Colorectal cancer (CRC) is the third leading cause of cancer-related death globally(Reference Rawla, Sunkara and Barsouk1). Approximately, 1 200 000 new cases and 609 000 deaths occur across the globe in each year. At the same time, CRC accounts for about 10 % of all cancers in men and women worldwide(Reference Sümbül and Akkız2). Alarmingly the global burden of CRC is presumed to rise by 60 % in the coming years exceeding 2·2 million new cases and 1·1 million cancer deaths by 2030(Reference Bray, Ferlay and Soerjomataram3). The situation is becoming frightening as the CRC incidence rate increased by 1·6 % in adults aged below 50 years during the period of 2000–2013. Mortality is also increased by 13 % in the same period(Reference Siegel, Miller and Fedewa4). CRC originates from the epithelial cell lining of the colon or rectum in the gastrointestinal tract under the influence of genetic and environmental factors along with other factors like diet, lifestyle, genomic mutation, inflammatory bowel disease and an imbalance in gut microbiota(Reference Wang, Cui and Pan5). However, more than 70 % of the cases are still considered sporadic with no family history or genetic predisposition(Reference Yamagishi, Kuroda and Imai6). Inflammatory bowel disorder is one of those reasons and deemed as the third-highest risk factor for CRC only after the familial adenomatous polyposis and hereditary non-polyposis CRC(Reference Kim and Chang7). The stage of diagnosis is one of the principal determinants of the outcome of any cancer, including CRC. Therefore, the search for early diagnosis is ever demanding in clinical set-up and regarded as the principal determinant of fruitful outcome post-treatment.
Currently, CRC is clinically treated by surgery and subsequent chemotherapy. Unfortunately, chemotherapeutics are always associated with unavoidable toxicity which worsens the quality of an individual’s life(Reference Nurgali, Jagoe and Abalo8). Adverse side effects arise as chemotherapeutic agents can leave their mark on the fast-dividing non-malignant cells like hair follicle cells or digestive tract cells, along with the tumour cell(Reference Redondo-Blanco, Fernández and Gutiérrez-Del-Río9). The first line of CRC chemotherapy is based on 5-fluorouracil which can cause adverse side effects like nausea, vomiting, diarrhoea, mucosal and submucosal tissue damage, inhibition of the haematopoietic function of the bone marrow, leukopenia, etc.(Reference Gillis and Eminger10). Therefore, search for an alternative treatment strategy with minimal side effect to treat or prevent CRC is always on. In this context, olive oil and its phenolic compounds find their place as one of the alternative strategies used by the different research groups as this is a part of the natural diet, various ethnic groups all the world, especially Mediterranean people follow. A voluminous literature focused on the activity of different biologically active compounds present in the diet, resisting different cancers. Accumulating evidence suggests regular intake of olive oil may protect against developing CRC. The main aims of this review are to accumulate and critically asses the chemopreventive activities of olive oil as well as some of the phytochemicals originating from it, including hydroxytyrosol, tyrosol, oleuropein, oleocanthal (OC), apigenin, luteolin, etc. and the mechanisms behind the protection.
An extensive search in the PubMed, Google Scholar and Medline databases carried out using relevant keywords, for example, ‘colorectal cancer’ or ‘colon cancer’ combined with other terms including ‘olive’, ‘olive oil’, ‘virgin olive oil’, ‘extra virgin olive oil’, ‘hydroxytyrosol’, ‘oleuropein’, ‘oleocanthal’, ‘apigenin’, ‘luteolin’ and ‘olive phenolic extract’; we filtered our search within the literature by sticking to the time frame January 2010–April 2021.
