Maternal and child malnutrition, which is generally caused by food insecurity that led to nutritional deficiencies, was the ninth leading cause of death worldwide in 2019(Reference Murray, Aravkin and Zheng1). Micronutrient deficiency has a negative impact on different aspects of human health, but major effects are observed during childbearing years in women and childhood(Reference Carazo, Macáková and Matoušová2). Vitamin A deficiency is the most important cause of preventable blindness and a risk factor for morbidity and mortality during childhood and in reproductive period, especially in low- and middle-income countries(Reference Murray, Aravkin and Zheng1–3). Vitamin A deficiency is diagnosed mainly in surveys to estimate the prevalence of low serum retinol concentrations, a population biochemical indicator(4) and the presence of night blindness, a functional indicator(5).
In areas where vitamin A deficiency is considered a public health problem, the intervention measures that are commonly applied include supplementation, encouragement of consumption and the fortification of foods that are sources of vitamin A. Among the available strategies, vitamin A supplementation used to be recommended during pregnancy, the postpartum and childhood(Reference Oliveira, Allert and East6,7) . The current WHO guidelines do not recommend vitamin A supplementation for postpartum women due to the lack of benefits in the long term(8).
In Brazil – an emerging economy marked by high inequality – nationwide surveys are needed to assess the population’s nutritional status and thus guide public policies, but their occurrence is infrequent. The last national survey carried out in Brazil on the population’s food consumption was the Family Budget Survey in 2017–2018, which showed a prevalence of 89 % of inadequate vitamin A intake in the Brazilian population aged 19 to 59 years in both sexes, an increase compared with the 2008–2009 survey, which showed an intake deficiency of 85 % in the same group(9,10) . Nationally, vitamin A deficiency was assessed more than 15 years ago, and 12 % of Brazilian women of reproductive age presented vitamin A deficiency(11).
The lack of updated nationwide prevalence of vitamin A deficiency requires an effort to estimate the proportion of Brazilian women with this deficiency during their reproductive period. The aim of this research was to estimate the vitamin A deficiency prevalence in Brazilian women during their reproductive age.
Methods
The protocol containing the detailed methods of this systematic review was registered in the International Prospective Register of Systematic Reviews (CRD42020171856). This report followed the recommendations of the 2020 Preferred Reporting Items for Systematic Review and Meta-analysis(Reference Page, McKenzie and Bossuyt12).
Eligibility criteria
We considered eligible observational or experimental studies that assessed vitamin A deficiency in women of childbearing age (15–49 years of age) in Brazil(13), regardless of population representativeness. Case–control studies were excluded because reliable prevalence data cannot be derived from this study design due to the nature of the sample (i.e. one group previously known with the condition of interest and one group without). All vitamin A statuses were assessed at the serum retinol levels’ cut-off points: normal (< 1·05 µmol/l or < 0·30 µg/dl), deficiency (< 0·70 µmol/l or < 0·20 µg/dl) and critical (< 0·35 µmol/l or < 0·10 µg/dl).
Information sources
The searches were carried out in MEDLINE, Embase, Scopus, LILACS, SciELO and Brazilian theses and dissertations databases (Brazilian Coordination for the Improvement of Higher Education Personnel and repositories of Brazilian universities with a postgraduate programme of collective health and nutrition). We screened the references of relevant publications to identify additional potentially eligible studies. No language or publication status restrictions were applied.
Search strategy
A pilot strategy was developed for MEDLINE database (via PubMed) and adapted for the other databases, following the recommendations of the Peer Review of Electronic Search Strategies guidance(Reference McGowan, Sampson and Salzwedel14). The final strategy for bibliographic databases is shown in online Supplementary Table S1. For theses and dissertations, the following keywords in Portuguese, in combination or separately, were used: ‘deficiência’, ‘vitamina’, ‘hipovitaminose’ and ‘retinol’. The search results in compatible formats were imported into the Covidence platform (www.covidence.org) to remove duplications and further review steps. Searches in all databases were carried out on 2 June 2020 and fully updated on 31 March 2022.
Selection process
Six researchers (CMF, DAH, DFSA, JMO, MTS, TFG) in pairs independently selected the studies from title and abstracts and then in full-text assessment using the Covidence platform. Discrepancies were resolved in consensus meetings. Calibration of the selection process was carried out in meetings to assess discordances after the selection of 100 studies. For theses and dissertations, one reviewer (CMF) screened the search results, and eligibility was confirmed by a second researcher (DFSA), using an Excel spreadsheet.
