Folate as a cofactor in one-C metabolism is essential for all cellular processes, especially in conditions of rapid cell replications and tissue growth, such as pregnancy. The role of synthetic folic acid (FA) supplements in the prevention of neural tube defects (NTD) has been well documented( Reference Grant, Chalmers and Lucock 1 , Reference Cragan, Roberts and Edmonds 2 ). Several countries with policies of FA food fortification have reported a 30·0–70·0 % reduction in the incidence of NTD( 3 ). As reported by the International Clearinghouse for Birth Defects, Surveillance and Research, Japan has experienced an increase in the prevalence of spina bifida over the past few decades. Although in countries such as the USA and England the prevalence of NTD is about 3/10 000 births, Japan has a prevalence of 5·2( Reference Takimoto and Tamura 4 , Reference Kondo, Kamihira and Ozawa 5 ). Efforts have been made to determine folate status of pregnant Japanese women in other regions of Japan but not in Hokkaido( Reference Kondo, Kamihira and Shimosuka 6 – Reference Ihara, Watanabe and Aoki 9 ). However, probable small sample sizes, different folate assay techniques and varied folate status definitions/cut-off levels might have yielded inconsistent results. For instance, three previous studies have reported a wide range of folate status among pregnant Japanese women. A study from Aichi Prefecture that defined normal v. inadequate plasma folate status as ≥6·80 and <6·80 nmol/l, respectively, reported folate inadequacy in 1·0 % of forty-one pregnant and 154 non-pregnant participants( Reference Kondo, Kamihira and Shimosuka 6 ). Another study from Tokyo metropolis among pregnant women in all trimesters reported low serum folate status in 67·0 % of 118 women in their first trimester and 79·3 % overall. The study defined normal, low and deficient folate statuses as having >13·60, 6·80–13·60 and <6·80 nmol/l, respectively( Reference Matsuzaki, Haruna and Ota 8 ). The third study from Tokyo metropolis was conducted among fifty-eight young university women. Normal serum folate status was categorised as having ≥13·60 nmol/l, low status as having 6·80–13·59 nmol/l and folate deficiency as having <6·80 nmol/l of folate concentration. Results showed 12·1 % had folate deficiency and 58·6 % had low folate status( Reference Ihara, Watanabe and Aoki 9 ). In Hokkaido Prefecture, such reports are scarce. We recently conducted a genetic study using the first part of this cohort’s data. Low folate status was reported among 28·7 % of the study population (n 1784), but the scope of the study excluded a detailed exploration of the demographic and lifestyle predictors of folate status( Reference Yila, Sasaki and Miyashita 10 ). In contrast to previous smaller studies in Japan, this study uses data from a large cohort to explore the various demographic and lifestyle factors that may influence first-trimester folate status of Japanese women in Hokkaido.
Methods
Study location and population
Participants of this study were pregnant women recruited during their first trimester (<13 weeks of gestation) from thirty-seven healthcare facilities across Hokkaido Prefecture. They are participants of the ongoing, large-scale birth cohort of the Hokkaido Study on Environment and Children’s Health. The study is broadly aimed at observing the health effects of intra-uterine exposures to various environmental and genetic factors on fetal development, outcomes of pregnancy and subsequent childhood health. Details of the study have been described elsewhere( Reference Kishi, Sasaki and Yoshioka 11 , Reference Kishi, Kobayashi and Ikeno 12 ). In brief, the ongoing, large-scale cohort started in 2002, with a full-blown, large-scale version from February 2003. A total of 20 816 women were recruited between 2002 and March 2012. All pregnant Japanese women who presented at any of the participating healthcare facilities for prenatal care during their first trimester were considered eligible for the study. However, only those who agreed to participate in the study were contacted and recruited. Data were generated from these participants by means of baseline questionnaires, biochemical assays, hospital birth records and 4-month postpartum health records. We finally used a total of 15 266 participants; details of the selection process are shown in the flowchart (Fig. 1).

Fig. 1 Study selection chart: the Hokkaido Study on Environment and Children’s Health, 2002–2012, Japan.
