Vitamin D is a precursor of a system of hormones that assists in the active absorption of Ca, thereby facilitating skeletal mineralisation. Exposure to UV radiation (UVR) from the sun increases vitamin D synthesis in the skin, which is the source of approximately 90 % of circulating vitamin D in the body( Reference Holick 1 ). Few foods naturally contain vitamin D. Thus, only small amounts of vitamin D are usually obtained through diet. Vitamin D is metabolised in the liver to form 25-hydroxyvitamin D (25(OH)D), the major circulating metabolite. Serum 25(OH)D is then converted in the kidney into the highly active metabolite 1,25-dihydroxyvitamin D (1,25D). Serum 25(OH)D levels are used to determine vitamin D status as this biomarker reflects both dietary intake (food, supplements) and endogenously synthesised vitamin D. Serum 25(OH)D has a much longer half-life than 1,25D, implying that it is the more stable metabolite. Circulating 25(OH)D levels are almost 1000-fold more than 1,25D, making 25(OH)D the major circulating substrate. The liver has a high capacity for 25-hydroxylation, which is loosely regulated compared with the production of 1,25D in the kidneys( Reference Feldman, Pike and Adams 2 ). Therefore, vitamin D nutritional status is better reflected by the more available substrate, 25(OH)D.
Adolescence and young adulthood are critical times in a young woman’s life as independent behaviours and lifestyle choices are established( Reference Sawyer, Afifi and Bearinger 3 ). These choices made as an emerging adult lay the foundation for future health trajectories not only for individuals but also for their future partners and families( Reference Patton, Sawyer and Santelli 4 ). Vitamin D deficiency (VDD) is more common in females than males( Reference Daly, Gagnon and Lu 5 ). In adults, inadequate 25(OH)D impacts adversely on musculoskeletal health (e.g. osteoporosis, secondary hyperparathyroidism, osteomalacia). Observational studies suggest that VDD during pregnancy may also be a risk factor for a number of reproductive health outcomes. Therefore, serum 25(OH)D concentrations of >50 nmol/l are recommended by the WHO, National Institutes of Health and the Royal Australia New Zealand College of Obstetricians and Gynaecologists( Reference Mousa, Misso and Teede 6 ). Clinical, behavioural and lifestyle factors associated with vitamin D status in young females of child-bearing age require further attention as previous studies have focused largely on elderly populations, where the risk factors for and consequences of chronically low vitamin D levels are better established. A better understanding of the determinants of vitamin D status and addressing VDD in young women are likely to improve their overall well-being, productivity and long-term health outcomes, as well as the health of their potential future offspring.
Serum 25(OH)D has been most commonly been found to correlate with season, personal sun exposure, obesity and demographic factors such as age, country of birth or socioeconomic status( Reference Daly, Gagnon and Lu 5 , Reference Kimlin, Lucas and Harrison 7 ). Less commonly, concentrations have been associated with cardiometabolic markers such as lipids and markers of insulin resistance( Reference Black and Rosen 8 ), creatinine levels( Reference Vieth, Ladak and Walfish 9 ) and medication use including hormonal contraception( Reference Harmon, Umbach and Baird 10 ), which may be interlinked with changes in reproductive hormones.
Studies assessing vitamin D status in young Australian adults have previously been limited to clinical populations (e.g. oncology, psychiatry) or have focused on associations with a specific health outcome, such as CVD risk. Despite large sample sizes, other studies have had relatively small sample sizes across the late adolescent and young adult age range( Reference Daly, Gagnon and Lu 5 , Reference Pasco, Henry and Nicholson 11 – Reference Pasco, Henry and Nicholson 13 ). Therefore, the first objective of this study was to examine the prevalence of VDD, defined as serum 25(OH)D <50 nmol/l, in a community sample of young women aged 16–25 years. The second aim was to explore the clinical, demographic and lifestyle determinants of vitamin D status in this under-represented demographic.
Methods
Study design and population
Part A of the Safe-D study was a cross-sectional study of vitamin D and related health in females aged 16–25 years at the time of recruitment, living in Victoria, Australia (latitude 34–39°S). A detailed description of the study methodology has been reported elsewhere( Reference Callegari, Reavley and Garland 14 ). In brief, participants were recruited through the online social networking site, Facebook. Individuals were recruited into the study if they were able to provide verbal and written consent, and complete all three components of the study: an online questionnaire, wearing an UV dosimeter for 14 consecutive days and a study site visit, including phlebotomy. Pregnant or breast-feeding women were excluded from the study.
Ethics
The study was approved by the Melbourne Health Human Research Ethics Committee, Melbourne Health, Victoria, Australia (project no. 2013·007). The study was carried out in accordance with the National Statement on Ethical Conduct in Research Involving Humans (2007) produced by the National Health and Medical Research Council of Australia (NHMRC). The study was supported by NHMRC project grant APP1049065.