Molecular insight of colorectal cancer
Genomic instability is a major driving force behind CRC(Reference Grady and Carethers11) and major molecular events include chromosomal instability (CIN), microsatellite instability and CpG island methylation that may lead to genomic instability(Reference Mármol, Sánchez-de-Diego and Pradilla Dieste12). In ˜85 % of CRC cases, CIN prominently presents either in the form of loss of tumour suppressor genes (TSG) or activation of oncogenes(Reference Tsang, Cheng and Wong13,Reference Damilakis, Mavroudis and Sfakianaki14) . TSG like adenomatous polyposis coli (APC), TP53 and SMAD4 are either physically lost from the genome or mutated in CRC. CIN can also drive the activation of different oncogenes like KRAS (Kirsten Rat Sarcoma viral oncogene homolog), BRAF (v-raf murine sarcoma viral oncogene homolog B1) and PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha) by accumulating mutations (Fig. 1)(Reference Wang, Cui and Pan5,Reference Nguyen and Duong15) . Microsatellite instability presents in 15–20 % of sporadic CRC and more than 95 % in hereditary non-polyposis colon cancer(Reference Nguyen and Duong15). Mismatch repair genes are also affected by microsatellite instability which include silencing of MutL homolog 1 in HNPPC patients, already having a higher risk of developing CRC(Reference Ponz de Leon and Percesepe16,Reference Turano, Delrio and Rega17) . Inactivation of DNA mismatch repair results in the alteration of different key regulatory genes like TSG (e.g. TGFBR2, TCF4) and apoptosis-pathway related genes (e.g. BAX, caspase 5) (Fig. 1)(Reference Turano, Delrio and Rega17,Reference Mori, Yin and Rashid18) . Another important driving factor of CRC tumourigenesis is CpG island methylator phenotype, an epigenetic alteration that causes aberrant methylation of CpG islands and present in around 20–30 % of all CRC (Fig. 1)(Reference De Palma, D’Argenio and Pol19,Reference Advani, Advani and Brown20) . It is characterised by DNA hypermethylation at promoter-associated CpG islands of TSG results in transcription inhibition of the particular TSG(Reference Curtin, Slattery and Samowitz21).

Fig. 1. Schematic representation of key molecular events that drive colorectal carcinoma. APC mutation acts as the stepping stone in the process of transforming normal colorectal epithelium to adenoma, whereas the adenoma–carcinoma sequential progression is supported by alteration in three crucial events: CIN, microsatellite instability and CpG island methylator phenotype. Once the oncogenesis initiated, further accumulation of genetic changes by mutations of regulatory genes, such as DNA repair genes drive the progression. Finally, modifications of the genes related to epithelial–mesenchymal transition, basement membrane disruption, cell motility and angiogenesis contribute to metastasis.
New factors coming to the mix include microRNA (miRNA) and long non-coding RNA (lncRNA), which are thought to play a significant role in the carcinogenesis process of CRC as the expression of both miRNA and lncRNA altered in CRC(Reference To, Tong and Wu22,Reference Yao, Wang and Chen23) . Aberrant expressions of miRNA (e.g. miR-106a, miR-143) and lncRNA (e.g. HOTAIR, MALAT1) can lead carcinogenesis by altering the expression of different key regulatory genes (e.g. RB1 (retinoblastoma), BCL2 (B-cell lymphoma 2), KRAS, etc.)(Reference Lin, Chuang and Zuo24,Reference Galamb, Barták and Kalmár25) . Recently, it has been shown that the miR-200 family including miR-141, miR-200a, miR-200b, miR-200c and miR-429 is down-regulated in CRC and linked to epithelial-to-mesenchymal transition of cancer cells(Reference Ranković, Zidar and Žlajpah26). Similarly, lncRNA such as H19 or MALAT1 can promote metastasis and invasion in CRC(Reference Bermúdez, Aguilar-Medina and Lizárraga-Verdugo27). More fascinating connections are coming through the rank as microRNA, along with lncRNA, shown to have a role in the acquisition of post-treatment drug resistance(Reference Corrà, Agnoletto and Minotti28).
Alterations of key signalling pathways: driving force behind colorectal cancer development
It is a well-known fact that impairment of cell signalling pathways help tumour cells to survive within the microenvironment(Reference Wood, Parsons and Jones29,Reference Pennel, Park and McMillan30) . Some of the key signalling pathways documented to be involved in CRC include Wnt/β-catenin pathway, epidermal growth factor receptor (EGFR)/MAPK pathway, PI3K pathway, NF-κβ pathway, TGFβ signalling pathway and JAK/STAT pathway(Reference Wang, Cui and Pan5,Reference Koveitypour, Panahi and Vakilian31) . All the more, these intracellular pathways do not work in an isolated manner within the cancer milieu rather their crosstalk with each other fuel the progression and invasiveness of CRC and responsible for increased drug resistance(Reference Koveitypour, Panahi and Vakilian31–Reference Yuan, Tao and Zhang33).