Data collection process
Six researchers (CMF, DAH, DFSA, JMO, MTS, WWGS) in pairs independently extracted the data from the studies using an extraction form customised in Covidence. Calibration of the collection process was carried out in meetings to assess discordances after the extraction of two studies to standardise the process and adapt the extraction form as needed. One author (TFG) compared the independent extraction and resolved discrepancies to define the final extracted data to be included. To harmonise the data from studies with multiple publications, the main study information was compared in an Excel spreadsheet by one author (WWGS), and the report with the most complete data was defined by another author (TFG). The study’s authors were contacted to obtain additional data in case of absence in the reports or to clarify conflicting information included in different reports of the same study.
Data items
We extracted data about the study (author, year of data collection, research location, sample source, study design), characteristics of the population (age, number of women, reproductive status) and outcome (biological matrix, technique for analysis, cut-off point adopted, number of women with deficiency of vitamin A and total of women assessed).
Study risk of bias assessment
Paired and independent researchers (CMF, DAH, DFSA, JMO, MTS, WWGS) assessed the methodological quality of the included studies using the Joanna Briggs Institute’s checklist for prevalence data(Reference Munn, Moola and Lisy15), loaded in the extraction form in Covidence consisting of the items: (i) sample frame, (ii) sampling method, (iii) sample size, (iv) setting and participants description, (v) coverage of data analysis, (vi) methods for outcome measurement, (vii) the condition measured in a standard and reliable, (viii) statistical analysis and (ix) response rate (online Supplementary Table S2). Reviewers assigned one point for each item properly addressed by the studies, with a maximum score of nine per study. One author (TFG) checked the independent ratings of the risk of bias and assigned the final assessment, resolving discrepancies.
Effect measures
The primary outcome was the prevalence of vitamin A deficiency (serum retinol < 0·70 µmol/l or < 0·20 µg/dl) and 95 % CI. Secondary outcomes included the prevalence of normal (serum retinol < 0·30 µg/dl or < 1·05 µmol/l) and critical (serum retinol < 0·10 µg/dl or < 0·35 µmol/ l) levels, prevalence of deficiency by reproductive status (pregnant, postpartum, breast-feeding and not pregnant women), Brazilian geographic regions (North, Northeast, Midwest, Southeast and South) and decade of research (≤ 1990s, 2000s and 2010s).
Synthesis methods
Meta-analysis of proportions was calculated with Freeman–Tukey double arcsine transformation (metaprop command, ftt option in Stata software v. 14.2)(Reference Newcombe16,Reference Barendregt, Doi and Lee17) . Heterogeneity was estimated by the assessment of inconsistency between studies (I²) and χ 2 tests, with a significance level of P < 0·10(Reference Higgins and Thompson18).
Meta-regressions were calculated using the modified Knapp–Hartung method to investigate the effects of independent variables (year of research, decade, quality score, Brazilian region) on the variability of the prevalence of vitamin between studies(Reference Knapp and Hartung19). Sensitivity analysis to assess the robustness of the results against the removal of studies with discrepant results was performed.
Reporting bias assessment
Publication bias was assessed by visual inspection of funnel plots of the log odds of vitamin A deficiency against standard error of the estimate and of the log odds of vitamin A deficiency against the study size(Reference Hunter, Saratzis and Sutton20) and Egger’s test at the significance level of P < 0·05(Reference Egger, Smith and Schneider21).
Results
Study selection
Out of 3529 retrieved records, 108 were selected for full-text assessment and seventy-six reports from thirty-two studies were included in the review (Fig. 1). We contacted fourteen authors (regarding twenty studies), and eleven (regarding seventeen studies) provided clarification or additional data based on our request. The reasons for exclusions of the studies sought for full-text assessment are shown in online Supplementary Table S3, and the list of reports of included studies is available in online Supplementary Table S4.