Online questionnaire
Participants were emailed links to an extensive, online questionnaire( Reference Callegari, Reavley and Garland 14 ). Demographic, health and lifestyle information collected included date of birth, country of birth, location of residence, education level, hormonal contraception including combined oral contraceptive pill (COC) use, self-reported Fitzpatrick skin type that categorises an individual’s skin type by their response to sun exposure (e.g. skin type I represents skin that always burns, rarely tans and is pale white, whereas skin type VI never burns and is deeply pigmented)( Reference Fitzpatrick 15 ), sun behaviours (exposure, sun-protection measures and tanning preference), physical activity using a modified Active Australia Survey( Reference Brown, Burton and Marshall 16 ), smoking status, vitamin D supplementation and multivitamin use. Daily alcohol consumption and Ca intake were sourced from the Cancer Council Victoria FFQ, which collects data on usual eating habits in the past 12 months( Reference Giles and Ireland 17 ). A sun tanning attitude score was calculated from a range of statements relating to tanning behaviour( Reference Hill, White and Marks 18 ). The statements included the following: (1) I feel more healthy with a suntan, (2) a suntan makes me feel more attractive to others, (3) this coming summer I intend to sunbathe regularly to get a suntan, (4) most of my friends think that a suntan is a good thing, (5) a suntan makes me feel better about myself, (6) most of my close family think that a suntan is a good thing and (7) a suntan protects you against melanoma and other skin cancers. The higher the score the more likely the participant felt positively about sun tanning. A score for the use of sun-protection measures was calculated from responses to how often a participant: (1) sought shade between 11.00 and 16.00 hours, (2) covered their head, (3) wore clothing to protect their skin from the sun, (4) wore sunglasses and (5) used sunscreen on skin exposed to the sun. These questions were adapted from the Cancer Council Australia SunSmart recommendations ‘Slip Slop Slap Seek Slide’( 19 ). A higher sun-protection score indicated that a participant was less likely to regularly use sun-protection measures.
Sun exposure
Personal, real-time UVR exposure was measured using UV dosimeters developed at the National Institute of Water and Atmospheric Research in New Zealand (Scienterra). Dosimeters were set up and calibrated at the Australian Radiation Protection and Nuclear Safety Agency (ARPANSA). Data from the UV dosimeter were downloaded and analysed to calculate the average standard erythemal dose (SED) for each participant for the previous fortnight (1 SED=100 J/m2).
Biochemical measures
Participants were instructed to fast overnight for a minimum of 8 h before their allocated site visit. Site visits were conducted in the morning between 08.00 and 11.00 hours at the Royal Melbourne Hospital, Parkville, Australia. Blood samples were processed by the Melbourne Health Shared Pathology Service. Serum biochemistry was measured using an Abbott ARCHITECT c16000 integrated system (Abbott Diagnostics) in real time. Parathyroid hormone (PTH) was measured using an Abbott ARCHITECT i2000 SR immunoassay connected to a FlexLab track (Abbott Diagnostics). The CV for PTH was 4·7 % at 2·90 pmol/l.
A serum aliquot was stored at −80°C for 25(OH)D analysis (25(OH)D3 plus 25(OH)D2), which was measured using liquid chromatography-tandem MS (LC-MS/MS) at VivoPharm Laboratories. The D2 metabolite had a detection limit of 4·91 nmol/l, whereas for 25(OH)D3 it was 6·71 nmol/l. Tri-Level Vitamin D metabolite Quality Control samples from UTAK Laboratories (PM Separations) were used for quality control in each assay run. The CV for 25(OH)D3 was 2·0 % at 24·74 nmol/l, 1·6 % at 72·72 nmol/l and 1·4 % at 163·33 nmol/l. The CV for 25(OH)D2 was 4·9 % at 21·35 nmol/l, 2·5 % at 63·48 nmol/l and 2·5 % at 152·47 nmol/l.
Physical measurements
Height and weight were measured using standard procedures, from which BMI (BMI) was calculated (kg/m2) and categorised according to WHO criteria. Cutaneous melanin density was measured at the upper inner arm, hand and cheek using a CM-2500d Konica Minolta portable spectrophotometer (Konica Minolta) coupled with a skin analysis program (CM-SA; Konica Minolta Sensing Inc.)( Reference Dwyer, Blizzard and Ashbolt 20 ).
Dual-energy X-ray absorptiometry
Dual-energy X-ray absorptiometry (DXA; QDR 4500 A densitometer; Hologic Inc.) was used to quantify body fat as a percentage of body weight, fat mass and lean mass. Scans were analysed using QDR software version 9.1D.
Statistical analysis
Participants were excluded from the analysis if they had not completed the medical history section of the questionnaire, had abnormal pathology results, were previously diagnosed with relevant medical conditions, had undergone relevant surgery, were taking medication/s that may affect 25(OH)D levels or had a diagnosis of osteoporosis before commencing the study. VDD was defined as serum 25(OH)D level <50 nmol/l according to the Australian and New Zealand Bone and Mineral Society (ANZBMS) position statements( Reference Diamond, Eisman and Mason 21 , Reference Nowson, McGrath and Ebeling 22 ). This cut-off is supported by the Endocrine Society of Australia, Osteoporosis Australia and The National Academy of Medicine (formerly the Institute of Medicine). The 2005 ANZBMS position statements further categorises deficiency as mild (25(OH)D 25–50 nmol/l), moderate (12·5–25 nmol/l) or severe (<12·5 nmol/l)( Reference Diamond, Eisman and Mason 21 ).
