Preterm birth (PTB), which is defined as a live baby younger than 37 weeks of gestation by the WHO, has become a major global health problem pertaining to perinatal mortality and morbidity( Reference Blencowe, Cousens and Oestergaard 1 , 2 ). Over one-third of the 2·76 million global neonatal deaths each year are ascribed to PTB complications, and PTB is the leading cause of death in children younger than 5 years of age( Reference Liu, Oza and Hogan 3 , 4 ). Furthermore, many surviving preterm children have an increased risk of neurodevelopmental dysfunction and chronic disease in adulthood, which can bring huge economic burden to families and society( Reference Blencowe, Cousens and Oestergaard 1 ). Therefore, effective prevention and reducing the incidence of PTB are crucial for public health improvement.
Traditional risk factors for PTB include maternal demographic characteristics, nutritional status, pregnancy history, infection, uterine contractions and cervical length in the gestation period( Reference Goldenberg, Culhane and Iams 5 ). Among these factors, maternal abnormal Hb status, including anaemia and high Hb level, has long been a hot research issue on the aetiology of PTB. However, previous studies have reported conflicting results for the association between maternal anaemia or high Hb level and PTB risk( Reference Levy, Fraser and Katz 6 – Reference Abeysena, Jayawardana and de A Seneviratne 11 ). A systematic review published in 2013 reported that anaemia in the first trimester increased the risk of PTB, and that Hb above 140 g/l in the third trimester decreased the risk of PTB( Reference Sukrat, Wilasrusmee and Siribumrungwong 12 ), whereas another recent review reported a U-shaped relationship of maternal Hb concentration with PTB risk( Reference Dewey and Oaks 13 ).
Assessing haematological indices before pregnancy can detect abnormal Hb status in the preconceptional period, which makes primary prevention and intervention before pregnancy possible. However, little has been done to investigate the relationship between maternal preconception Hb concentration and PTB risk. Therefore, we conducted a large population-based cohort study in over 2·7 million reproductive-aged women in rural China to assess the association between maternal Hb concentration before pregnancy within 6 months and the risk of PTB.
Methods
Population and study design
A large population-based retrospective cohort study was conducted based on National Free Pre-Pregnancy Checkups Project (NFPCP), which is a national free health service for reproductive-aged couples who planned to conceive within 6 months. The project has been administered by the National Health and Family Planning Commission and the Ministry of Finance of the People’s Republic of China. The service began with 100 rural counties in 2010 and was further expanded to 2907 counties in mainland of China after 2013. Detailed design, organisation and implementation of NFPCP are described elsewhere( Reference Zhang, Wang and Sheng 14 , Reference Yang, He and Li 15 ). The study was approved by the Institutional Research Review Board at the National Health and Family Planning Commission. Written informed consent in Chinese was obtained from all NFPCP participants.
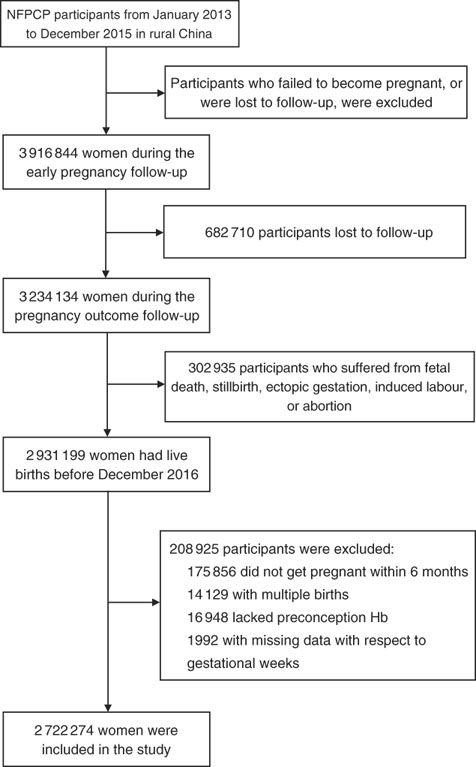
A total of 2 931 199 women aged 20–49 years old completed the NFPCP from January 2013 to December 2015 and pregnancy outcome follow-up before December 2016 in a rural household registration. We then further excluded participants who gave multiple births, did not get pregnant within 6 months and had missing information of preconception Hb concentration and last menstrual period (LMP) date or delivery date. As a result, 2 722 274 women were included in the current analysis. Detailed information on the study population recruitment, and derivation of the population used in the final analysis, is shown in Fig. 1.