Wnt/β-catenin signalling serves as the central organiser of epithelial stem cell identity and crypt maintenance(Reference Koch34) and highly interlinked with several other signalling pathways (e.g. Notch, Hedgehog, BMP). The combinatorial signalling events shape the homoeostasis of the intestinal epithelium and responsible for tissue regeneration (Fig. 2)(Reference Vanuytsel, Senger and Fasano35,Reference Kaemmerer, Jeon and Berndt36) from the stem cells reside at the lower crypt of the intestine. β-catenin-mediated canonical Wnt signalling drives proliferation at the lower crypts(Reference Komiya and Habas37) whereas the non-canonical Wnt signalling (β-catenin independent) operates predominantly in the upper crypt area, where the proliferation comes to a halt and differentiation becomes essential. β-catenin gets accumulated and stabilised as a result of the Wnt activation. Subsequently, β-catenin-dependent transcription of several target genes controls the proliferation of intestinal stem cells(Reference Martini, Krug and Siegmund38). This pathway is one of the most significant pathways as APC gene is the most often mutated in CRC and linked to both sporadic and hereditary carcinogenesis(Reference Kaemmerer, Jeon and Berndt36,Reference Armaghany, Wilson and Chu39,Reference Kirsanov, Fetisov and Lesovaya40) . Mutation at APC is one of the main factors in the development of familial adenomatous polyposis syndrome as well as found around 80 % in sporadic CRCs(Reference Cheng, Xu and Chen41). APC acts as an integral member of the β-catenin destruction complex and thereby prevents β-catenin accumulation in cytoplasm(Reference Hankey, Frankel and Groden42). So, in the absence of APC or in case of mutated APC condition, β-catenin accumulates to a higher level and translocates into the nucleus. In the nucleus, β-catenin binds to DNA and activates the transcription of different proto-oncogenes linked to CRC, like c-myc, cyclin D1 and matrix metalloproteinase-7(Reference Cheng, Xu and Chen41). Recently, Yaegar et al. (Reference Yaeger, Chatila and Lipsyc43) observed several alterations in the core Wnt regulator genes within a set of 400 genes and identified oncogenic Wnt activation in 96 % of human CRCs. Similarly, Wnt signalling in tumour microenvironment linked to tumour immunomodulation and immune suppression(Reference Goldsberry, Londoño and Randall44). So, it is quite evident that targeting Wnt/β-catenin is always a major focus of the CRC research.

Fig. 2. Schematic depiction of colonic epithelium structure and components. The colonic crypt can be subdivided into three zones depending on the presence of different types of cells: stem cell zone, transit-amplifying (TA) cell zone and the differentiated zone. Reg4+ (regenerating islet-derived family member 4) deep crypt secretory cells (DCS) reside at the bottom of the colonic crypt and provide necessary support to the Lgr5+ (leucine-rich repeat-containing G-protein coupled receptor 5) stem cells, similar to the Paneth cells present in the small intestines. Quiescent stem cells or label-retaining cells (LRC) are located at the +4 position of the stem cell zone. TA cells are rapidly dividing and eventually differentiate into functional cells. The presence of Wnt, Notch, BMP, BMP antagonists, and Hedgehog and their respective concentration gradient in different zones is indicated by upward and downward triangles.
Apart from the Wnt/β-catenin pathway, several other signalling pathways like EGFR/MAPK signalling pathway, phosphatidylinositol-3-kinase (PI3K) signalling pathway and NF-κβ pathway also contribute to the development and progression of CRC. Different key players of the EGFR/MAPK signalling pathway (e.g. KRAS, BRAF, etc.) are mutated in CRC(Reference Krasinskas45) which limits the efficacy of EGFR inhibitors like cetuximab in metastatic CRC(Reference Zhang, Roberts and Shivdasani46). On the other hand, PI3K pathway influences the initiation and progression of CRC. Mutations in PIK3CA and PIK3CB (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit beta) gene and loss of function of TSG PTEN (phosphatase and tensin homolog) can accord the process of benign to malignant transformation(Reference Papadatos-Pastos, Rabbie and Ross47). Akt, which acts as the downstream effector of the PI3K pathway, also involved in the proliferation as well as apoptosis inhibition in CRC(Reference Koveitypour, Panahi and Vakilian31). Under the influence of Akt, further downstream effector mTOR supports angiogenesis, protein translation, growth and metabolism(Reference Koveitypour, Panahi and Vakilian31). PIK3CA mutation even confers resistance to first-line chemotherapy (FOLFOX regimen) in CRC as survival and proliferation of CRC stem cells are up-regulated by PI3K/Akt signalling(Reference Wang, Shi and Zhou48). Different types of inhibitors like pan PI3K inhibitors, Akt inhibitors and PI3K/mTOR dual inhibitors are being tested in clinics to restrain PI3K/Akt/mTOR axis. On the other hand, the NF-κβ signalling pathway serves as a major regulator of inflammation and activated NF-κβ is linked to DNA damage, carcinogenic mutations and redox imbalance. All these can lead to CIN, aneuploidy and epigenetic changes related to tumourigenesis(Reference Yang, Dang and Ji49). Along with the STAT3 signalling pathway, NF-κβ plays an integral role in the transformation of inflammation into CRC by regulating cellular signal transduction(Reference Yang, Dang and Ji49). NF-κB action also promotes the proliferation and invasion and metastasis by regulating signalling pathways including epithelial-to-mesenchymal transition(Reference Li, Lin and Chen50).