Certain repeated self-reported information obtained from birth records and postpartum questionnaires were compared with baseline questionnaires in order to improve quality and reduce missing data in the whole data set. Otherwise, these data were not used in the analysis of this report. The Institutional Ethical Board for Human Gene and Genome studies at Hokkaido University Graduate School of Medicine approved the study protocol.
Biochemical assays
Non-fasting, whole blood samples were collected from participants, pre-treated and serum samples were obtained. Serum samples were stored promptly at 4°C until they were transported on ice to a commercial laboratory (SRL Corporation Inc.) for folate assay. The ADVIA Centaur Folate Assay Protocol is one of the automated competitive protein binding Immunoassay Technologies. Folate is quantified by direct chemiluminescent acridinium ester technology( 13 ). This technique has an acceptable imprecision of <10·0 %, with an advanced quality control package. It has an analytical sensitivity of 0·91 nmol/l. It can detect from small volumes of as low as 10 ul of biological specimen, making it a method of choice in large epidemiological studies( Reference Sujaku, Ogiwara and Kawasaki 14 ). Specimen preparations, shipping and assays were carried out in batches, depending on new recruitments. All laboratory analysts were blinded to participant information. As there is no standard classification of folate status from automated immunoassay techniques, we adopted the WHO classification guidelines(15). Nicotine is the toxic chemical in tobacco products and its predominant metabolite is cotinine. Cotinine can be detected in biological specimens as a biomarker of exposure to tobacco. In this study, we used plasma cotinine concentrations to quantitatively classify active and passive smoking status. The details of measurements of plasma cotinine are described in our previous report( Reference Sasaki, Braimoh and Yila 16 ).
Definition of variables
The dependent variable was folate status. Folate status was classified as follows: folate deficiency (<6·80 nmol/l), suboptimal status (6·80–13·59 nmol/l) and optimal folate status (≥13·60 nmol/l)( Reference Sauberlich 15 ). Folate deficiency was reported in 0·5 % of the study population. To improve study power, and because non-fasting serum was used for folate assay, we merged this group with the suboptimal category. Active and passive exposure to tobacco smoking statuses were classified based on plasma cotinine cut-off points established in a previous report( Reference Sasaki, Braimoh and Yila 16 ). A non-smoker was defined as having plasma cotinine concentrations of <1·19 nmol/l, a person exposed to environmental tobacco smoke (ETS) as having 1·19–65·21 nmol/l and an active smoker as having >65·21 nmol/l of plasma cotinine concentration. Prenatal FA supplement use was defined as ‘a report on the use of FA supplements before or after conception’. Other nutritional supplements use was defined as ‘any report on intake of nutritional supplements other than FA, before or after conception’. Ingestion of alcoholic beverages was categorised based on frequency of intake: monthly, weekly or daily. Self-reported active tobacco smoking was categorised based on the number of cigarette sticks smoked per day – light smokers (<10 cigarette sticks/d), moderate smokers (10–19 cigarette sticks/d) and heavy smokers (≥20 cigarette sticks/d). ETS exposure at home was defined as ‘living with one or more active smokers’. ETS at workplace referred to ‘working with one or more active smokers at workplace’. In this study, the lifestyle habits considered were alcoholic beverage consumption, nutritional supplements use and tobacco use. Potential predictors of folate status were identified based on previous reports. In this study, year of enrolment, maternal age, parity, BMI, educational level, household income, occupation, use of nutritional supplements, active and passive cigarette smoking, alcohol intake, season of the year and geographical location were identified as putative predictors.
