and 13-(Z) (24 %) isomers. Another objective was to determine if the tomato preparation can affect the intestinal isomerisation of (all-E) lycopene during absorption.
Experimental methods
Lycopene preparations
Tomato preparations rich in (Z)-lycopene isomers
Tomato preparation rich in 5-(Z) lycopene isomer
Produced by mixing 10 g of tomato oleoresin in 500 ml dichloromethane with 580 μl iodine solution (5·6 mg iodine in 596 μl methylene chloride). The mixture was photoisomerised for 10 h at room temperature. Photoisomerised tomato oleoresin was further enriched in 5-(Z) lycopene by successive fractionation in ethanol and in ethyl acetate. The solid fraction was then re-suspended in ethyl acetate (1:80 w/w) and the suspension centrifuged at 16 900 g for 5 min at room temperature. The solid fraction was discarded and the tomato preparation enriched in 5-(Z) lycopene was obtained by distilling ethyl acetate under reduced pressure at 30°C. This preparation provided 65 % of total lycopene as 5-(Z) isomer (Table 1).
Table 1 Lycopene isomer profile of the six lycopene preparations
(Percentage of the total lycopene)
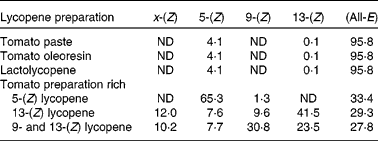
ND, not defined.
Tomato preparation rich in 13-(Z) lycopene isomer
A suspension of tomato oleoresin in ethyl acetate (1:10 v/v) was refluxed for 1 h under stirring. After cooling to room temperature, the precipitate (approximately 10 % w/w of initial raw material) was filtered off. This precipitate contained mainly (all-E) lycopene isomer. The isomerised tomato preparation was obtained by evaporating ethyl acetate from the filtrate under reduced pressure at 30°C and by azeotropic distillation after addition of water and ethanol (water:ethanol ratio: 8:2). This preparation provided 70 % of total lycopene as (Z)-isomers with 42 % as 13-(Z) lycopene (Table 1).
Tomato preparation rich in 9- and 13-(Z) lycopene isomers
A similar process as the one described for the tomato preparation rich in 13-(Z) lycopene was performed, with the exception that the heating duration had been increased up to 24 h. This preparation provided 72 % of total lycopene as (Z)-isomers, with 31 % as 9-(Z) lycopene and 24 % as 13-(Z) lycopene (Table 1).
Tomato preparations rich in (all-E) lycopene
Three tomato products, i.e. a commercial tomato paste packed in a 300 g tube (Thomy, Vevey, Switzerland), a tomato oleoresin (Indena, Milan, Italy) and a Lactolycopene™ preparation (Indena), contained about 95 % of total lycopene as the (all-E) isomer.
The lycopene isomer profiles of the tomato supplements are presented in Fig. 1 and Table 1. With the exception of tomato paste, the daily doses of test products were packed separately as aliquots in light-protected containers, sealed under N2 and stored at − 80°C during the course of the study. The lycopene content and profile of isomers were shown to be stable for 6 months at − 40°C in the tomato products, indicating that the storage conditions were adequate.

Fig. 1 HPLC chromatograms of the lycopene isomer profiles in the tomato paste rich in (all-E) lycopene and in the three tomato preparations rich in (Z)-lycopene isomers.
Subjects
A total of thirty healthy men were enrolled in the study. Of these, twenty-seven subjects, aged 24 (sem 1) years, completed the study. Their mean starting body weight was 70 (sem 1) kg and BMI 22·5 (sem 0·3) kg/m2. Subjects were normolipidaemic, i.e. they had a ratio of plasma cholesterol to HDL cholesterol < 5·0 and plasma TAG concentrations < 1·5 mmol/l. Because of the large amount of blood that was drawn during the study, subjects were required to have a blood Hb concentration >1·3 g/l as inclusion criteria. The subjects did not use medication; neither did they have a history of gastrointestinal disease or lipid metabolic disorders. Three volunteers abandoned the trial before the end for the following reasons: unavailability, medical treatment related to an eye injury, nausea related to the consumption of fatty meals.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the ethical committee of Marseille (Marseille, France). All subjects received information on background and design of the study and gave written informed consent before participation. They were free to withdraw from the study at any time.