Olive oil and its phenols: is it worthy of use in colorectal cancer?
Natural products including phytochemicals are gradually coming to the mix in search for inhibitors of aberrant cellular signalling networks and dietary modification could hold the key to prevent CRC by regulating cell signalling. Owing to the drug resistance and unanticipated side effects of chemotherapy, a voluminous quantity of research focused on the activity of different biologically active compounds present in the diet as an alternative strategy in CRC. The biologically active compounds from plants are defined as phytochemicals which include polyphenols, flavonoids, phytoalexins, phenolic acids, etc.(Reference Dariya, Rajitha and Alam51). Olive oil, the principal culinary fat in the traditional Mediterranean diet, is a bountiful source of phenolic compounds(Reference Papanikolaou, Melliou and Magiatis52). A number of phytochemicals isolated from olive oil polyphenols (OOP) have been shown to exert anti-inflammatory as well as anti-cancer properties(Reference Bassani, Rossi and De Stefano53) and the health claim of hydroxytyrosol (a phenolic compound present in olive oil) already approved by the EFSA (European Food Safety Authority) in 2017 (https://www.efsa.europa.eu/en/efsajournal/pub/4728, accessed on 6th July 2021). Over the years, it has been learned from in vitro and in vivo models and that OOP may evolve as a novel therapeutic strategy to avert and treat disease with minimal side effects. Additionally, the amalgamation of chemotherapeutic drugs and phenolic compounds present in olive oil could synergistically augment positive treatment outcome in cancer by reducing the undesirable side effects of conventional anticancer drugs(Reference Terzuoli, Nannelli and Frosini54,Reference Torić, Marković and Brala55) . In the past few decades, OOP been exploited effectively as preventive and therapeutic agents in a spectrum of diseases including CVD(Reference Pelucchi, Bosetti and Negri56) obesity(Reference Soriguer, Almaraz and Ruiz-de-Adana57), diabetes mellitus(Reference Schwingshackl, Lampousi and Portillo58), Alzheimer’s disease(Reference Román, Jackson and Reis59) and different cancers like breast, liver, lungs and CRC(Reference Borzì, Biondi and Basile60). Thus, it is worthy to analyse the current developments of olive oil effects on CRC and future strategies to include olive oil components in the treatment protocol.
Protective role of different forms of olive oil against colorectal cancer
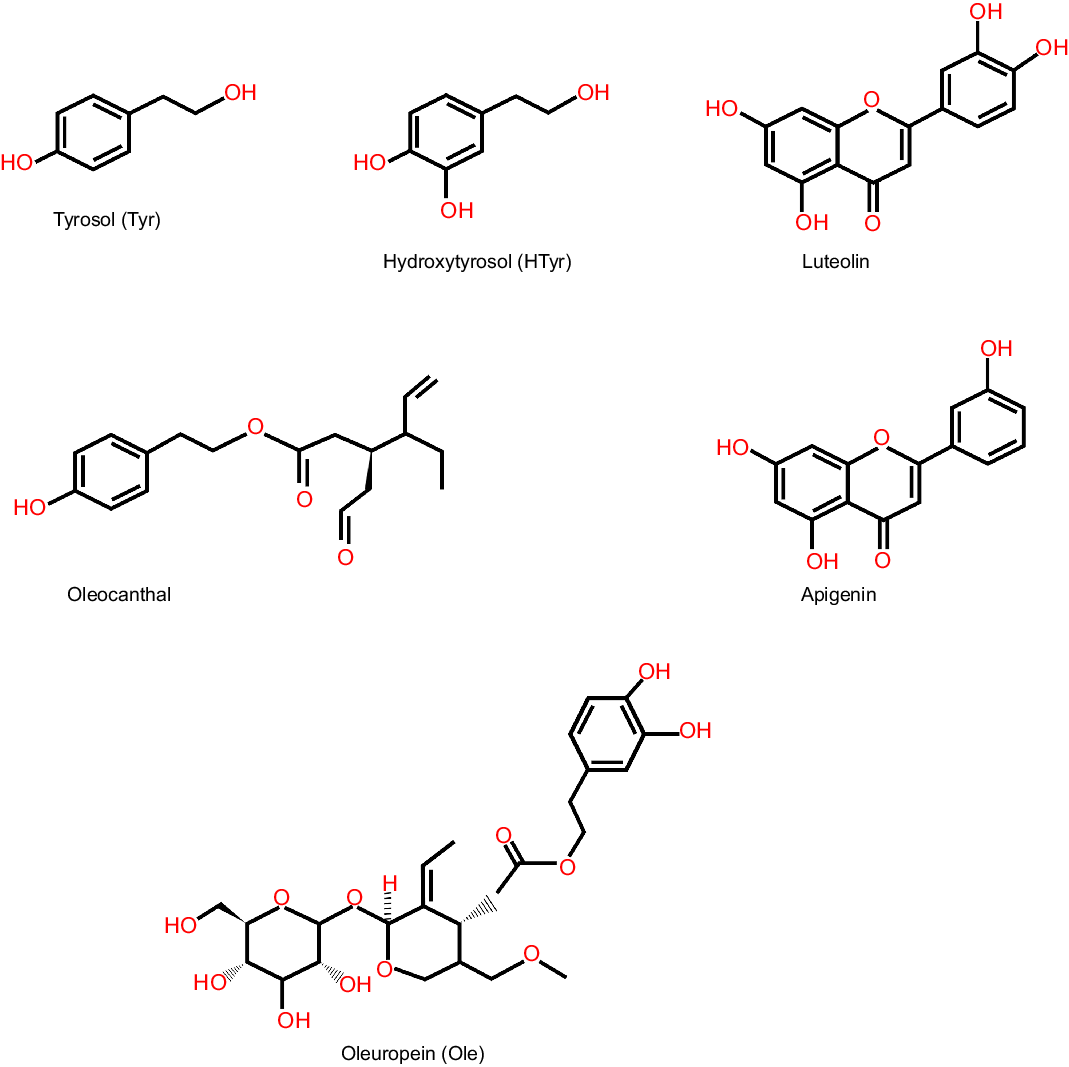
Olive oil is regarded as the plentiful source of phenolic compounds. Among all the phenolic compounds present in olive oil, tyrosol (Tyr), hydroxytyrosol (HTyr) (the concentration of total tyrosol and hydroxytyrosol is 100–400 mg/kg oil)(Reference Romero and Brenes61), oleuropein (Ole) (3·8 mg/kg oil)(Reference Medeiros de Azevedo, Ferreira Ribeiro de Oliveira and Alves Alcântara62) and its aglycone (222·62–537·83 mg/kg)(Reference Ouni, Taamalli and Gómez-Caravaca63) are well characterised and most studied (Fig. 3).