Each of the following factors was categorised as follows: country of birth as Australia or elsewhere; education as high school only or further education; location of residence as urban or regional; season as summer (December–February), autumn (March–May), winter (June–August) or spring (September–November); BMI category as underweight (<18·5 kg/m2), normal (18·5–24·9 kg/m2), overweight (25–29·9 kg/m2) and obese (>30 kg/m2); Fitzpatrick skin type as type I–IV and V–VI; COC use as yes/no; physical activity as minimal-low (0–599 metabolic equivalent of task (MET)-min) or moderate-high levels (600+ MET-min); daily alcohol consumption as 0, 1–14, 15–29 or ≥30 g; smoking as current smoker or ex-/non-smoker; vitamin D supplementation in the last 2 weeks as yes/no; use of a multivitamin in the last 2 weeks as yes/no; Ca intake as above or below 1000 mg/d; use of SPF30+ sunscreen use as yes/no; took a holiday in the most recent summer period as yes/no; and reported spending >2 h in the sun on a typical day in summer or winter as yes/no. The Socio-Economic Indexes for Areas (SEIFA) percentile was used to determine socioeconomic status( Reference Pink 23 ).
Scatterplot smoothing (Lowess) curves were used to examine the relationships between serum 25(OH)D and continuous variables. Continuous variables were checked for normality using the Shapiro–Wilk test. Pearson’s correlation was used to test associations between 25(OH)D levels and continuous variables that were normally distributed (Spearman’s correlation was used for data that were not normally distributed). Either Student’s t test or an ANOVA was used to examine associations between serum 25(OH)D and categorical variables. A multivariate linear regression model was used to explore associations between serum 25(OH)D and relevant variables. Participants with data missing for a particular variable were excluded from analysis where that variable was required in analysis. A P value of <0·05 was considered statistically significant. All statistical analysis was performed using StataSE 13 (StataCorp LP).
Sample size
It was necessary for the sample size for part A of the Safe-D study to provide sufficient eligible participants to recruit adequate numbers for part B of the study, a randomised-controlled trial aiming to assess the effectiveness of an mHealth-based behavioural intervention to improve 25(OH)D levels and related health in young women with 25(OH)D levels ranging from 25 to 75 nmol/l( Reference Tabesh, Garland and Gorelik 24 ). Sample-size calculations have been reported previously and yielded a recruitment target of 468 participants( Reference Callegari, Reavley and Garland 14 ). This sample size provides 80 % power at a 5 % significance level to detect small-medium effect sizes (Cohen’s d=0·25–0·30) in outcome measures.
Results
In all, 557 participants were recruited into part A of the Safe-D study by November 2015. Serum 25(OH)D concentrations were available for 407 participants. We excluded fifty-nine participants for the following reasons (note: some participants fulfilled multiple exclusion criteria): the participant had not completed the medical history section of the questionnaire (n 4), corrected Ca >2·60 mmol/l (n 2), thyroid-stimulating hormone <0·35 mIU/l (n 4), C-reactive protein >10 mg/l (n 31), was previously diagnosed with one of the following conditions: hyperthyroidism (n 2), hypothyroidism (n 1), cystic fibrosis (n 1), coeliac disease (n 8), inflammatory spondyloarthritis (n 1), congenital heart defects (n 1), anorexia, bulimia or other eating disorders (n 7), or malabsorption conditions (n 1), had undergone previous surgery potentially affecting relevant outcomes (n 3) and the participant was taking specific medications (prednisolone (n 3), hydroxychloroquine (n 1), phenothiazine (n 1) or immunosuppressive drugs (n 1)). Two participants were excluded as they were diagnosed with osteoporosis before commencing the study. After exclusions were applied, data for 348 (85 %) participants were included in the analysis. Adequate data for personal sun exposure measured by the UV dosimeters were available for 258 (74 %) participants.
Participant characteristics
Participant characteristics are presented in Table 1. In all, 84 % of the participants were born in Australia. Participants born outside Australia (n 55) reported their country of birth as in Europe (31 %), New Zealand (7 %), China, Japan or in Southeast Asia (29 %), in Southern Asia (11 %), America or Canada (9 %), in South America (7 %) or in Africa (5 %). A third of the participants were educated to a high school level only, whereas 14 % were from the lowest socioeconomic status quartile according to SEIFA.
Table 1 Characteristics of Safe-D participants (n 348) (Mean values and standard deviations; medians and interquartile ranges (IQR))

25(OH)D, 25-hydroxyvitamin D; PTH, parathyroid hormone; SED, standard erythemal dose; MET, metabolic equivalent.