Study design
The study was a randomised, six-period, six-treatment crossover clinical trial with a washout period of 3 weeks minimum. Subjects and technicians were blinded while the investigator was blinded for five tomato products but not for the tomato paste which was a commercial product. Subjects were asked to refrain for 48 h before the postprandial tests from eating lycopene-rich products: tomatoes (crude, cooked and tomato sauces including ketchup and harissa), pizza, ratatouille, lasagna, pasta including tomato sauce, watermelon, pink grapefruits and guava. In addition to this dietary restriction, the subjects ate a standard meal the evening before the postprandial tests consisting of green vegetables, a source of cereals (pasta, bread or rice), lean meat or fish, low-fat natural yoghurt, one fruit and mineral water. They had to consume this dinner in the evening between 19.00 and 20.00 hours. These recommendations were checked by the investigator on the postprandial test day.
After an overnight fast, subjects arrived at the Clinical Pharmacology and Therapeutic Trial Center of the University of Marseille (Marseille, France) and consumed a standard meal consisting of semolina (70 g) cooked in 200 ml hot water, white bread (40 g), egg-whites (60 g), groundnut oil (40 g), natural yoghurt (125 g), sugar (5 g) and water (330 g). This standard meal provided 842 kcal (3520 kJ) with the following nutrient composition: protein (11·7 %), carbohydrates (39·3 %) and lipids (49·0 %). A portion of one of the six lycopene preparations adapted to provide 25 mg of lycopene was incorporated into the test meal just before its consumption. This meal was consumed within 30 min. No other food was allowed over the following 6 h, but the subjects were allowed to drink bottled water up to a maximum of 330 ml. Fasting blood was drawn from an anticubital vein by venepuncture into an evacuated tube containing potassium EDTA/K3 that was immediately placed in an ice-water-bath and covered with an aluminium foil to avoid light exposure. Blood samples were collected 20 min and 5 min before consumption of the standard meal as well as after 2, 3, 4, 5 and 6 h post-absorption.
This short postprandial time frame, i.e. 6 h, was chosen to assess lycopene isomerisation within the intestine with as low contamination by lycopene isomers coming from other organs (mainly the liver) as possible. Indeed, during the postprandial period, chylomicrons secreted by the intestine, which contained lycopene coming from the meal, are transformed into chylomicron remnants following TAG hydrolysis by lipoprotein lipase. These chylomicron remnants are taken up by the liver. A fraction of dietary lycopene is then incorporated in VLDL and released in the blood. It is not known, but possible, that an isomerisation of lycopene also occurs in the liver leading to an increase in blood concentration of lycopene isomers. Because liver VLDL start to be produced some hours after a fat meal intake, the longer the postprandial period, the higher the level in the blood of VLDL coming from the liver. As, technically, it is quite complicated to isolate the chylomicron remnants from the VLDL, we decided to collect the TRL during the initial postprandial period so that they contain mostly lipoproteins of intestinal origin.
Blood sample preparation
Tubes containing the blood were protected from light, stored at 4°C and then centrifuged within 2 h (10 min, 4°C, 878 g) to separate the plasma. On the test day, plasma (6 ml) was overlaid with 0·9 % NaCl solution and centrifuged for 28 min at 130 000 g at 10°C in an SW41Ti rotor (Beckman, Fullerton, CA, USA) in an L7 ultracentrifuge (Beckman). The upper phase containing TRL, i.e. mainly chylomicrons and chylomicron remnants, was collected. Immediately after recovery, TRL were divided into aliquots and immediately stored at − 80°C before lycopene isomer profile determination.