Fig. 3. Structure of major phenolic compounds present in olive oil. Major phenolic compounds present in olive oil and their structure are shown here, which include simple phenol like hydroxytyrosol, secoiridoid like oleuropein and flavone like apigenin.
Dietary habit is related to cancer and accumulating pieces of evidence hint at a link between the consumption of red meat and CRC risk(Reference Aykan64). Consumption of red meat may lead to an increased level of secondary bile salt in the gut(Reference Trefflich, Marschall and Giuseppe65), that may in turn inhibit the action of diamine oxidase, an enzyme present in a high level at ileal mucosa and colon. All these actions can lead to mucosal proliferation as well as carcinoma(Reference Imran, Nadeem and Gilani66). Stoneham et al.(Reference Stoneham, Goldacre and Seagroatt67) first demonstrated that olive oil consumption could protect against CRC development by influencing polyamine metabolism in the colon through altering secondary bile acid patterns. However, the missing link between consumptions of olive oils and effect of its constituents on normal healthy cells’ metabolism yet to be documented which requires extensive research as polyamines are vital for normal cell growth as well. Similarly, in human colon adenocarcinoma cell line (Caco-2), extra virgin olive oil (EVOO) polyphenols protect against inflammation induced by oxysterol (present in cholesterol containing food items) by reducing the NF-κB pathway(Reference Serra, Incani and Serreli68). Hence, diet containing olive oil could protect gut epithelium from potentially harmful components present in food like oxysterols and help in maintaining gut homoeostasis. Protective role of EVOO against intestinal inflammation is well documented as EVOO protects against intestinal inflammation induced by 5 % (w/v) of dextran sodium sulphate in drinking water for 10 d in mice by reducing the expression of pro-inflammatory genes (e.g. IL-1β, TGFβ, IL-6)(Reference Cariello, Contursi and Gadaleta69). Further olive oil may bring about cancer cell death by inducing apoptosis in CRC cells in vitro by virtue of its antioxidant properties(Reference Centrone, D’Agostino and Difonzo70) and interfere in colorectal carcinogenesis by reducing COX-2 (cyclooxygenase-2) and Bcl-2 level(Reference Llor, Pons and Roca71). It is also shown to interfere all the three stages of CRC development including initiation, promotion and metastasis(Reference Costea, Hudiţă and Ciolac72). Another aspect of protective role of olive oil against colon carcinogenesis is possible through improving barrier function, reducing DNA damage and decreasing invasiveness as shown in in vitro (HT-29, HT-119 and Caco-2)(Reference Gill, Boyd and McDermott73) as well as in colon carcinoma in vivo rat model. In rat model, olive oil potentially acts on arachidonic acid metabolism and PGE2 synthesis to protect against colon carcinogenesis(Reference Bartolí, Fernández-Bañares and Navarro74). In a very recent study, it has been further solicited that EVOO-rich diet is capable to prevent colorectal carcinogenesis virtue of its ability to modify gut microbiota in mice(Reference Rodríguez-García, Sánchez-Quesada