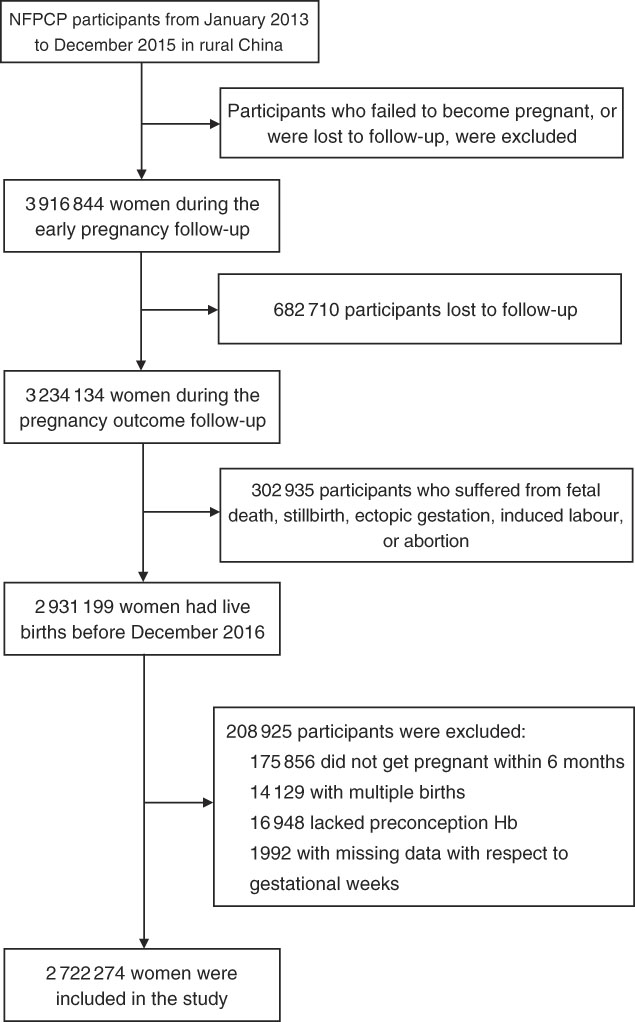
Fig. 1 Flow chart of the study population. NFPCP, National Free Pre-Pregnancy Checkups Project.
Measurements and data collection
All enrolled participants completed a standard questionnaire to collect baseline information about demographic characteristics, history of chronic diseases, family history, reproductive history and other relevant factors such as smoking and alcohol consumption through face-to-face interviews by trained staff in the local family planning service agencies or maternal and child care service centres in each county.
During the pre-pregnancy physical examination, body weight and height of participants wearing light, indoor clothing and no shoes were measured. Next, BMI was calculated. Seated blood pressure (BP) was measured using an automated BP monitor on a single occasion after participants rested for ≥10 min. Blood sample after at least 8 h of fasting was taken from each participant and immediately sent to the local laboratories. Hb concentration was measured with haematology analysers immediately in accordance with National Guide to Clinical Laboratory Procedures. Serum glucose level and thyroid-stimulating hormone (TSH) were also measured. The accuracy and stability of Hb measurements and other laboratory tests were ensured through the establishment of quality assurance system of the NFPCP( Reference Wang, Zhang and Zhang 16 ). Hypertension was defined as systolic BP≥140 mmHg or diastolic BP≥90 mmHg or self-reported hypertension. Diabetes was defined as a fasting blood glucose ≥7·0 mmol/l or a history of diabetes. Thyroid dysfunction was defined as current serum TSH level of <0·44 mIU/ml or >3·45 mIU/ml, or a history of thyroid disease.
Follow-up and outcome
Two follow-up interviews were conducted after baseline examination by trained nurses using telephone. The first follow-up was conducted within three months after baseline examination to track pregnancy status of the participants. If the participants did not get pregnant at the first interview, repeated investigations were conducted subsequently within the next three months until 12 months after baseline examination. Information about the LMP and any lifestyle changes in the first trimester of pregnancy was collected. Within 1 year after the first follow-up survey was completed, the second interview was carried out to find out the pregnancy outcomes of the subjects who had become pregnant. Information regarding the delivery date and delivery mode was self-reported.
PTB is defined as any birth before 37 completed weeks of gestation. PTB can be further subdivided based on gestational age: moderate PTB (MPTB, 32 to <37 weeks) and very PTB (VPTB, <32 weeks). The gestational age (weeks) was calculated as the number of weeks between the date of delivery and the 1st day of the LMP using relevant information collected in the two follow-up surveys.
Statistical analysis
Baseline characteristics were presented as mean values and standard deviations for continuous variables and numbers and percentages for categorical variables. The χ 2 test or Kruskal–Wallis test was used to compare the distributions of baseline characteristics according to different preconception Hb status.