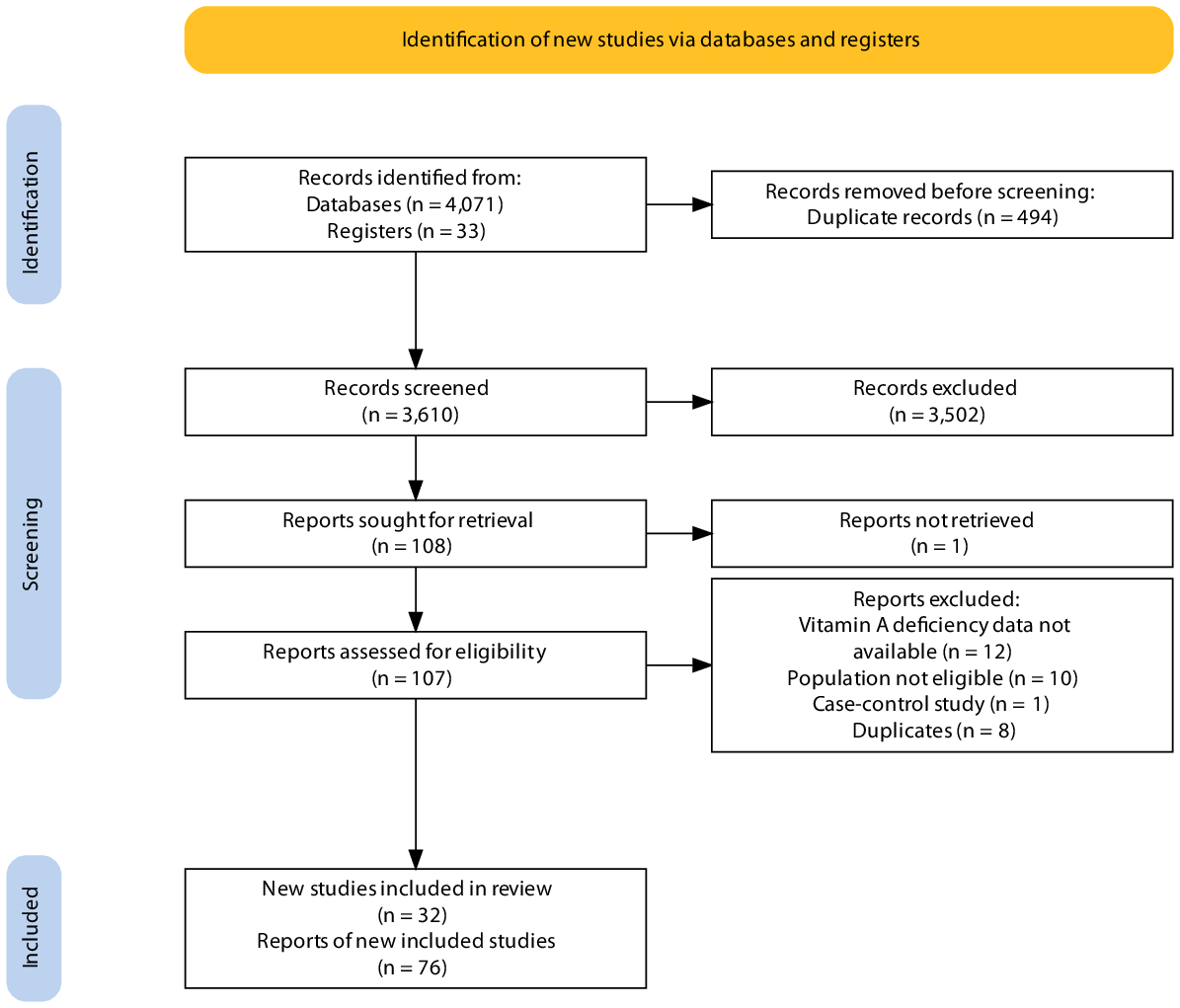
Fig. 1. Process of selection and inclusion of studies in the review.
Study characteristics
In total, 12 577 women were assessed in all included studies conducted from 1965 to 2017. Most of the studies were cross-sectional, held at institutions such as hospitals, conducted in the Northeast and Southeast regions, and included women aged 10–60 years (Table 1). The methodological quality score ranged from 2 to 9 with a median of 6, with high risk being more frequent in the sample frame (30/32) and sampling method (29/32), while all studies met the requirements of valid methods to diagnose vitamin A deficiency and measurement of the outcome in a standard, reliable way for all participants (online Supplementary Table S5).
Table 1. Characteristics of included studies

PNDS, Pesquisa Nacional de Demografia e Saúde (National Demographic and Health Survey).
* Mean age.
† Frequency of age for most of the sample.
Results of syntheses
The prevalence of vitamin A deficiency in women was 13·1 % (95 % CI 9·4, 17·2; 29 studies; I² = 96·6 %). The Midwest region had higher prevalence of vitamin A deficiency (12·8 %; 95 % CI 11·0, 14·9; 1 study), similar to the Southeast region (11·7 %; 95 % CI 10·6, 12·8; 13 studies; I² = 97·4 %), while lower prevalence was noted in the South region (8·0 %; 95 % CI 6·6, 9·7; 1 study) (Fig. 2). After removal of a discrepant study with highest prevalence rate (Deminice 2018(Reference Deminice, Ferraz and Monteiro28)), prevalence and heterogeneity did not significantly change. Since no reason for exclusion was identified, the study was kept in the final result.