Statistical analyses
Statistical tests of associations included Pearson’s χ 2 tests and Fisher’s exact tests for categorical variables. Skewed serum folate and plasma cotinine concentrations were log-transformed during the preliminary descriptive analyses, thereafter back-transformed. Differences in mean folate levels were explored using ANOVA with post hoc analyses to correct for multiple comparisons. However, the main regression analyses were performed using qualitative folate status. We imputed the missing values present in the data via multivariate imputation by chained equations (MICE), as implemented in the R package mice, obtaining m=10 imputed data sets. MICE is a Markov chain Monte Carlo method that uses the correlation structure of the data and imputes missing data values for each incomplete variable m times by regression of incomplete variables on the other available variables iteratively. We used Bayesian logistic regression and fitted the model to the m=10 imputed data set, with dichotomised folate status as the outcome variable, and the following as potential predictor variables: age, BMI, parity, educational level, income, occupation, region, year of enrolment, season of the year at enrolment, FA supplements use, other nutritional supplements use, alcohol intake, active cigarette smoking and exposure to ETS both at home and at workplace. We used results of plasma cotinine concentration to quantitatively classify active smoking and passive exposure to tobacco products and regressed against folate status, with adjustment for all other potential predictors. We reported pooled estimates for the main effects of the predictor variables in the model. P values for testing the presence of a linear trend are also reported for predictor variables with more than two categories. Reported effects, CI and p values are pooled over the m=10 imputed data sets. In addition, we reported the value of the McFadden’s pseudo R 2 pooled over these data sets. All statistical analyses were performed using JMP 11 Pro Statistical Software Package (SAS), except for the binary logistic regression model, which required multiple imputation of missing data and was performed using R version 3.2.2. An α level of significance was set at <0·05.
Results
Overall, the geometric mean of serum folate concentration was 17·77 (sd 3·58) nmol/l. Among women with optimal folate status, the geometric mean was 20·67 (sd 3·26) nmol/l and was 10·83 (2·65) nmol/l among participants with suboptimal folate status. One-sided lower-limit tolerance interval at 95 % of the population was 8·47 nmol/l. Prevalence of folate deficiency was 0·5 %. Suboptimal folate status constituted 25·7 %, whereas optimal folate status was reported in 73·8 % of the population (Table 1). Initial descriptive analyses using folate as a continuous variable revealed that mean serum folate concentrations increased with increasing maternal age (P<0·001), educational status (P<0·001), annual income (P<0·001), FA supplements use (P<0·001) and other nutritional supplements use (P<0·001). Mean serum folate concentrations decreased with increasing number of cigarette sticks smoked per day (P<0·001), ETS exposure at home (P<0·001) and increasing plasma cotinine concentrations (P<0·001). Exposure to ETS at both home and work was associated with low folate status, P<0·001. About 60·0 % of those with folate deficiency were exposed to ETS both at home and at workplace. Other associations were geographical region, year of enrolment into the study and season of the year (data not shown). Serum folate inversely correlated with plasma cotinine concentration (r –0·2000, P<0·001, data not shown). Significant differences were observed in mean plasma cotinine concentrations between non-users of FA supplements and users, with geometric means of 46·41 (sd 23·23) nmol/l and 25·27 (sd 15·32) nmol/l, P<0·001, respectively. In addition, geometric means for non-users and users of other nutritional supplements were 42·49 (sd 21·91) nmol/l and 34·99 (sd 20·17) nmol/l, P=0·028, respectively (Fig. 2). Users of FA supplements were likely to be those with chronic inter-current medical conditions, those who had fertility treatments and those who were also users of other nutritional supplements; 7·0 % of FA users started intake more than 3 months before conception. Another 8·0 % started 1 month before conception, whereas the majority (more than 60·0 %) started use following confirmation of pregnancy. The average frequency of use per week was three times. Multivitamins reported were found to contain various doses of FA in the range of 100–200μg/tablet (data not shown).

Fig. 2 Mean plasma cotinine concentrations by nutritional supplements use among participants: the Hokkaido Study on Environment and Children’s Health, 2002–2012, Japan. FA, folic acid; OS, other nutritional supplements.
Table 1 Distribution of maternal characteristics by folate status: the Hokkaido Study on Environment and Children’s Health 2002–2012, JapanFootnote † (Numbers and percentages; n 15 266)

JPY, Japanese Yen; ETS, environmental tobacco smoke.
* P<0·050; ** P<0·010; *** P<0·001.
† P values were derived from Pearson’s χ 2 tests and Fisher’s exact tests. All percentages are row percentages. Values may not add up to 100 % due to missing values.