In this study, Hb concentrations of women who resided in areas with altitude≥1000 m were adjusted by subtracting the adjustment values from the original Hb concentrations according to the recommendation by the WHO( 17 , 18 ). Detailed adjustments are presented in the online Supplementary Table S1. Then we classified women into three groups (anaemia: <110 g/l; normal Hb level: 110–149 g/l; and high Hb level: ≥150 g/l) and seven groups (severe anaemia: <70 g/l; moderate anaemia: 70–99 g/l; mild anaemia: 100–109 g/l; normal Hb level: 110–149 g/l; mild high Hb level: 150–159 g/l; moderate high Hb level: 160–169 g/l; and severe high Hb level: ≥170 g/l). Hb categorisation was based on WHO guidelines for the grading of anaemia( 17 , 18 ) and one previous study, which indicated that high Hb level can be defined as Hb concentration ≥160 g/l( Reference Yip 19 ). Because the definition of anaemia in China is 10 g/l lower than the criterion of the WHO( Reference Ge and Xu 20 ), all of the cut-off values in our study were reduced by 10 g/l as compared with the recommendations.
We examined associations between the two categories of preconception Hb concentration and the risk of PTB, as well as MPTB and VPTB. The OR and their corresponding 95 % CI were estimated by age-adjusted and multivariate-adjusted logistic regression models separately, using normal Hb level (110–149 g/l) as the reference group. Covariates in the multivariate-adjusted logistic regression model included baseline age, education, ethnicity, occupation, pre-pregnancy BMI, smoking, passive smoking, alcohol consumption, hypertension, diabetes, thyroid dysfunction, parity, region with GDP per capita, history of adverse pregnancy outcomes and sex of the child. In addition, we assessed the dose–response relationships between preconception Hb concentrations and the risk of PTB, MPTB and VPTB using restricted cubic spline models( Reference Hu, Xia and Pan 21 ), and plotted smooth curves with four knots at the fifth, 35th, 65th and 95th percentiles of preconception Hb concentrations. Subgroup analysis was performed according to the residence altitude of participants. To examine the robustness of our findings, we performed sensitivity analyses by using the original Hb concentration with or without additional adjustment of residence altitude in the models. All analyses were performed using R, version 3.2.2, with the ‘speedglm’ and ‘rms’ packages (Development Core Team, 2015). All statistical tests were two-sided, and values of P<0·05 were considered statistically significant.
Ethical statements
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving humans were approved by the Institutional Research Review Board at the National Population and Family Planning Commission. Written informed consent in Chinese was obtained from all NFPCP participants.
Results
In the current study, the median age of the participants was 25·0 years (interquartile range (IQR): 23·0–27·4). Only 2·32 % of the participants were older than 35 years, and 69·90 % were primipara. Overall, 387523 (14·20 %) women had anomalous preconception Hb concentration: 267 372 (9·80 %) were anaemic and 120 151 (4·40 %) had high Hb concentration (Table 1). Those with abnormal Hb concentrations were more likely to be with less educational attainment, have pre-existing diabetes or thyroid dysfunction.
Table 1 Maternal characteristics with respect to preconception status of Hb concentrations (Numbers and percentages; mean values and standard deviations)
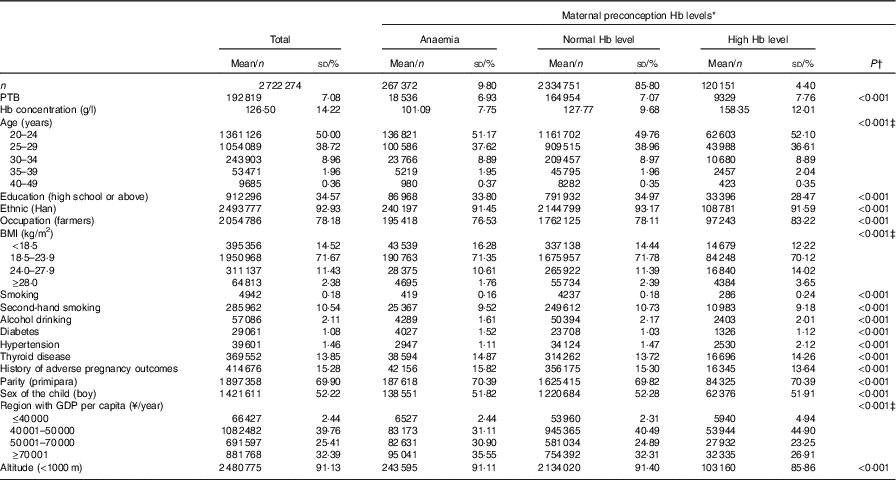
Anaemia, Hb concentration<110 g/l; normal Hb level, Hb concentration in the range of 110–149 g/l; high Hb level, Hb concentration≥150 g/l; PTB, preterm birth.
* Preconception Hb concentrations of women who lived in areas with altitude≥1000 m were adjusted by subtracting the adjustment values from the measured Hb concentrations according to the recommendation by the WHO( 17 , 18 ).