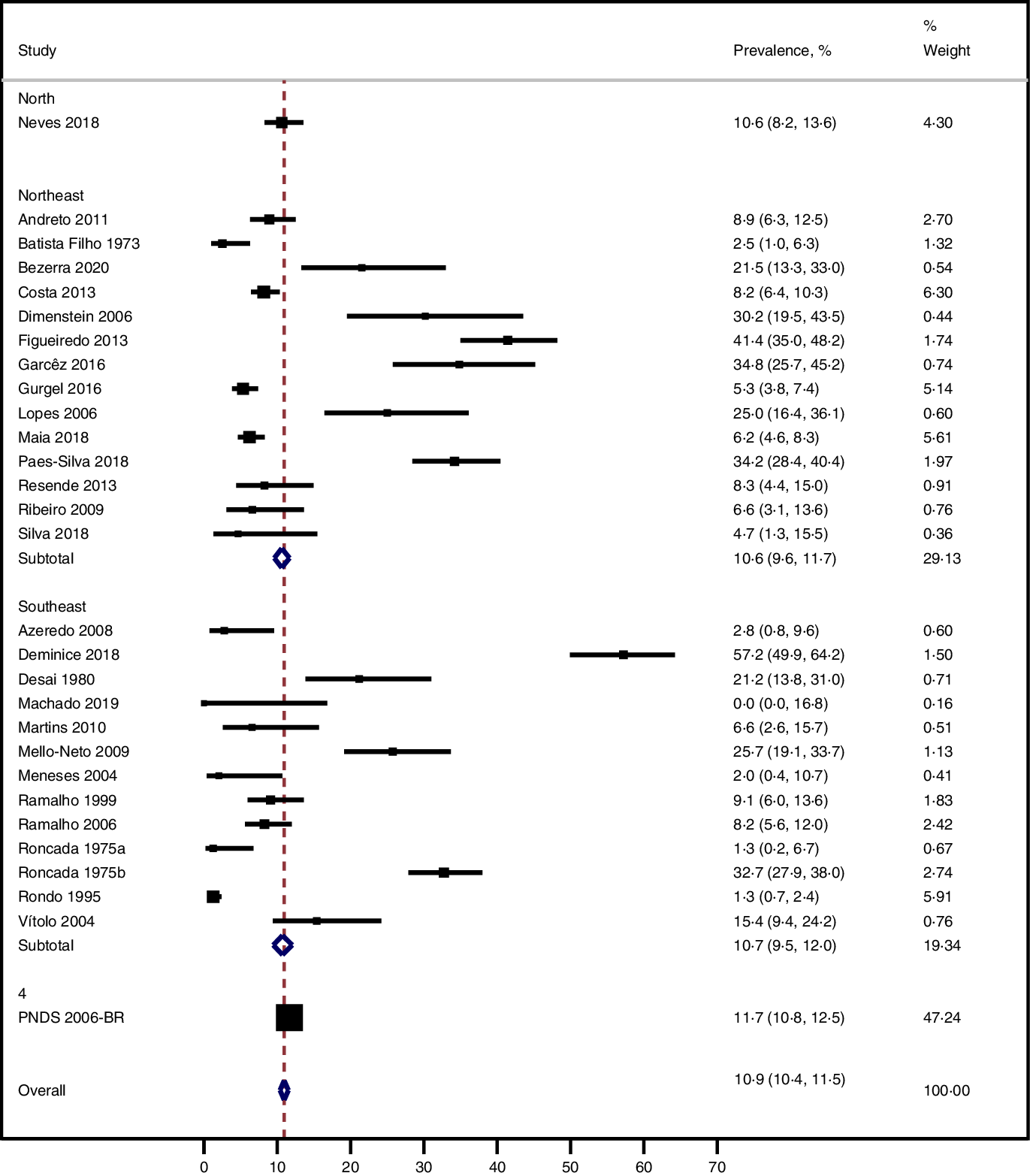
Fig. 2. Prevalence of vitamin A deficiency in women by Brazilian region.
A higher prevalence of vitamin A deficiency was noted in subgroups of studies held in schools, with cross-sectional design, assessments based on serum samples and by HPLC, in the 2010s and in pregnant women (Table 2). Homogeneous results were observed in cohort studies, clinical trials and studies held in schools, while no homogeneity was observed in subgroups of high or low risk of bias in the methodological quality domains (online Supplementary Table S6).