In the regression model, the value of McFadden’s pseudo R 2 pooled over the m=10 imputed data sets was 8·7 %. In Table 2, the demographic determinants of low folate status are shown: as lower maternal age (adjusted OR (AOR) 1·48; 95 % CI 1·32, 1·66, P<0·001), lower educational level (AOR 1·27; 95 % CI 1·17, 1·39, P<0·001), lower annual income (AOR 1·11; 95 % CI 1·01, 1·22, P=0·024), residing in the south and eastern regions (AOR 1·25; 95 % CI 1·14, 1·38, P<0·001) and (AOR 1·15; 95 % CI 1·05, 1·25, P=0·003), respectively. Being enrolled into the study between 2005 and 2007 was associated with an increase in the risk of low folate status (AOR 1·23; 95 % CI 1·12, 1·35, P<0·001), whereas recruitment between 2008 and 2010 reduced the likelihood of having low folate status (AOR 0·81; 95 % CI 0·73, 0·90, P<0·001), respectively. Being enrolled during summer, autumn and winter were associated with higher likelihood of low folate status (AOR 1·12; 95 % CI 1·02, 1·24, P=0·023), (AOR 1·13; 95 % CI 1·02, 1·25, P=0·015) and (AOR 1·13; 95 % CI 1·01, 1·27, P=0·037), respectively. Lower BMI (AOR 0·84; 95 % CI 0·74, 0·94, P=0·006) and unemployment were associated with risk reduction (AOR 0·87; 95 % CI 0·80, 0·94, P=0·001).
Table 2 Estimated effects of demographic characteristics and lifestyle factors on folate status: the Hokkaido Study on Environment and Children’s Health 2002–2012, JapanFootnote † (Numbers and percentages; adjusted odds ratios (AOR) and 95 % confidence intervals; n 15 266)

Ref., referent values; JPY, Japanese Yen; ETS, environmental tobacco smoke.
* P<0·050; ** P<0·010; *** P<0·001.
† Regression model adjusted for maternal age, parity, BMI, educational level, annual income, occupation, geographical region, year of enrolment into the study, season of the year at enrolment, nutritional supplements use, alcohol intake and active and passive smoking. McFadden’s pseudo R 2=8·7 %. All percentages are row percentages. Values may not add up to 100 % due to missing values.
‡ Other nutritional supplements used included multivitamins, trace elements, herbs, proteins, ginseng and energy drinks.
Lifestyle factors that reduced the odds of low folate status were the use of FA supplements (AOR 0·19; 95 % CI 0·17, 0·22, P<0·001), other nutritional supplements (AOR 0·55; 95 % CI 0·48, 0·64, P<0·001) and weekly alcohol consumption (AOR 0·75; 95 % CI 0·62, 0·90, P=0·003). Lifestyle factors that increased the odds of low folate status were active cigarette smoking and ETS exposure. Smoking <10 cigarette sticks/d was associated with increased odds (AOR 1·42; 95 % CI 1·23, 1·64, P<0·001), whereas smoking between 10 and 19 cigarette sticks/d was associated with an increased risk (AOR 2·28; 95 % CI 1·92, 2·71, P<0·001). However, smoking ≥20 cigarette sticks/d was not statistically significant, P trend<0·001. Exposure to ETS at home increased the odds of low folate status (AOR 1·23; 95 % CI 1·13, 1·34, P<0·001), and exposure to ETS at the workplace also increased the odds of low folate status (AOR 1·16; 95 % CI 1·02, 1·31, P=0·026).
Using plasma cotinine concentrations to classify active and passive exposure to tobacco products, Table 3 shows that participants with plasma cotinine levels between 1·19 and 65·21 nmol/l were 1·20 times more likely to have low folate status (AOR 1·20; 95 % CI 1·10, 1·31, P<0·001), whereas those with levels>65·21 nmol/l had a 2-fold increase in risk (AOR 1·91; 95 % CI 1·70, 2·14, P<0·001); P trend<0·001.
Table 3 Estimated effects of active and passive cigarette smoking based on plasma cotinine concentrations on folate status: the Hokkaido Study on Environment and Children’s Health 2002–2012, JapanFootnote † (Numbers and percentages; adjusted odds ratios (AOR) and 95 % confidence intervals; n 15 266)
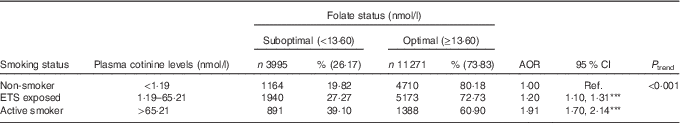
Ref., referent values; ETS, environmental tobacco smoke.