† Multiple comparison with Bonferroni-adjusted P value<0·05 (anaemia group v. normal Hb group; high Hb group v. normal Hb group).
‡ The Kruskal–Wallis H test was used to examine the differences of baseline characteristics among Hb groups. Others used the χ 2 test.
The median length of time from baseline examination to pregnancy was 1·54 months (IQR: 0·64–3·04). A total of 192 819 (7·08 %) PTB events were documented. The incidence of PTB was 6·93, 7·07 and 7·76 % for women who were anaemic, with normal Hb level and high Hb level, respectively (Table 2). Compared with women with Hb level of 110–149 g/l, the multivariable-adjusted OR for PTB was 0·98 (95 % CI 0·96, 0·99) and 1·06 (95 % CI 1·04, 1·09) for women with Hb <110 and ≥150 g/l, respectively. Increased risk of PTB was also observed for women with mild, moderate and severe high Hb levels (OR 1·04; 95 % CI 1·01, 1·06; 1·11; 95 % CI 1·05, 1·17; 1·19; 95 % CI 1·11, 1·27), whereas decreased risk of PTB was observed for women with mild anaemia (OR 0·96; 95 % CI 0·95, 0·98).
Table 2 Associations between maternal preconception Hb concentrations and risk of preterm birth (Odds ratios and 95 % confidence intervals)
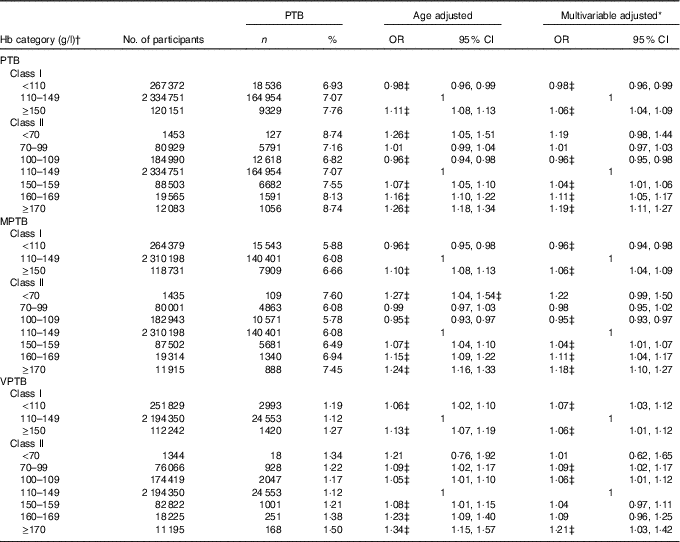
PTB, preterm birth (<37 weeks of gestation); MPTB, moderate preterm birth (32 to <37 weeks of gestation); VPTB, very preterm birth (<32 weeks of gestation).
* Multivariable-adjusted OR (95 % CI) were adjusted for characteristics of women (age, education, ethnic, occupation, region with GDP per capita), smoking, passive smoking and alcohol drinking status at baseline, history of diseases (diabetes, hypertension and thyroid dysfunction), pre-pregnancy BMI, parity, history of adverse pregnancy outcomes and sex of the child.
† Class I refers that women were classified into three groups (anaemia:<110 g/l; normal Hb level: 110–149 g/l; and high Hb level:≥150 g/l); class II refers that women were classified into seven groups (severe anaemia:<70 g/l; moderate anaemia: 70–99 g/l; mild anaemia: 100–109 g/l; normal Hb level: 110–149 g/l; mild high Hb level: 150–159 g/l; moderate high Hb level: 160–169 g/l; and severe high Hb level:≥170 g/l).
‡ Statistical significance (P<0·05).
Table 2 also summarises the associations between the maternal preconception Hb categories and risk of MPTB, as well as VPTB. Compared with women with Hb level of 110–149 g/l, the multivariable-adjusted OR for MPTB was 0·96 (95 % CI 0·94, 0·98) and 1·06 (95 % CI 1·04, 1·09) for women with Hb <110 and ≥150 g/l, respectively. The corresponding OR for VPTB was 1·07 (95 % CI 1·03, 1·12) and 1·06 (95 % CI 1·01, 1·12), respectively. When anaemia and high Hb concentration were further subdivided into mild, moderate and severe status, increased risk of VPTB was also observed for mild and moderate anaemia (OR 1·06; 95 % CI 1·01, 1·12; 1·09; 95 % CI 1·02, 1·17), and for severe high Hb level (OR 1·21; 95 % CI 1·03, 1·42).