Table 2. Prevalence of vitamin A deficiency in women, 95 % confidence interval (CI) and heterogeneity (I²) according to the study design, sample source and geographic region of the studies

* One study(Reference Roncada49) was carried out in a migrant reception centre.
† Includes postpartum and lactating women in some studies.
The variability in the prevalence of vitamin A deficiency was not significantly affected by the year of research (P = 0·114; residual I² = 99·6 %), the methodological quality score of the studies (P = 0·180; residual I² = 99·6 %), women’s ages (P = 0·711; residual I² = 99·7 %) and Brazilian region (P = 0·407; residual I² = 99·6 %) (online Supplementary Fig. S1). A symmetric distribution was noted in the funnel plots (online Supplementary Fig. S2), and a small studies effect was discarded (P = 0·217).
Discussion
More than one in ten Brazilian women of reproductive age were estimated to have vitamin A deficiency according to the available evidence at all time periods. Pregnant women had a higher prevalence of deficiency than non-pregnant or breast-feeding women. Prevalence seems to be increasing over time. In the 2010s, nearly 20 % of Brazilian women of reproductive age had deficient vitamin A levels. This estimate of almost one in five women of childbearing age presenting vitamin A deficiency in the more recent studies is regarded as a moderate public health problem and requires proper nutritional public policies to avoid the related disease burden(4).
Our findings are based on over thirty different studies conducted over a wide range of times that mainly employed convenience sampling to recruit women from highly selected sources, such as hospitals, which impact the representativeness of the sample. The estimates were highly heterogeneous, and attempts to identify the sources of heterogeneity did not elucidate the causes, which may be inherent to prevalence studies held in different settings(Reference Barendregt, Doi and Lee17). Assessing only more recent studies with better methodological quality, for example, was not feasible due to the low number of studies meeting these criteria. Publication bias was not suspected and improves the confidence in the completeness of our results.
The overall prevalence of vitamin A deficiency in women of childbearing age is higher in low- and middle-income countries. While 2 % of vitamin A deficiency was estimated for women in North America, in the Caribbean (9 %) and Latin America (10 %) nearly five-fold of women had this deficiency, with a higher burden for maternal and childhood health(Reference Murray, Aravkin and Zheng1). A Pakistani national survey conducted in 2017–2018 observed a prevalence of vitamin A deficiency of 27 % in women aged 15–49 years, with 22 % experiencing moderate deficiency and 5 % severe deficiency(53).
Vitamin A deficiency was shown to increase over the decades in the present study. The global prevalence of vision loss associated with vitamin A deficiency increased by 9 % from 1990 to 2017, rising from 69 to 75 % in this period(Reference Xu, Shan and Lin54). In Ethiopia, although dietary vitamin A deficiency has reduced from 1990 to 2017, the age-standardised incidence rate of vitamin A deficiency increased by 9 %, and the proportion of disability-adjusted life years due to this deficiency increased from 0·6 % in 1990 to over 1 % in 2017(Reference Hassen, Ali and Gebreyesus55).
Pregnant women had a higher prevalence of vitamin A deficiency than non-pregnant or lactating women. During pregnancy, physiological adaptations include an increase in plasma volume with haemodilution and higher demands of vitamin A due to fetal needs, mainly during the third trimester(Reference Bastos Maia, Rolland Souza and Costa Caminha56–Reference West, Christian and Labrique59). The placental barrier is highly selective for vitamin A transfers to the conceptus to avoid teratogenic effects(Reference Azaïs-Braesco and Pascal60). These modifications could lead to lower plasmatic levels of vitamin A(Reference Bastos Maia, Rolland Souza and Costa Caminha56). After birth, retinol-binding protein transports most of the serum retinol to breast reaching the breast milk(Reference Green, Green and Akohoue61), and breast-feeding provides vitamin A during the first 6 months of life at a rate of sixty-fold more amount than placental levels during the whole pregnancy(Reference Stoltzfus and Underwood62). Pregnancy itself is an inflammatory period due to the immunological conditions for proper development of the conceptus. The conventional cut-off points may underestimate vitamin A status, especially in the last trimester and in populations with a high prevalence of infections(Reference Bastos Maia, Rolland Souza and Costa Caminha56,Reference Kæstel, Martinussen and Aaby63–Reference Larson, Guo and Williams65) . Our findings could also be affected by these factors, and the higher deficiency observed in pregnant women could be due to overestimation of deficiency as a result of not considering these physiological changes that may artificially increase the prevalence. Despite this possible limitation, no official recommendation of different cut-offs for vitamin A deficiency during pregnancy is available.