Levels of significance: *** P<0·001.
† Regression model adjusted for maternal age, parity, BMI, educational level, annual income, occupation, geographical region, year of enrolment into the study, season of the year at enrolment, nutritional supplements use, alcohol intake and active and passive smoking. McFadden’s pseudo R 2=8·5 %. All percentages are row percentages. Values may not add up to 100 % due to missing values.
Discussion
To our knowledge, this is the first report to present robust information on demographic and lifestyle predictors of folate status in a relatively large cohort of pregnant Japanese women. The majority (73·8 %) of participants had optimal first-trimester folate status. Only 0·5 % had serum folate concentrations <6·80 nmol/l, a level clinically considered a negative folate balance, whereas 25·7 % of the population had marginal folate status. Lower tolerance limit of 8·47 nmol/l implies a negative folate balance for this population. Our findings contrast those from Tokyo where >50·0 % of the study population of pregnant women had low folate status.
Demographic predictors of folate status
Low folate status was associated with younger maternal age, higher BMI, lower educational level and lower annual income. Cigarette smoking rate is on the increase among young Japanese women, and a quest to achieve a lower BMI via dieting is in vogue among women of reproductive age. These factors may invariably compromise nutritional status including folate among younger women( Reference Takimoto and Tamura 4 , Reference Takimoto, Yoshiike and Kaneda 17 ). Micronutrient deficiencies including folate in overweight/obese people have been reported by some previous studies( Reference Damms-Machado, Weser and Bischoff 18 ). Possible mechanisms postulated have been decreased dietary intake, current cigarette smoking and possible low serum/plasma concentrations as a result of increased intravascular volume( Reference Aasheim, Hofso and Hjelmesaeth 19 ). Consistent with our findings, socio-economic status has been reported to influence folate intake among Japanese workers( Reference Miyaki, Song and Taneichi 20 ). In addition, educational attainment was reported to do so in Belgium( Reference Vandevijvere, Amsalkhir and Van Oyen 21 ) and Australia( Reference Gall, Seal and Taylor 22 ). In the USA, older maternal age, higher education and higher income status have been reported to predict the use of FA supplements( Reference Branum, Bailey and Singer 23 ). In this study, these factors might have favoured higher folate status. Other demographic factors associated with suboptimal folate status have been reported from other countries, and these include household size( Reference Thoradeniya, Wickremasinghe and Ramanayake 24 ), season of the year( Reference Hao, Ma and Stampfer 25 ), rural residence( Reference Garcia-Casal, Osorio and Landaeta 26 ) and region( Reference Zhao, Hao and Zhang 27 ). We observed that residing in the southern and eastern regions and seasons of the year were associated with the risk of low folate status.
Traditionally, most Japanese women are full-time house wives. This may explain why the unemployed had lower risk. Working women are likely to skip their meals and may prefer fast foods as reported among children of working women( Reference Gaina, Sekine and Chandola 28 ). Of note here is that employment status was broadly classified. Further exploration based on job types may shed more insight on this observation.
Unfavourable lifestyle predictors of folate status
We report self-reported active cigarette smoking and ETS exposure as major modifiable unfavourable predictors of folate status. Although we could not demonstrate a dose–response pattern in the odds, especially among heavy smokers during pregnancy, this may probably be related to a small subgroup size. Using plasma cotinine as a biomarker, the risk of low folate status increased in a dose–response pattern. Contrary to this result, another study in Tokyo found no lifestyle habits as risk factors for suboptimal folate status( Reference Matsuzaki, Haruna and Ota 8 ). However, our result is consistent with reports from other developed countries, where lifestyle factors are commonly observed as predictors of folate status. Folate-depleting effects of active smoking and ETS exposure have been reported( Reference Mathews, Yudkin and Smith 29 , Reference Trobs, Renner and Scherer 30 – Reference Vardavas, Linardakis and Hatzis 35 ). Possible biological mechanisms of folate depletion in active and passive smokers include decreased intake( Reference Mathews, Yudkin and Smith 29 , Reference Ortega, Requejo and Lopez-Sobaler 33 ), inactivating effects of organic nitrites, cyanates and nitrous oxide on circulating folates( Reference Erdemir and Bergstrom 34 , Reference Ozerol, Ozerol and Gokdeniz 36 ) and direct effects of oxidative stress or increased folate turnover( Reference Ulvik, Ebbing and Hustad 31 , Reference Yanbaeva, Dentener and Creutzberg 37 ). We observed lower mean plasma cotinine concentrations among nutritional supplements users. Nutritional supplements users are more likely to practise healthy lifestyles.