* 25(OH)D2 detected in thirty-one samples (9 %). The remaining samples were below the assay limit of detection.
† The minimum possible Fitzpatrick skin type is 1, whereas the maximum possible is 6.
‡ The minimum possible sun tanning attitude score is 7, whereas the maximum possible score is 42.
§ The minimum possible sun-protection score is 5, whereas the maximum possible score is 25.
Vitamin D status and parathyroid hormone
The prevalence of VDD in the Safe-D cohort was 26·2 %. Less than 1 % of the participants had severe deficiency (25(OH)D <12·5 nmol/l), 5·5 % had moderate deficiency (12·5–29·9 nmol/l) and 20·4 % had mild deficiency (30–49·9 nmol/l). In all, ten participants (2·9 %) had 25(OH)D ≥125 nmol/l. The mean serum 25(OH)D was 68 (sd 27) nmol/l. A total of thirty-one samples (9 %) had detectable 25(OH)D2 with a median level of 6 (interquartile range (IQR) 5, 7) nmol/l (Table 1). The median PTH concentration was 6 (IQR 4, 7) pmol/l. Serum 25(OH)D showed seasonal variations (see Fig. 1 and Table 3). The prevalence of VDD was 8 % in summer, 25 % in autumn, 37 % in winter and 21 % in spring (P<0·001).
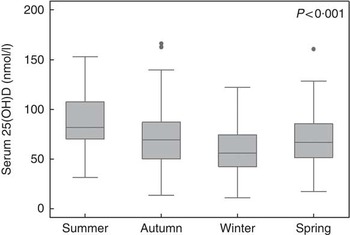
Fig. 1 Box plot of seasonal variations in 25-hydroxyvitamin D (25(OH)D) levels.
Serum 25(OH)D was negatively correlated with PTH (ρ=−0·31, P<0·001; Table 2). The median PTH levels were 7·6 (IQR 5·8–9·4) pmol/l in participants with serum 25(OH)D <12·5 nmol/l, 6·2 (IQR 5·1–7·8) pmol/l with 25(OH)D 25–49·9 nmol/l, 5·5 (IQR 4·4–7·1) pmol/l with 25(OH)D 50–74·9 nmol/l and 4·7 (IQR 3·9–5·9) pmol/l with 25(OH)D >75 nmol/l (P<0·001).
Table 2 Univariate associations between serum 25-hydroxyvitamin D and continuous variables in young women

SEIFA, Socio-Economic Indexes for Areas; SED, standard erythemal dose; PTH, parathyroid hormone; eGFR, estimated glomerular filtration rate; SHBG, sex hormone-binding globulin; TIBC, total Fe-binding capacity.
* Calculated using Pearson’s correlation.
† Calculated using Spearman’s correlation.
‡ Serum Ca corrected for albumin. Ca +((40−albumin)×0·02).
Association between 25-hydroxyvitamin D and demographic variables
A negative association was found between serum 25(OH)D levels and chronological age (Table 2). Serum 25(OH)D and SEIFA percentile were positively associated (Table 2). Participants born outside of Australia had, on average, 26 nmol/l lower 25(OH)D compared with Australian-born participants (Table 3). Participants with university or further education had, on average, 7 nmol/l lower 25(OH)D levels compared with those with high school education only (Table 3). No association was found between 25(OH)D levels and location of residence (urban v. rural; P=0·095; data not shown).
Table 3 Univariate associations between serum 25-hydroxyvitamin D (25(OH)D) and categorical variables in young women (Mean values and standard deviations)

COC, combined oral contraceptive pill.
* Differences between groups were analysed using Student’s t test. If data were grouped into more than two groups ANOVA was used.
Association between 25-hydroxyvitamin D and sun exposure
A summary of sun exposure-related variables is presented in Table 1. In all, 7 % of the participants had been sunburnt >5 times in the previous 12 months. In summer, 55 % of the participants reported spending >2 h in the sun on a typical day, whereas in winter 20 % reported spending >2 h in the sun daily. A total of 62 % of the participants with adequate 25(OH)D levels reported spending >2 h in the sun on a typical day in summer compared with 37 % with VDD (P<0·001).
Serum 25(OH)D was positively associated with daily personal exposure measured by UV dosimetry (R 2=0·08; see online Supplementary Fig. S2), the sun tanning attitude score and reported number of times sunburnt in the previous 12 months (Table 2). In addition, serum 25(OH)D levels were higher in participants who reported spending >2 h in the sun in summer on a typical day and in those who took a holiday in the most recent summer period (see Table 3). Serum 25(OH)D was not associated with spending >2 h in the sun in winter (P=0·098), the sun-protection score (P=0·067) or the use of a sunscreen with SPF30+ or higher (P=0·416; data not shown).