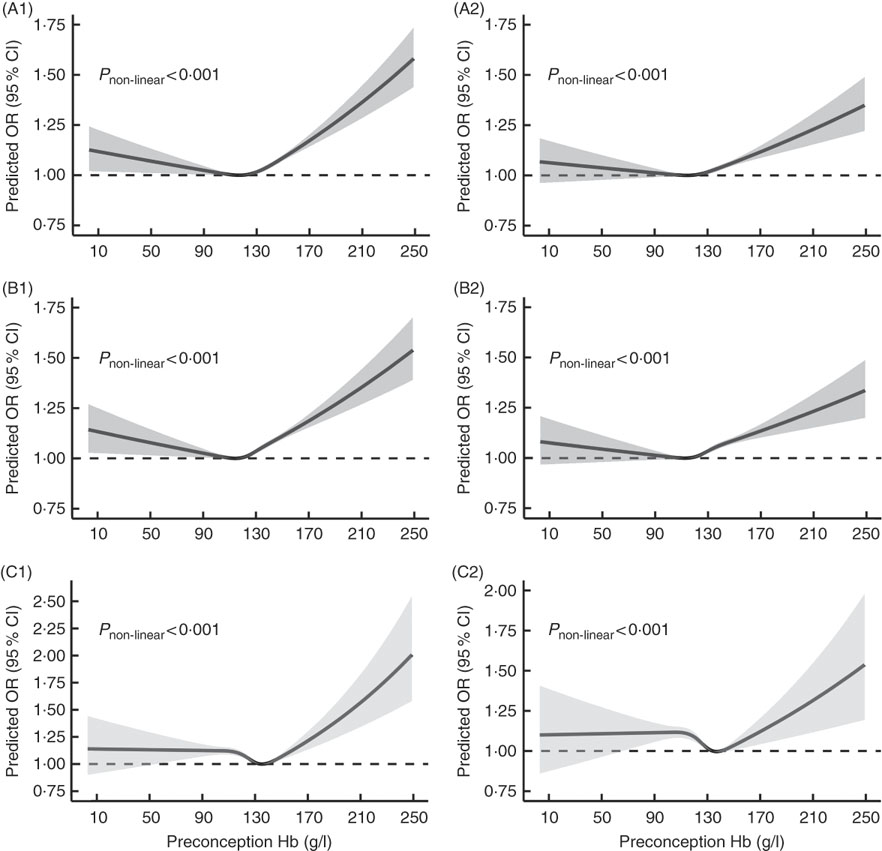
Fig. 2 shows U-shaped dose–response relationships of maternal preconception Hb concentration with PTB and MPTB (P non-linear<0·001), even though the multivariable-adjusted OR were not significant when Hb concentration was below 110 g/l. Although the OR remained stable when Hb concentration was lower than 130 g/l in the dose–response curve of maternal preconception Hb concentration with VPTB, the relationship was also approximately U-shaped (P non-linear<0·001).
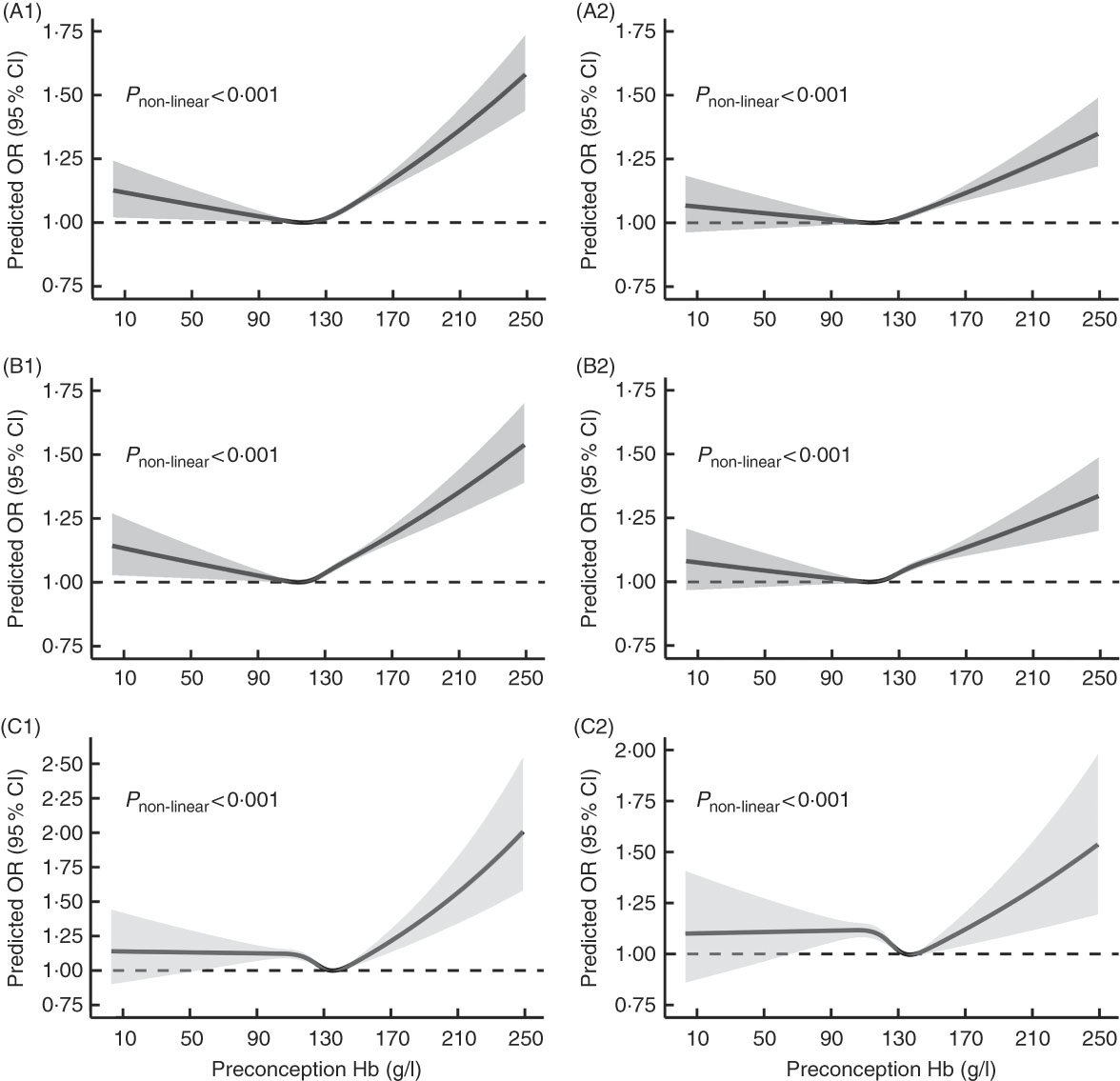
Fig. 2 Dose–response relationship between maternal preconception Hb concentrations and the risk of preterm birth (PTB, <37 weeks of gestation), moderate preterm birth (MPTB, 32 to <37 weeks of gestation) and very preterm birth (VPTB, <32 weeks of gestation). Graphs show the age-adjusted and multivariable-adjusted OR of associations between maternal preconception altitude-adjusted Hb concentrations and the risk of PTB (A1, A2), MPTB (B1, B2) and VPTB (C1, C2), respectively. In the graph, black lines and shaded grey areas represent predicted OR and 95 % CI, respectively. Multivariable-adjusted OR and 95 % CI were adjusted for characteristics of women (age, education, ethnic, occupation, region with GDP per capita), smoking, passive smoking and alcohol drinking status at baseline, history of diseases (diabetes, hypertension and thyroid dysfunction), pre-pregnancy BMI, parity, history of adverse pregnancy outcomes and sex of the child.