Favourable lifestyle predictors of folate status
FA supplements use is the major modifiable predictor of optimal folate status. This report further confirms the well-documented role of FA supplements in improving folate status. Other nutritional supplements used also correlated positively with folate status, probably because most multivitamins also contain FA. Other nutritional supplements used included multivitamins, trace elements, herbs, proteins, ginseng and energy drinks. Over-the-counter multivitamins used contained various doses of FA in the range of 100–200μg/tablet according to the brand names reported by study participants – the majority of whom were recruited between 2002 and 2010. However, lately, the FA content seems to have been increased by drug makers (up to 480 μg/tablet). This may reflect in our findings of an increase in mean folate concentrations of participants enrolled from 2010 and beyond and a reduction in the risk of having low folate status. In this study, the majority of FA supplement users did not use it because of pregnancy. Those who used it for prenatal purpose started only after confirming that they were pregnant. This information may impact on the crucial periconceptional period for prevention of NTD. Within Japan, some smaller studies outside Hokkaido did report that using FA supplements increased blood folate concentrations more than using dietary sources of folate only. They also observed that Japanese women of reproductive age do not meet the daily RDA of 440 μg for folate( Reference Kondo, Kamihira and Ozawa 5 , Reference Mito, Takimoto and Umegaki 7 , Reference Matsuzaki, Haruna and Ota 8 , Reference Kondo, Shimosuga and Oguchi 38 , Reference Kondo, Asada and Shibata 39 ). Although the Japanese Government has recommended that women of reproductive age or those who plan to become pregnant should take 400μg/d of FA supplements, scholars have reported that the levels of awareness and compliance with the recommendations are still low( Reference Kondo, Kamihira and Ozawa 5 , 40 ). Furthermore, across the Asian subregion, prenatal FA supplements use is not a routine prenatal care practice( Reference Oi 41 ). Our findings are similar to other reports emerging from China, Malaysia and Indonesia. Of these three, mandatory fortification is legislated only in Indonesia( Reference Ren, Zhang and Li 42 – Reference Green, Skeaff and Venn 44 ). Internationally, studies from other developed countries without food fortification policies are reporting increasing incidence of suboptimal folate concentrations( Reference Vandevijvere, Amsalkhir and Van Oyen 21 , Reference Brough, Rees and Crawford 45 , Reference McNulty, Pentieva and Marshall 46 ). Our result on the role of alcoholic beverage consumption on folate status is consistent with a previous study in the Czech Republic, where moderate beer consumption correlated with higher plasma folate( Reference Mayer, Simon and Rosolova 47 ). Conversely, chronic heavy alcohol consumption is associated with folate deficiency via numerous mechanisms( Reference Halsted, Villanueva and Devlin 48 ). We stand with the universal recommendation that pregnant women should abstain from consuming alcoholic beverages, because of adverse fetal effects( 49 ).