Association between 25-hydroxyvitamin D and other lifestyle variables
Serum 25(OH)D levels were, on average, 8 nmol/l higher in participants who reported moderate-to-high physical activity levels compared with those who reported minimal-to-low activity levels (see Table 2). Serum 25(OH)D levels were lower in those who reported drinking <15 g of alcohol daily or abstained from drinking (Table 3). Participants who had reported taking a multivitamin in the previous week had on average 12 nmol/l higher 25(OH)D levels than those who did not (Table 3). A trend towards higher 25(OH)D with vitamin D supplementation was observed, but did not reach statistical significance. Serum 25(OH)D levels were not associated with dietary Ca (P=0·172) or energy intake (P=0·722) as continuous variables, nor were 25(OH)D levels associated with current smoking status (P=0·464; data not shown).
Association between 25-hydroxyvitamin D and biomarkers
Serum 25(OH)D was positively associated with serum creatinine (R 2=0·11), Ca corrected for albumin, sex hormone-binding globulin (SHBG; R 2=0·15), Fe, transferrin and TIBC values (Table 2). The positive association between 25(OH)D and creatinine remained significant after adjustment for lean mass and height (β=1·4; 95 % CI 0·99, 1·84, P<0·001). A positive trend was observed between 25(OH)D and transferrin saturation (P=0·090; data not shown). Serum 25(OH)D was inversely associated with estimated glomerular filtration rate and prolactin (Table 2). No association was observed between 25(OH)D and the following analytes: C-reactive protein (ρ=0·05, P=0·357), thyroid stimulating hormone (r 0·06, P=0·289), ferritin (ρ=0·09, P=0·103), luteinising hormone (ρ=−0·05, P=0·547), follicle-stimulating hormone (r −0·07, P=0·401), oestradiol (ρ=−0·06, P=0·466), progesterone (ρ=0·14, P=0·076), testosterone (r 0·08, P=0·307) and dehydroepiandrosterone sulphate (r −0·03, P=0·289).
Association between 25-hydroxyvitamin D and clinical variables
Serum 25(OH)D was positively associated with height, whereas 25(OH)D was negatively associated with percent body fat, BMI and fat mass (Table 2). Serum 25(OH)D levels were significantly lower in those who were categorised as either underweight or obese (Table 3). Serum 25(OH)D levels were on average 19 nmol/l higher in COC users compared with non-users (R 2=0·12; Table 3). Serum 25(OH)D was not associated with body weight (r −0·10, P=0·215), but tended to be positively associated with lean mass (ρ=0·10, P=0·057).
Serum 25(OH)D was positively associated with melanin density of the hand (Table 2). No association was observed between 25(OH)D and melanin density of the upper, inner arm (ρ=−0·01, P=0·905) or facial cheek (ρ=−0·02, P=0·654). A trend towards an association was observed between 25(OH)D levels and Fitzpatrick skin type (P=0·077). Serum 25(OH)D levels were, on average, 17 nmol/l lower in participants with Fitzpatrick skin type V–VI compared with skin types I–IV (Table 3).
Stepwise regression model
Factors found to be significantly associated with 25(OH)D in univariate analyses were included in a stepwise elimination regression model (Table 4). Serum SHBG, creatinine, daily sun exposure measured by UV dosimetry, a holiday taken in the most recent summer period, multivitamin use, spending >2 h daily in the sun in summer, Fe concentrations, height and the sun tanning attitude score were independently associated with higher serum 25(OH)D levels. Factors independently associated with lower serum 25(OH)D were season (autumn, winter or spring compared with summer) and fat mass. The final model explained 56 % of the variation in serum 25(OH)D.
Table 4 Stepwise regression model assessing a number of potential correlates of 25-hydroxyvitamin D in young womenFootnote * (Regression coefficients (β) and 95 % confidence intervals)

SHBG, sex hormone-binding globulin, COC, combined oral contraceptive pill; SED, standard erythemal dose.
* The following variables were entered into a single stepwise elimination regression model: age (years), Socio-Economic Indexes for Areas percentile, creatinine (μmol/l), estimated glomerular filtration rate (ml/min/1·73 m2), corrected Ca (mmol/l), prolactin (μmol/l), SHBG (nmol/l), Hb (g/l), Fe (μmol/l), height (m), fat mass (kg), melanin density index of the hand, personal sun exposure measured using UV dosimeters (SED), sun tanning attitude score, reported number of times sunburnt in previous 12 months, country of birth, education, season, Fitzpatrick skin type V–VI, COC use, reported spending >2 h in the sun in summer, reported going on holidays in the most recent summer period, physical activity, alcohol consumption and multivitamin use.
† Estimates are given as nmol/l per unit of covariate.