In the subgroup analysis, the associations between maternal preconception Hb levels and risk of PTB appeared to be modified by altitude (Table 3). For women living in areas≥1000 m, anaemia was associated with an increased risk of PTB (OR 1·15; 95 % CI 1·10, 1·21), but high Hb level was observed to be associated with a decreased risk of PTB (OR 0·92; 95 % CI 0·87, 0·98). Mild, moderate and severe anaemia had 13, 19 and 30 % higher risks of PTB, respectively, whereas no significant association was found between different grades of high Hb level and PTB risk. In the sensitivity analyses, the associations between preconception Hb categories and risk of PTB, as well as MPTB and VPTB, did not change appreciably by using original Hb concentrations (online Supplementary Tables S2 and S3).
Table 3 Subgroup analysis by altitude of the association between maternal preconception Hb concentrations and preterm birth (PTB) risk (Odds ratios and 95 % confidence intervals)
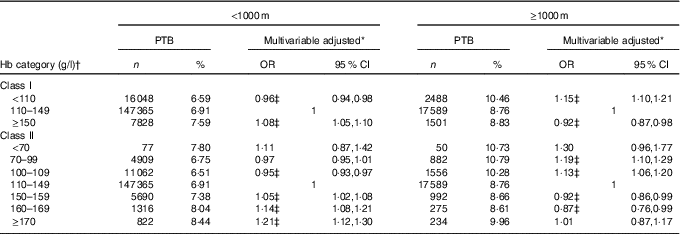
* Multivariable-adjusted OR and 95 % CI were adjusted for characteristics of women (age, education, ethnic, occupation, region with GDP per capita), smoking, passive smoking and alcohol drinking status at baseline, history of diseases (diabetes, hypertension and thyroid dysfunction), pre-pregnancy BMI, parity, history of adverse pregnancy outcomes and sex of the child.