and Algarra75). Involvement of olive oil containing diet on gut barrier health should be explored critically as leaky gut and altered gut microbiome are proved to be critical in colon carcinogenesis. Not only the VOO alone, but the metabolites generated from VOO by gut microbiota such as HTyr and phenylacetic and hydroxyphenylpropionic acids also help cell cycle arrest and promote apoptosis(Reference López de Las Hazas, Piñol and Macià76). Therefore, next line of research should be focused on the olive oil metabolites as well. Both virgin olive oil and OVP (virgin olive oil phenolics extract) have shown the anti-invasive properties in vitro (HT-115)(Reference Hashim, Rowland and McGlynn77) and in vivo (SCID BALB/c mice) model by reducing different integrin protein expression to control metastasis(Reference Hashim, Worthington and Allsopp78). Pampaloni et al.(Reference Pampaloni, Mavilia and Fabbri79) revealed that EVOO inhibits CRC cell growth by acting on oestrogen receptor-β. However, the precise role of the individual phenolic compounds on oestrogen receptors is yet to be discovered. Effectiveness of olive oil is shown against a common environmental toxicant, benzo[a]pyrene (B[a]P)-induced colon carcinogenesis in mouse model where it accelerates B[a]P detoxification in the liver and thereby decreases oxidative damage caused by otherwise harmful metabolites generated via B[a]P biotransformation(Reference Banks, Amoah and Niaz80). In this context, effect of olive oil on phase II metabolism of carcinogens should be studied in great detail to establish olive oil-based diet as a preventive strategy against common carcinogens. Similarly, EVOO-enriched diet could have a preventive role in ulcerative colitis-associated colon carcinogenesis(Reference Sánchez-Fidalgo, Villegas and Cárdeno81). Epigenetic modifications of key regulatory genes by changing the DNA methylation are also quite possible as evidenced in pre-clinical DMH (1, 2-dimethylhydrazine) treated colon cancer in rat model where olive oil treatment inhibited the NF-κB inflammatory pathway and restored apoptotic pathways by altering miRNA and methylation pattern(Reference Nanda, Mahmood and Bhatia82). Thus, epigenetic therapy based on olive oil components could be a reality in coming years although the effect of olive oil on methylation pattern of regulatory genes essential for normal cell function should be characterised in great detail. In another remarkable study, EVOO polyphenols alone shown to inhibit the colon cancer cell (SW480) growth, but in combination with anticancer drugs such as carboplatin, cisplatin, 5-fluorouracil and irinotecan enhance the metabolic activity and survival of cancer cell which imply cautious intake of olive oil in patients under chemotherapy(Reference Torić, Brozovic and Baus Lončar83). Therefore, to better evaluate the efficacy of olive oil in CRC, more clinical research should be designed to evaluate the role of individual components of olive oil and their metabolites. Table 1 summarises the outcomes of several studies where various form of olive oil used as intervention.