Discussion
The Safe-D study is the first to evaluate vitamin D status in young women recruited through Facebook advertising, a novel, non-traditional method of recruitment. The prevalence of VDD in 16–25-year-old females was 26 %. The following variables were found to be positively associated with serum 25(OH)D (Table 4): SHBG levels, creatinine levels, personal sun exposure in the previous 2 weeks, holiday taken in the most recent summer period, blood drawn in the summer season, multivitamin use, reporting spending more than 2 h in the sun in summer on a typical day, Fe levels, body height and having a positive attitude towards sun tanning. Fat mass was negatively correlated with serum 25(OH)D. Our final model was able to explain more than 50 % of the variation in serum 25(OH)D concentrations in a community sample of 348 healthy young women. By contrast, Kimlin et al. ( Reference Kimlin, Lucas and Harrison 7 ) were able to explain 40 % of the variance in 25(OH)D levels in 1002 Australians aged 18–75 years, living across 24° of latitude. We found that 26 % of the young women studied were vitamin D deficient, which is in close agreement with the current literature that has reported prevalence rates of 21–27 %( Reference Daly, Gagnon and Lu 5 , Reference Gill, Hill and Shanahan 25 ). A US report found that 25(OH)D levels were significantly lower in young adults compared with adults aged older than 60 years of age( Reference Schleicher, Sternberg and Looker 26 ). Collectively, these findings suggest that VDD may be as common in adolescents and young adults as in older populations, who are usually considered the most at risk for VDD. The lower prevalence of VDD in older adults may be, in part, due to the higher vitamin D supplement use in older adults. Current Australian Bureau of Statistics (ABS) data suggest that 20 % of adults aged >75 years have VDD compared with 31 % in 18–34 year olds. However, 14 % of older adults use vitamin D supplements compared with <3 % in 18–34 year olds( 27 ). Nonetheless, these results support the need for future vitamin D research, including supplementation trials, in this currently understudied demographic.
We found no association between 25(OH)D and education levels or socioeconomic status in the final model, suggesting that they are not strong correlates of 25(OH)D in this demographic. In univariate analyses, 25(OH)D was positively associated with socioeconomic status, whereas 25(OH)D concentrations were lower in participants receiving further education. It should be noted that there was no association between education levels and SEIFA percentile in the current study. The SEIFA index used in this study is calculated from a number of socioeconomic status variables including, but not limited to, education, assets, income, debt, occupation and housing details. The education aspect of the SEIFA index looks at the proportion of individuals with an education of year 11 (approximately 16–17 years old) or lower. Due to the age range studied and cohort demographics, participants in the Safe-D study in current education would be in late high school (year 11–12) or in tertiary/further education. The discrepancy between the education measures is likely the reason for the contradictory direction of the association between socioeconomic status and 25(OH)D, as well as between 25(OH)D and education level.
As expected, serum 25(OH)D was inversely associated with PTH. Serum 25(OH)D was positively associated with serum creatinine in the final model, a marker of renal function, which has been previously reported( Reference Vieth, Ladak and Walfish 9 , Reference Shirazi, Almquist and Malm 28 ). Vieth et al. ( Reference Vieth, Ladak and Walfish 9 ) found the same association in participants <51 years old (P<0·001), but not in participants older than 70 years of age. Treatment with calcitriol has been shown to cause an increase in creatinine, but the mechanism is unclear( Reference Andreev, Koopman and Arisz 29 ). The lack of association between 25(OH)D and age in the final model is likely due to the narrow age range studied. The Safe-D participants were primarily Caucasian and Australian-born, which might explain the lack of an observed association between 25(OH)D and country of birth in the final model (Table 4), despite the significant association observed in univariate analyses.
Serum 25(OH)D showed a seasonal variation, which has been consistently demonstrated and is predominantly due to increased ambient UVR in summer, thereby facilitating cutaneous vitamin D synthesis( Reference Webb, Kline and Holick 30 – Reference Gies 33 ). Although season plays an important role in influencing vitamin D status, behavioural factors also contribute( Reference van der Mei, Ponsonby and Engelsen 12 ). We demonstrated that daily sun exposure, a higher sun tanning attitude score, spending >2 h daily in the sun in summer and taking a holiday in the summer period, as well as height were positively associated with 25(OH)D in the final model. The association between 25(OH)D and height has been described previously in young American women aged 16–22 years( Reference Kremer, Campbell and Reinhardt 34 ). It correlates with a larger body surface area and therefore a greater potential capacity to synthesise vitamin D. In addition to increasing vitamin D synthesis, UVR exposure is the cause of 95–99 % of skin cancers. The association between 25(OH)D and spending >2 h in the sun is particularly striking as this amount of sun exposure would be considered excessive and likely increase the risk of skin cancer significantly. Melanoma remains the most common cancer among 15–24-year-old Australians; therefore, it is crucial that efforts are made to achieve a balance between safe sun exposure, to minimise the risk of skin cancer, and sufficient sunlight exposure to achieve adequate vitamin D status( 35 , Reference Armstrong and Kricker 36 ).