Within the Brazilian territory, a heterogeneous distribution of deficiency was observed, and the lowest prevalence was observed in the South and more developed region of Brazil, which was assessed in the nationwide survey – no local study to assess hypovitaminosis A has been performed in this region. This may indicate that most investigations on vitamin A deficiency in Brazil may focus on more vulnerable regions, and this research interest may impact the findings. Another hypothesis is publication bias (which was discarded in our overall assessment): studies of low prevalence could refrain from being published or end up testing associations with a higher threshold to indicate sub-optimal levels of vitamin A, instead of the recommended cut-off point for deficiency.
Women of childbearing age, especially pregnant and lactating women, should practise a healthy diet that includes natural vitamin A food sources, which in turn requires proper economic conditions of the individual and communities. In Brazil, in 2009, 72% of women aged 19–59 years had inadequate intake of vitamin A. In the following decade, this percentage increased to 80%; higher frequencies were observed in the North region (87%) and lower in the Southeast region (76 %), while the average intake of vitamins – equivalent to the activity of retinol – decreased from 410 to 342 in this period(Reference Verly Junior, Marchioni and Araujo66).Contrary to the healthy eating needs of Brazilians, costs of healthy diet increased from 2009 to 2018, mainly for the poorer individuals, and acquisition of expensive foods richer in vitamin A was low in Brazil(Reference Verly Junior, Oliveira and Sichieri67). Hunger, once a solved problem in Brazil, is on the rise due to dismantling of major social programmes that affect food security such as family agriculture incentives(Reference Ferreira Costa68). The conditional cash transfer Bolsa Família – which led to reduction of poverty and reaching sustainable development goals, such as maternal mortality – was terminated in 2021 after 18 years of existence(Reference Rasella, Alves and Rebouças69). Vitamin A supplementation during pregnancy had no impact on maternal and child outcomes in low- and middle-income countries(Reference Keats, Oh and Chau70), as well as in the postpartum(Reference Oliveira, Allert and East6), which led to the discontinuation of the former Brazilian recommendation of vitamin A megadose (200 000 IU) supplementation in the immediate postpartum period for women living in risk areas(Reference Brasil71). Food security policies and nutrition education strategies targeted to priority groups – especially during pregnancy and lactation – should be a priority in Brazil in order to improve vitamin A food sources availability and ingestion and reduce the burden of vitamin A deficiency in the country.
Conclusion
Over one in ten women of reproductive age were estimated to suffer from vitamin A deficiency in Brazil, and this prevalence seemed to be increasing over the decades. Pregnant women had a higher prevalence of deficiency than non-pregnant or postpartum women. The results are limited by the low representativeness of the studies, mainly based on convenience sampling, and would be better addressed by future representative research that assesses this deficiency. Food and nutrition security policies should be prioritised to avoid the burden of vitamin A deficiency in women of childbearing age in Brazil.
Acknowledgements
The authors of this study thanks the authors of eligible manuscripts for providing data or clarification upon our request: Vilma de Azevedo, Roberto Dimenstein, Renan Éboli, Ilma Kruse, Alexandre Torres, Patrícia Rondó, Claudia Saunders, Nádia Trugo, Márcia Regina Vitolo, Ana Godoy, Ivan Savioli Ferraz and Taciana Fernandes.
This research was funded by the Brazilian National Council for Scientific and Technological Development (CNPq) (grant number: 442709/2019–6). Galvao TF receives productivity scholarship from CNPq (grant number: 310238/2020–0).
C. M. F., M. T. S., J. M. O., D. A. H., D. F. S. A. and T. F. G. conceived the work, formulated the research question and designed the study (Conceptualisation), C. M. F., M. T. S. and T. F. G. analysed the data (Data curation and Formal Analysis), T. F. G. conceived the funding acquisition, supervision and project administration, C. M. F., M. T. S., J. M. O., D. A. H., D. F. S. A., W. W. G. S. and T. F. G. collected the data, interpreted the findings, drafted and revised the work (Investigation, Methodology, Resources and Software). C. M. F. and T. F. G. concieved the validation and visualization this project. All authors approved the final version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved (Writing – original draft, review and editing).
There are no conflicts of interest.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114522001714.
The full dataset of this research is available at https://osf.io/4fjq7.