Analytical procedures
The profile of lycopene isomers was determined according to the method described previously by Schierle et al. (Reference Schierle, Bretzel and Bühler18) for lycopene products (Fig. 1), and according to the method of Ferruzzi et al. (Reference Ferruzzi, Nguyen and Sander24) for TRL. The main lycopene isomers identified were 5-(Z), 9-(Z), 13-(Z) and (all-E) lycopene. Minor compounds eluting between 24 and 48 min (Fig. 1) were identified as (Z)-lycopene by LC–MS/MS using an Applied Biosystems APCI 4000 LC–MS/MS (Foster City, CA, USA). The following conditions were used: isocratic flow, 1 ml/min; declustering potential, 130 V; 60 pounds per square inch (414 kPa) N2; capillary voltage, 22 V; vaporiser temperature, 400°C; corona needle, 5 mA; the fragmentation conditions used were those described by dos Anjos Ferreira et al. (Reference dos Anjos Ferreira, Yeum and Russell25). The peak areas of unidentified (Z)-lycopene isomers were summed up and reported as x-(Z) lycopene (Table 1).
Data analyses
The raw data consisted of TRL-lycopene isomer concentrations (expressed in mmol/l) of five lycopene isomers, i.e. x-(Z), 5-(Z), 9-(Z), 13-(Z) and (all-E), measured at six time points (before and, 2, 3, 4, 5 and 6 h after ingestion of the standardised meal containing lycopene) for each of the six different test meals and each of the twenty-seven subjects. These raw data were averaged for the twenty-seven subjects and presented as means with their standard errors for each time point (Fig. 2). The baseline was the average of the two lycopene concentrations measured in plasma samples collected before consumption of the meal. As our goal was to determine lycopene isomerisation during the absorption phase, we voluntarily limited the measurement of lycopene isomer profiles to a short period of time after lycopene intake. Thus, we measured mainly lycopene present in chylomicrons in the TRL fraction, with only some lycopene present in the VLDL fraction coming from the liver. These purely descriptive analyses confirmed that lycopene concentrations did not return to baseline 6 h after ingestion of the test meal. In consequence, no formal kinetics modelling (e.g. area under the curve, T max or C max) was further considered. In order to complete this descriptive data analysis and to simplify the discussion of results, individual lycopene isomers were expressed as relative percentage of the total lycopene calculated as the sum of the five lycopene isomers, i.e. x-(Z), 5-(Z), 9-(Z), 13-(Z) and (all-E) (Fig. 3).

Fig. 2 TAG-rich lipoproteins-lycopene isomer concentrations over 6 h post-consumption of the standard meals providing 25 mg lycopene from various sources. (a) Preparation rich in 5-(Z) lycopene, (b) preparation rich in 13-(Z) lycopene, (c) preparation rich in 9-(Z) and 13-(Z) lycopene, (d) tomato paste rich in (all-E) lycopene, (e) lactolycopene rich in (all-E) lycopene and (f) oleoresin rich in (all-E) lycopene. Values are means, with their standard errors represented by vertical bars (nmol/l, n 27). x-(Z) lycopene (♦); 5-(Z) lycopene (■); 9-(Z) lycopene (▲);13-(Z) lycopene ( × ); (all-E) lycopene (*).

Fig. 3 TAG-rich lipoproteins-lycopene isomer profiles over 6 h post-consumption of the standard meals providing 25 mg lycopene from various sources. (a) Preparation rich in 5-(Z) lycopene, (b) preparation rich in 13-(Z) lycopene, (c) preparation rich in 9-(Z) and 13-(Z) lycopene, (d) tomato paste rich in (all-E) lycopene, (e) lactolycopene rich in (all-E) lycopene and (f) oleoresin rich in (all-E) lycopene. Values are means, with their standard errors expressed as percentage of the total lycopene represented by vertical bars (n 27). x-(Z) lycopene (♦); 5-(Z) lycopene (■); 9-(Z) lycopene (▲); 13-(Z) lycopene ( × ); (all-E) lycopene (*).