Only 9 % of samples tested had detectable 25(OH)D2, suggesting that plant-based dietary sources of supplemental forms of vitamin D2 contribute very little to circulating 25(OH)D in young Australians. In contrast, a US population-based study found that individuals with a vitamin D intake of >5 µg/d had significantly higher 25(OH)D levels( Reference Schleicher, Sternberg and Looker 26 ). This observation suggests that a possible approach to improve vitamin D levels might be to adopt more active food-fortification strategies. Alternatively, increasing total vitamin D intake with supplementation is a strategy that has been demonstrated to improve 25(OH)D levels( Reference Nowson, McGrath and Ebeling 22 ). In Australia, multivitamins are the most commonly used dietary supplement and contain about 5–10 μg (200–400 IU) vitamin D( 37 ). We demonstrated that participants who reported taking a multivitamin had approximately 12 nmol/l higher 25(OH)D levels in the final model. Multivitamins have been shown to be safe to use; however, data on the benefits of multivitamin use in the general population are limited( Reference Huang, Caballero and Chang 38 , Reference Macpherson, Pipingas and Pase 39 ). It is likely that we did not find a significant association between 25(OH)D and vitamin D supplement use due to the low proportion of use (approximately 8 %) and data were limited to the previous 2 weeks, rather than the previous month, for example. In Australia, most vitamin D supplements contain 25 μg (1000 IU) vitamin D. Randomised-controlled trials would provide an opportunity to gain insights into the potential benefits of a multivitamin or vitamin D supplementation on vitamin D status and health outcomes in young women( Reference Tabesh, Garland and Gorelik 24 ).
The association between 25(OH)D and Fe levels is consistent with previous literature, suggesting that low 25(OH)D levels are associated with an increased risk of anaemia( Reference Monlezun, Camargo and Mullen 40 , Reference Blanco-Rojo, Pérez-Granados and Toxqui 41 ). Haeme-bound Fe is used in the hydroxylation of vitamin D metabolites by the cytochrome P450 enzymes, providing a probable link between vitamin D and Fe metabolism( Reference Jones, Prosser and Kaufmann 42 ). Other proposed links between vitamin D and Fe status include vitamin D modulating inflammation, stimulating erythropoiesis, changes in hepcidin levels and associations with fibroblast growth factor 23( Reference Smith and Tangpricha 43 , Reference Smith, Alvarez and Kearns 44 ).Vitamin D supplementation trials may be able to resolve whether increasing 25(OH)D is beneficial to Fe status in young women.
We demonstrated that serum 25(OH)D levels were inversely associated with fat mass. The relationship between reduced 25(OH)D and obesity has consistently been reported and is commonly explained by increased 25(OH)D storage in adipose tissue as vitamin D metabolites are lipophilic, thereby reducing circulating concentrations( Reference Daly, Gagnon and Lu 5 , Reference Black and Rosen 8 , Reference Blum, Dolnikowski and Seyoum 45 , Reference Wortsman, Matsuoka and Chen 46 ). Other mechanisms for reduced 25(OH)D levels with higher fat mass may be more specific to personal choices that reduce personal sun exposure, such as reduced outdoors activity or covering up, limiting vitamin D synthesis. The increasing prevalence of obesity in young women is likely to continue to contribute towards the prevalence of VDD( Reference Moodie 47 ).
Serum 25(OH)D was positively associated with SHBG levels, which has been demonstrated to increase with COC oestrogen dose( Reference Zimmerman, Eijkemans and Bennink 48 ). In addition, COC use was associated with 19 nmol/l higher 25(OH)D levels in univariate analysis, which is in agreement with previous literature( Reference Harmon, Umbach and Baird 10 , Reference Møller, Jensen and Mosekilde 49 , Reference Bouillon, Baelen and Moor 50 ). Harmon et al. ( Reference Harmon, Umbach and Baird 10 ) recently found that COC use was associated with a 20 % increase in 25(OH)D levels in 1662 African American women aged 23–34 years. Some studies have suggested that vitamin D binding protein (DBP) levels increase with COC use, varying the proportion of free and protein-bound 25(OH)D( Reference Møller, Jensen and Mosekilde 49 , Reference Bouillon, Baelen and Moor 50 ). Alternatively, increased DBP binding may protect 25(OH)D from 24-hydroxylation, thereby increasing circulating 25(OH)D( Reference Møller, Jensen and Mosekilde 49 , Reference Bouillon, Baelen and Moor 50 ). The use of COC should be taken into account when interpreting 25(OH)D results and a review of vitamin D status might be considered when a young woman ceases COC use. The latter is particularly important for women planning to conceive, where ceasing COC use may further exacerbate VDD and affect pregnancy outcomes adversely.
The Safe-D study has a number of methodological strengths. We successfully recruited a broadly representative sample of young women and were able to produce a data set of generally healthy young women through extensive health data collection( Reference Fenner, Garland and Moore 51 ). In addition, LC-MS/MS was employed by National Association of Testing Authorities, Australia, accredited laboratory to assay serum 25(OH)D as this method has the highest sensitivity and specificity compared with other 25(OH)D assays and is therefore often considered the current ‘gold standard’ for 25(OH)D measurement( Reference Fraser and Milan 52 – Reference Ashwell, Stone and Stolte 54 ). The objective measurement of sun exposure through UV dosimeters has previously been shown to be a feasible and more accurate measure of time spent outdoors compared with self-reported data( Reference Køster, Søndergaard and Nielsen 55 ). Finally, the study could explain 56 % of the variability in 25(OH)D levels by assessing a wide range of clinical, behavioural and lifestyle factors associated with 25(OH)D and indicated that SHBG levels, creatinine levels, sun exposure, holiday in the most recent summer period, season, fat mass, multivitamin use, Fe levels, height and attitudes towards sun tanning were the major sources of vitamin D status in young women.