Table 1. Summary of studies involving different forms of olive oil as intervention in CRC

Olive oil polyphenols against colorectal cancer
Hydroxytyrosol and tyrosol
Hydroxytyrosol and tyrosol are two phenolic compounds which are found abundantly in olive oil and both are known for their antioxidant attributes(Reference Karković Marković, Torić and Barbarić84). Different studies have weighed on their possible effect in different cancers(Reference Toteda, Lupinacci and Vizza85,Reference Chimento, Casaburi and Rosano86) . Tyrosol can potentially curb intestinal inflammation by attenuating IL-8 secretion as shown in human colon adenocarcinoma cells, WiDr(Reference Ye, Chang and Tseng87). Anti-invasive property of tyrosol explored in HT-115 colon carcinoma cells, where tyrosol reduced invasion by ˜30–70 %(Reference Hashim, Rowland and McGlynn77). However, the effect of tyrosol as sole intervention is not studied in great detail in animal model of CRC which could be important to assess the potential of OOP as possible preventive measures. On the other hand, HTyr can induce apoptosis in human colon cancer cells (DLD1) possibly by generating reactive oxygen species and destabilising the intrinsic redox status of cancer cell through PI3K/Akt signalling pathway(Reference Sun, Luo and Liu88). It also shown to stimulate apoptotic cell death of CRC cells (HT-29) in a p53-dependent way(Reference Cárdeno, Sánchez-Hidalgo and Rosillo89). Olive oil polyphenolic extract containing both Tyr and HTyr along with Ole led to cell cycle arrest in colon adenocarcinoma cells as these phenols have a strong negative effect on CRC cell proliferation by blocking the cell cycle at the G2/M phase(Reference Corona, Deiana and Incani90). Authors further suggested that interference in the cell cycle is due to obstructive COX-2 expression through inhibition of p38 and transcription factor, CREB (cAMP response element-binding protein)(Reference Corona, Deiana and Incani90). Another study by the same group pointed out that HTyr is able to reduce the level of cyclin D1 through inhibition of extracellular signal-regulated kinase (ERK)1/2 phosphorylation and therefore CRC cell proliferation(Reference Corona, Deiana and Incani91). G1 phase blockade of human colon cancer cells (Caco-2 and HT-29) was possible with HTyr and it instigated caspase-dependent apoptosis in CRC cells(Reference López de Las Hazas, Piñol and Macià76). It seems that HTyr has both anti-proliferative and pro-apoptotic properties against CRC cells, but the effect of HTyr on cell survival pathways like autophagy should be studied at the same time to evaluate possible resistance against HTyr by the cancer cells. Another feature of HTyr protection against CRC may be through its anti-metabolic properties as it can influence the activity of a major anabolic enzyme fatty acid synthase, an important regulator of the AMPK/mTOR pathway in human colon cancer cells(Reference Notarnicola, Pisanti and Tutino92). Fatty acid synthase plays a critical role during cancer cell growth transformation, that is, from two-dimensional to three-dimensional growth(Reference Bueno, Jimenez-Renard and Samino93). In a different mechanism proposed by Di Francesco et al.(Reference Di Francesco, Falconi and Di Germanio94) HTyr alters the function of TSG CNR1 that codes for type 1 cannabinoid receptor (CB1) by reducing the level of DNA methylation at the promoter region of CNR1 gene which subsequently leads to the increased CB1 expression (up to 4-fold) in colon of Sprague–Dawley rats. HTyr also increased the CNR1 expression through reduction of the CNR1 targeting miRNA (e.g. miR23a and miR-301a). This is the initial hint of epigenetic modification of regulatory genes by HTyr. Although epigenetic modifications of other oncogenes or TSG by HTyr not documented yet, HTyr can also exert its action on cancer cells through cell surface receptors or intracellular receptors. It reduces CRC cell proliferation via intracellular oestrogen receptors as lyophilised extracts containing HTyr minimised human colon cancer cell proliferation, through oestrogen receptor-β (Reference Bernini, Carastro and Palmini95). In a recent study, it is unveiled that HTyr can hinder the activity of cell surface receptor EGFR which is strongly associated with CRC progression. Treatment with HTyr in colonic adenocarcinoma cells (CaCo2, HT-29 and WiDr) resulted in a decrease in EGFR expression through lysosomal and proteasomal machinery and subsequent halt in cell proliferation. HTyr further directs EGFR degradation by inducing ubiquitination of EGFR through phosphorylation of the docking site of Cbl (E3 ubiquitin-protein ligase), pY1045. Inhibition of EGFR and subsequent decrease in tumour growth by HTyr have been shown in animal model (HT-29 xenografts) as well(Reference Terzuoli, Giachetti and Ziche96). HTyr even capable of mounting cetuximab (EGFR inhibitor) action against CRC cells. The combination of HTyr and cetuximab showed stronger cytotoxicity against CRC adenocarcinoma cells (WiDr and HT-29) compared with cetuximab alone. This combinational treatment resulted in the cell cycle blockade at G2/M phase by down-regulating various cell cycle regulators such as cyclins B, D1 and E, and cyclin-dependent kinase (CDK)2, CDK4 and CDK6. Enhanced apoptosis (caspase-independent) and autophagy were also observed in colon cancer cells after the combination treatment. Remarkably, normal colon cells or human keratinocytes were least affected from this combinational therapy(Reference Terzuoli, Nannelli and Frosini54) which indicates diet containing HTyr during cetuximab therapy might protect healthy cells, for example, skin or haematopoietic cells from severe side effects of cetuximab in CRC patients receiving cetuximab. So, there is a possibility that hydroxytyrosol supplement to the patients receiving cetuximab therapy might improve the quality of patients’ life in the clinic. On this backdrop, it should be noted that HTyr action depends on its concentration being used in the experimental set-up as it may act as both anti- and pro-oxidant within the physiological system. When given at a higher dose (100 µM), HTyr showed pro-oxidant effects in CRC cells (SW480 and HCT116) and generated H2O2 to kill cancer cells(Reference Fabiani, Sepporta and Rosignoli97). On the other hand, at low doses (10µM), it is potent to counteract the DNA damage in peripheral blood mononuclear cells induced by external H2O2 treatment(Reference Rosignoli, Fuccelli and Sepporta98). It is also possible that the sensitivity of different cancer cells to HTyr treatment is inversely proportional to the ability of the different cells to remove hydrogen peroxide from the cell culture medium(Reference Rosignoli, Fuccelli and Sepporta98). However, different scientific communities disagreed with this hypothesis and they argued that sodium bicarbonate which is commonly present in cell culture media is responsible for pro-oxidant behaviour of HTyr at higher concentrations(Reference Odiatou, Skaltsounis and Constantinou99). Therefore, dosing of HTyr should be determined by considering the fact in mind that HTyr may act as either antioxidant or pro-oxidant depending on the concentration.