Results
Following the ingestion of the six standardised meals supplemented with lycopene, TRL-lycopene concentrations increased promptly, reaching a first maximum concentration between 2 and 4 h, and started to increase again after 5 h (Fig. 2). Since lycopene isomer profiles were determined over a short period of time only, the second maximum was not well characterised. These two rises of lycopene concentrations are in agreement with the known lycopene absorption processes and show a first appearance of dietary lycopene in chylomicrons (before 4 h) and a subsequent incorporation of dietary lycopene chylomicrons into newly released VLDL from the liver (after 5 h). In consequence, our choice for a short postprandial time frame (0–6 h), which accounts for the appearance of lycopene in chylomicrons, is ideal to determine lycopene isomerisation within the intestine.
Interestingly, the profile of lycopene isomers expressed in percentage (Fig. 3) did not vary over the 6 h post-absorption. Moreover, the inter-individual variability of concentrations (Fig. 2) and percentages of lycopene (Fig. 3) were very small.
Proportion of lycopene isomers in TAG-rich lipoprotein after consumption of tomato preparations rich in (Z)-lycopene
Consumption of a tomato preparation rich (65 %) in 5-(Z) lycopene resulted in a close proportion (60 %) of this isomer in TRL, suggesting a good absorption and a low isomerisation of this isomer in the enterocytes (Figs. 2(a) and 3(a)). The other lycopene isomers observed in TRL consisted of 20 % of (all-E), 9 % of x-(Z), 7 % of 13-(Z) and 2 % of 9-(Z). Since these (Z)-isomers were not present in the diet, their appearance in TRL suggests that they were formed during absorption by isomerisation of the (all-E) lycopene.
Only a low amount (9 %) of the 13-(Z) isomer was measured in TRL following the intake of a tomato preparation which contained 42 % of this isomer (Figs. 2(b) and 3(b)). Other lycopene isomers measured in TRL were mainly the (all-E) (about 50 %) and the 5-(Z) (25 %) isomers, suggesting that part of the 13-(Z) lycopene was isomerised into these isomers during absorption.
Eating the tomato preparation rich in a mixture of 9-(Z) and 13-(Z) lycopene induced a release of lycopene in TRL containing mostly the (all-E) (42 %) and 5-(Z) (27 %) isomers (Figs. 2(c) and 3(c)), suggesting that the 9-(Z) and 13-(Z) isomers were isomerised into the (all-E) and 5-(Z) isomers.
Proportion of lycopene isomers in TAG-rich lipoprotein after consumption of tomato preparations rich in (all-E) lycopene
Consumption of the tomato paste rich in (all-E) lycopene (about 95 %) resulted in observing various lycopene isomers in TRL, i.e. roughly 60 % (all-E) and 40 % (Z)-isomers, with about 18 % of 5-(Z), 10 % of 13-(Z) and x-(Z), and 2 % of 9-(Z) (Figs. 2(d) and 3(d)). The appearance of isomers that were not detected in the tomato paste, i.e. 9-(Z), 13-(Z) and x-(Z), strongly suggests that part of the (all-E) was isomerised during absorption.
The same profile of lycopene isomers in TRL was also observed with the two other tomato preparations, i.e. lactolycopene and oleoresin, containing about 95 % (all-E) lycopene (Figs. 2(e), (f) and 3(e), (f)). These results indicate that the three tomato preparations rich in the (all-E) isomer were similarly isomerised during their absorption in human subjects and, therefore, that the lycopene food matrix did not influence it.
Discussion
We have recently demonstrated that, during absorption, a significant isomerisation of (all-E) lycopene into various (Z)-lycopene isomers takes place in human enterocytes(Reference Richelle, Sanchez and Tavazzi22). The first objective of the present study was to determine if the lycopene isomer profile in the blood circulation was affected by the isomer profile in the meal.
Consumption of the tomato preparation rich in 5-(Z) lycopene led to a very high proportion of 5-(Z) lycopene isomer in TRL (about 60 %), which indicates either a good absorption, or a greater thermodynamical stability(Reference Chasse, Mak and Deretey26) of this isomer, or both.