Our study is not without limitations. A number of countries use a cut-off of <30 nmol/l to define VDD. Due to the small proportion of participants with serum 25(OH)D <30 nmol/l (20/348; 6 %), it is difficult to make conclusive judgements about associations between health outcomes within this concentration range. Although the cohort was broadly representative of young Victorian women( Reference Fenner, Garland and Moore 51 ), there were some slight differences. Participants were primarily Australian born (84 v. 78 %), had a higher education level (67 v. 43 %) and more resided in urban areas (91 v. 79 %) compared with the ABS Census of Population and Housing 2011 data in 16–25-year-old Victorian females. The underrepresentation of overseas-born individuals and those from regional areas was likely due to language/cultural barriers and difficulties travelling to the study site centre in Melbourne, respectively. The greater proportion of higher educated participants is common in volunteer samples in research and may also be contributed to by the close vicinity of a number of universities to the study site centre. The Victorian Population Health Survey 2011–2012 found that 14·6 % of 18–24-year-old Victorian women were current smokers and 21·9 % were insufficiently active, which are similar to our results (9·5 and 28·7 %, respectively)( 56 ). In terms of body composition, 18·2 % of Victorians were considered overweight or obese in the 2011–2012 survey, which was lower than our data (28·5 %), which could potentially reflect population trends in obesity levels since the precious census( Reference Moodie 47 ). Nonetheless, slight differences in participant demographics are not necessarily biologically significant. Due to loss, damage or inaccurate use of UV dosimeters, the number with eligible UV data was reduced, thus reducing analytical power. We measured a large number of possible 25(OH)D correlates, which may lead to identifying significant correlations by chance alone. By using a stepwise elimination regression model in our final model we could present the variables that were the strongest statistical determinants of 25(OH)D. This is also why weaker correlates may have been significant correlated with 25(OH)D in univariate analyses alone as confounding variables may have been eliminated from the final model. Finally, due to the study’s cross-sectional nature, causation cannot be inferred; however, we are examining these relationships in part B of the Safe-D study( Reference Tabesh, Garland and Gorelik 24 ).
In conclusion, VDD was found to be as prevalent in young women as reported previously in older Australian adults. We were able to explain over half of the variation in serum 25(OH)D levels using sun exposure-related, biochemical and anthropometric variables. A better understanding of the factors influencing vitamin D status may help better identify individuals at increased risk of VDD. Our findings support the need for further vitamin D research specifically in young women and the need to address modifiable risk factors for VDD such as low sun exposure and obesity. The feasibility of safely improving vitamin D levels through lifestyle interventions in young women requires further attention in this currently understudied demographic.
Acknowledgements
The authors thank the participants who took part in the Safe-D study. The authors also thank the Safe-D chief investigators Associate Professor Marie Pirotta, Professor Anthony Jorm, Associate Professor Shanton Chang and Professor George Varigos as well as associate investigator Professor Kim Bennell, study coordinator Ms Adele Rivers and other members of the Safe-D research team. The authors thank the Young Female Health Initiative (YFHI) associate investigators Dr Yasmin Jayasinghe, Dr Catherine Segan, Dr Asvini Subasinghe and past YFHI coordinator Dr Elisa Young. The authors thank Anna Scobie, Marjan Tabesh, Miaowen Zhou, Lauren Gilbert and Skye Maclean for assisting with the Safe-D study. The authors would like to acknowledge the following people for their help with various components of the study: Adrian Bickerstaffe (The University of Melbourne); Maria Bisignano (Melbourne Health Shared Pathology Service); Alison Brodie (Queensland University of Technology); Dr Peter Gies (Australian Radiation Protection and Nuclear Safety Agency); Dr Ashwini Kale (The University of Melbourne); Dr Kerryn King (Australian Radiation Protection and Nuclear Safety Agency); and Jen Makin (Cancer Council Victoria).
The Safe-D study was funded by the Australian National Health and Medical Research Council (NHMRC) project grant APP1049065. The Safe-D study (part B) has received in-kind support from Swisse Wellness. Swisse Wellness did not play a role in study design, the implementation of these studies or the interpretation of the findings.
J. D. W. conceived the study. E. T. C. participated in the design of the study, establishment of the study methods, data collection and drafted the manuscript. J. D. W., S. M. G., A. G. and N. J. R. are study investigators and were involved in study design, study coordination and helped draft the manuscript. A. G. is the study statistician and was involved in sample size and power calculations and also advised on the statistical analysis of the data. All authors read, contributed to and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517002021