Strengths and limitations
This study is the first to utilise a large population of pregnant Japanese women who were recruited early enough within the stage of embryonic neurulation and organogenesis. Epidemiologically, the study identified demographic and lifestyle determinants of folate status at this critical stage of neural tube formation. Identifying modifiable lifestyle factors as favourable and unfavourable determinants can lay a sound foundation for public health intervention policies. All information about the type or brand name of nutritional supplements used as well as the timing and duration of use were self-reported, and hence the risk of bias. However, nutritional supplements use and smoking status were validated by biomarkers to avoid misclassification bias. For instance, the difference observed in folate biomarker concentrations among FA users and non-users was an indication of valid self-reported use. In addition, comparable results were obtained with plasma cotinine and self-reported cigarette smoking or ETS exposure. Serum folate was used as an indicator of folate status. Erythrocyte folate signifies tissue folate reserves and is not subject to dietary fluctuations exhibited by serum/plasma folate concentrations, thus making it a more reliable choice. However, because erythrocyte folate determination is more complex, the serum folate assay was preferred to conduct this large epidemiological study; two previous studies have justified its use in epidemiological studies( Reference Galloway and Rushworth 50 , Reference Drogan, Klipstein-Grobusch and Wans 51 ). This study involved only women who presented at the designated healthcare facilities and consented to participate; therefore, it may not be representative of the general population. Finally, our findings are more of statistical correlations and not in any way signifying causality. Future randomised-controlled trials using erythrocyte folate and known dosages of FA supplements may be more informative.
Implications
The implication of active and passive tobacco smoking for the determination of folate status is of public health importance because an increasing prevalence of tobacco smoking among younger Japanese women is being reported( Reference Takimoto, Yokoyama and Yoshiike 52 ). Optimal first-trimester folate status is central in this subpopulation. It may be helpful to consider policies that could improve folate status in this group. Mandatory food fortification with FA might be a great precautionary measure. Although there are emerging controversies about prenatal FA exposure and epigenetic effects( Reference Dolinoy 53 ), the folate-depleting effects of tobacco smoke may constitute a huge public health challenge in the prevention of NTD and other birth defects in Japan. Although this Hokkaido cohort data recorded only eight (0·04 %) cases of isolated NTD, the national rate is the second highest in developed countries after Germany.
In conclusion, demographic and lifestyle factors likely predict the folate status of Hokkaido women. Active cigarette smoking and ETS exposure are the major modifiable unfavourable predictors of folate status, whereas the use of FA supplements and FA-containing multivitamins are the major favourable predictors. FA supplementation may correct the folate deficits associated with tobacco smoking.
Acknowledgements
The authors express their profound gratitude to the study participants and to all of personnel of various hospitals and clinics that collaborate with the study: Keiai Hospital, Endo Kikyo Maternity Clinic, Shiroishi Hospital, Memuro Municipal Hospital, Aoba Ladies Clinic, Obihiro-Kyokai Hospital, Akiyama Memorial Hospital, Sapporo Medical University Hospital, Hokkaido University Hospital, Kitami Red Cross Hospital, Hoyukai Sapporo Hospital, Gorinbashi Hospital, Hashimoto Clinic, Asahikawa Medical College Hospital, Hakodate Central General Hospital, Ohji General Hospital, Nakashibetsu Municipal Hospital, Sapporo Tokushukai Hospital, Asahikawa Red Cross Hospital, Wakkanai City Hospital, Kushiro Rosai Hospital, Sapporo-Kosei General Hospital, Shibetsu City General Hospital, Nikko Memorial Hospital, Sapporo City General Hospital, Kohnan Hospital, Hakodate City Hospital, Hokkaido Monbetsu Hospital, Tenshi Hospital, Hakodate Goryoukaku Hospital, Nakamura Hospital, Kin-ikyo Sapporo Hospital, Kitami Lady’s Clinic, Engaru- Kosei General Hospital, Kushiro Red Cross Hospital, Nayoro City General Hospital and Obihiro-Kosei General Hospital.
Supported by Grant-in-Aid for Scientific Research from the Japanese Ministry of Health, Labour and Welfare (grant no. 717L000103) and Japan Society for the Promotion of Science (JSPS KAKENHI grant no. 25253050).
T. A. Y.: directly involved in the data interpretation and writing of the final manuscript; A. A.: funding preparation; S. S.: blood cotinine analysis; T. A. Y., C. M. and E. Y.: data analysis; K. I., H. M., T. E. and K. S.: data collection; T. I.: funding preparation; S. K.: sample collection; H. G., T. B.: data cleaning; T. B.: cotinine measurement; and R. K.: principal investigator.
There are no conflicts of interest.