† Class I refers that women were classified into three groups (anaemia:<110 g/l; normal Hb level: 110–149 g/l; and high Hb level:≥150 g/l); class II refers that women were classified into seven groups (severe anaemia:<70 g/l; moderate anaemia: 70–99 g/l; mild anaemia: 100–109 g/l; normal Hb level: 110–149 g/l; mild high Hb level: 150–159 g/l; moderate high Hb level: 160–169 g/l; and severe high Hb level:≥170 g/l).
‡ Statistical significance (P<0·05)
Discussion
In this large cohort study of over 2·7 million women in rural China, we found that high Hb level was associated with an increased risk of PTB, including MPTB and VPTB, and anaemia was associated with an increased risk of VPTB, independent of other vital risk factors. Furthermore, U-shaped curves for the risk of PTB, as well as MPTB and VPTB, with preconception Hb concentrations were identified. In areas with altitude≥1000 m, the OR of anaemia with PTB were larger than those of high Hb level with PTB.
In the current study, the incidence of PTB in the anaemia group, especially in the mild anaemia group, was lowest when compared with Hb of 110–149 g/l. In addition, we found that an increased risk of PTB, as well as MPTB, was only significantly associated with severe anaemia (<70 g/l) in age-adjusted models. The possible reason is that women with known anaemia before pregnancy would pay more attention to their Hb concentrations during pregnancy, and it is easier for women with mild anaemia than those with moderate or severe anaemia to improve their anaemia status through medical treatment, such as Fe supplements. Until now, there were only two studies that have assessed the association between preconception maternal anaemia and the risk of PTB( Reference Yi, Han and Ohrr 22 , Reference Ronnenberg, Wood and Wang 23 ). Owing to the different anaemia definitions and reference-level cut-off value adopted, our results were partly consistent with a previous retrospective cohort study conducted in Korean women, which found that moderate-to-severe anaemia (<100 g/l) before pregnancy was associated with PTB risk (OR 1·53; 95 % CI 1·05, 2·23) when compared with Hb of 120–149 g/l( Reference Yi, Han and Ohrr 22 ). However, another small-size cohort study (which included only 405 women) conducted in China found a null association between anaemia (<95 g/l) and PTB( Reference Ronnenberg, Wood and Wang 23 ). Our study also found that maternal preconception anaemia was associated with an increased risk of VPTB. However, the evidence on the relationship between maternal Hb concentration and VPTB risk was limited, possibly owing to the lower incidence of VPTB. The rate of self-reported VPTB events in the current study is 1·06 %, whereas that for overall PTB was 7·08 %. Our findings indicated that anaemia in the periconceptional period may have an adverse effect on PTB, especially on VPTB, which suggested that pre-pregnancy intervention for anaemia is very necessary.
Maternal high Hb level during pregnancy was suggested to have a more severe effect on birth outcomes than anaemia( Reference Chang, O’Brien and Nathanson 24 – Reference Steer 26 ). Previous studies have documented that high Hb concentration in the first( Reference Murphy, O’Riordan and Newcombe 27 ) or second trimester( Reference Gonzales, Tapia and Gasco 10 , Reference Chang, O’Brien and Nathanson 24 ) was significantly associated with an increased risk of PTB, because high Hb concentration may increase blood viscosity and harm placental blood flow( Reference Steer, Alam and Wadsworth 28 ). The current study demonstrated that women with preconception Hb ≥150 g/l had an increased risk of PTB, as well as MPTB and VPTB, when compared with those with preconception Hb of 110–149 g/l, and the associations remained significant when high Hb concentration was divided into different grades, except for the associations between mild and moderate high Hb concentration and VPTB. Our findings indicated that monitoring the risk of PTB for women with preconception high Hb concentration should not be ignored. However, another study conducted in Korean women found a null association between preconception high Hb level (≥150 g/l) and risk of PTB( Reference Yi, Han and Ohrr 22 ).The inconsistent results can be explained by the small sample size (2868 women) in the high Hb level group in their study, which limited the statistical power to detect a significant association( Reference Yi, Han and Ohrr 22 ).