The consumption of either a preparation rich in a mixture of 9-(Z) and 13-(Z) or a preparation rich in 13-(Z) lycopene was associated with the release of TRL which contained lower proportions of these two isomers as compared to those present in the tomato preparations. These results indicate either a low absorption of these isomers or an isomerisation of these isomers into more thermodynamically stable lycopene isomers, i.e. (all-E) and 5-(Z), during absorption. The second hypothesis seems the most likely, as the proportion of the (all-E) and 5-(Z) isomers measured in TRL after intake of the preparation rich in 9-(Z) and 13-(Z) isomers was higher than in the preparation itself.
These results show that although the human intestine is able to modify the profile of dietary lycopene isomers which is finally found in the body, the resulting profile is not the same for all isomer sources supplied but is dependent on the initial relative proportions of these isomers existing in the meal. This suggests that the mechanism of (E)-to-(Z) conversion can be overwhelmed by offering high amounts of dietary lycopene (Z)-isomers, e.g. 5-(Z). It is, therefore, possible to modulate the profile of (Z)-isomers which enter the human body via the intestinal lipoproteins by a lycopene isomer-rich diet.
Besides, it is well known that the bioavailability of (all-E) lycopene is dependent on the tomato preparation(Reference Richelle, Bortlik and Liardet21). In agreement with results reported in the literature(Reference Alien, Smith and Clinton15, Reference Holloway, Yang and Paganga17, Reference Fröhlich, Kaufmann and Bitsch27), we have shown that consuming a tomato paste which contained mainly (95 %) (all-E) lycopene resulted in secretion of TRL containing 40 % of lycopene (Z)-isomers(Reference Richelle, Bortlik and Liardet21, Reference Richelle, Sanchez and Tavazzi22), which suggests that part of the ingested (all-E) lycopene was converted into (Z)-isomers. The secondary objective of this work was to assess whether the preparation of (all-E) lycopene affects the isomerisation of the (all-E) lycopene during intestinal absorption. Present results show that consumption of the three tomato preparations rich in the (all-E) isomer (tomato paste, (all-E) oleoresin and lactolycopene) resulted in a similar isomerisation of the (all-E) lycopene. This shows that lycopene isomerisation occurring in the enterocytes is not significantly affected by the lycopene preparation. Another key observation is that the main (Z)-isomer recovered in TRL was the 5-(Z) lycopene (about 20 % of the total lycopene content). This suggests that either the 5-(Z) lycopene is specifically formed during isomerisation of the (all-E) lycopene, or the 5-(Z) isomer, being more stable than the others(Reference Chasse, Mak and Deretey26), leads to its preferential accumulation during absorption, or both.
Interestingly, there is a low inter-individual variation of lycopene isomerisation during intestinal absorption, which is in agreement with previous results observed with tomato paste(Reference Richelle, Sanchez and Tavazzi22).
(Z)-lycopene isomers have been discussed for their specific bioactivity. Indeed, individual (Z)-lycopene isomers exhibit different antioxidant activities(Reference Böhm, Puspitasari-Nienaber and Ferruzzi23) and some human organs like prostate(Reference Clinton, Emenhiser and Schwartz16) and skin accumulate high amounts of (Z)-lycopene isomers. More research is needed to evaluate how chronic supplementation with a diet enriched in (Z)-lycopene isomers will affect their distribution in tissues and subsequently will modulate lycopene bioactivity in humans.
Acknowledgements
The present study was funded in full by the Nestlé Research Center, Lausanne, Switzerland. M. R., P. L., C. J., P. B. and K. B. conceived and designed the study. I. T., P. L., A.-F. M. and K. B. achieved the design and production of formulations of the lycopene products. C. J. coordinated the trial and supervised the analytical aspects. C. J. and P. B. contributed to the development of analytical methods, lycopene analysis and data collection. M. R., P. L., C. J., P. B., K. B. and A. R. analysed the data. M. R. wrote the manuscript, and all authors were involved in interpreting the results and in critical revision of the paper. No author has any advisory board affiliation. The authors declare that they have no conflicts of interest.