The current study indicated that PTB rate was related to maternal preconception Hb concentrations in a U-shaped manner. There is substantial evidence for a U-shaped curve for the risk of adverse birth outcomes with maternal Hb concentration( Reference Chang, O’Brien and Nathanson 24 , Reference Steer, Alam and Wadsworth 28 – Reference Zhou, Yang and Hua 30 ), but some studies reported controversial results( Reference Yi, Han and Ohrr 22 , Reference Mohamed, Ahmad and Macri 31 ). The inconsistent results among previous studies might be related to the different criteria or cut-off thresholds for defining low and high Hb concentrations or caused by the various characteristics within the study population, such as different prevalence of abnormal Hb status and PTB. Hb concentration is highly correlated with the Fe stores in the body. Fe deficiency is a common cause of anaemia, and daily Fe supplementation may result in an increase in Hb values in 11 % of pregnant women( Reference Casanueva, Viteri and Mares-Galindo 32 ). A recent meta-analysis indicated that both Fe deficiency and Fe excess would increase the risk of gestational disease and pregnancy outcomes( Reference Iqbal and Ekmekcioglu 33 ). In China, free prenatal Fe supplements were not provided by the government. Some pregnant women may have Fe supplements during pregnancy by taking multi-vitamin products, such as Elevit. However, detailed information about Fe supplements of pregnant women was not collected in the current study. Therefore, the association between preconception Hb levels and PTB risk, taking Fe supplements or ferritin level during pregnancy into consideration, needs to be further explored in the future. Besides, our results showed that women with less educational attainment, pre-existing diabetes or thyroid dysfunction were more likely to be with abnormal Hb concentrations, which highlights that clinicians pay more attention to those with these risk factors to effectively reduce the risk of PTB.
As is well known, most populations living at high altitude characteristically have an increased Hb concentration as a compensatory mechanism to the effect of hypoxia( Reference Penaloza and Ariasstella 34 ). This Hb increase can ultimately misclassify anaemia or high Hb if it is not taken into consideration. Therefore, Hb concentration of residents living in areas≥1000 m should be adjusted according to the adjustment values recommended by the WHO( 17 , 18 ). However, no study had used adjusted Hb concentrations to explore its association with adverse pregnancy outcomes, except one study conducted in Bolivia( Reference Laflamme 35 ). Our study observed a higher risk of PTB associated with anaemia than that with high Hb level among 241 499 women, 8·87 % of the total participants, who lived in areas≥1000 m, which is consistent with the results of another cohort study conducted at high altitudes( Reference Gonzales, Steenland and Tapia 29 ). These findings indicated that anaemia is more harmful than high Hb level in high-altitude areas with respect to PTB.
In the study, over 2·7 million participants were recruited, and were followed up on pregnancy outcomes using strict quality controls. This large sample size enables us to divide Hb into different levels and ensure enough statistical power to detect the associations. Furthermore, Hb concentrations before pregnancy within 6 months were used in our study, which minimises the possibility of differential misclassification of the Hb level and confounding by haemodilution with gestational age.
However, several limitations should be addressed. First, changes in Hb concentrations were not monitored during pregnancy, which limited the estimation of Hb concentration during pregnancy or the effect of Hb changes on PTB rate. Second, other factors associated with Hb concentration, such as ferritin level or Fe supplements intake, were not collected, so we cannot distinguish which specific type of anaemia or high Hb level is associated with PTB. Third, owing to the lack of detailed information regarding specific types of PTB, such as spontaneous PTB or iatrogenic PTB, the association between maternal Hb concentration and various types of PTB cannot well be examined. Fourth, self-reported information on the LMP and delivery date was used in the current study, which may result in an inaccurate gestational age calculation and the misclassification of PTB. Finally, data on important maternal conditions during pregnancy that have significant effects on PTB, such as gestational hypertension or preeclampsia, were not collected, so we could not adjust for these factors in the multivariable analysis.
In summary, our study identified a U-shaped relationship between maternal preconception Hb concentration and the risk of PTB in Chinese, rural, reproductive-aged women. Both maternal anaemia and high Hb level before pregnancy can significantly increase the risk of VPTB. Early detection of Hb concentration and providing appropriate intervention for women with anaemia or high Hb concentration, before pregnancy, should be considered an important approach for primary prevention of PTB, and would be helpful in reducing perinatal mortality and morbidity.
Acknowledgements
The authors thank health workers and countless participants throughout thirty-one provinces in the NFPCP for great efforts and collaboration.
The study was supported by National Key Research and Development Program of China (no. 2016YFC1000307), National Natural Science Foundation of China (grant nos 81402757 and 21577119) and Operation Expenses for Basic Scientific Research of Central Authorities (grant no. 2016GJZ10). The sponsors had no role in study design, data collection, data analysis, data interpretation, writing of the report or the decision to submit the report for publication.
The corresponding author has full access to data in the study and takes responsibility for data integrity and the accuracy of data analysis. X. Z. and Q. X. searched the literature, analysed the data, interpreted the results and drafted the manuscript. L. W., Q. L., M. J., Y. H., Y. W., Y. Z., H. Z. and Z. P. collected the data. F. L. revised the manuscript. Y. Y., X. M. and Z. Y. conceived of the study, provided overall guidance and revised the manuscript. All authors revised the article for important intellectual content, and each approved the final manuscript as submitted.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518001721