Not only HTyr but also metabolites generated by HTyr are also shown to act as antioxidants to protect intestinal cells (Caco-2 monolayers) from the oxidising action of oxidised cholesterol in in vitro culture conditions(Reference Atzeri, Lucas and Incani100). Especially, glucuronide and sulphate metabolites of Tyr and HTyr are capable to protect intestinal cells against pathological overproduction of nitric oxides(Reference Serreli, Melis and Corona101). The anti-cancerous effect of hydroxytyrosol acetate (HTyr-Ac) in human CRC cells (Caco-2/TC7) further demonstrated by another group of scientists. HTyr-Ac impeded the cell cycle by increasing p21 and CCNG2 (encodes Cyclin-G2) and down-regulating the CCNB1 (encodes Cyclin B1) gene expression. HTyr-Ac action is not only limited to cell cycle blockade in CRC cells as it can modify transcription of programmed cell death associated genes (BNIP3, BNIP3L, PDCD4 and ATF3) and can activate caspase-3. Carcinogen detoxification could be enhanced upon HTyr-Ac exposure, as it enhances UGT1A10 and CYP1A1, known xenobiotic-metabolising enzymes(Reference Mateos, Pereira-Caro and Bacon102). Thus, the secondary metabolites of HTyr especially HTyr-Ac should be characterised in humans to rule out any possibility of their negative effect on cell cycle or cell death in other parts of the body except the tumour site.
Apart from olive oil, olive mill wastewater could be a cheap source of HTyr as the purified olive mill wastewater shown to have chemopreventive properties in both human (HCT116 and HT-29) and murine (CT-26) CRC cells. In animal model, olive mill wastewater shown to suppress IL-8 and vascular endothelial growth factor expression and reduce tumour growth(Reference Bassani, Rossi and De Stefano53). Key findings from different studies using HTyr as intervention are summarised in Table 2.
Table 2. Summary of studies employed hydroxytyrosol as intervention in CRC

Oleuropein
Oleuropein, another important phenolic compound present in high concentration in olive oil and leaves(Reference Caponio, Alloggio and Gomes103) has gained scientific attention recently due to the accounted health benefits(Reference Sun, Frost and Liu104). Oleuropein can reduce CRC cell proliferation as well as invasion as shown in LoVo, a human colon cancer cell line(Reference Hamdi and Castellon105). Metabolic inhibition in cancer cell with oleuropein also documented in human colon cancer cells (HCT116). Inhibition of glycolysis and reduced cell viability was seen under the influence of oleuropein in tumour cells(Reference Ruzzolini, Peppicelli and Bianchini106). It could be an alternative approach to target cancer cells specifically via glycolysis inhibition as cancer cells are known for their high glycolytic activity. Studies in animal model also indicated the efficacy of oleuropein against colorectal carcinogenesis as it protected C57BL/6 mice from azoxymethane (AOM)/dextran sodium sulphate/) induced colitis through down-regulation of signalling pathways including Wnt/β-catenin, P3IK/Akt, NF-κB and STAT3. Oleuropein reduced the pro-inflammatory mediators such as IL-6, TNF-α, IFN-γ and IL-17A in mice group treated with AOM/dextran sodium sulphate by influencing the signalling cascades(Reference Giner, Recio and Ríos107). Oleuropein treatment also decreased the level of COX-2, Bax and PCNA (proliferating cell nuclear antigen protein) expression. Therefore, it could be a possibility that a diet containing oleuropein might prevent the chronification of intestinal inflammation and might be useful in colitis patients. In another in vivo study, oleuropein supplementation (125 mg/kg) reduced the formation of preneoplastic lesions in different segments of colon in AOM-treated A/J mice(Reference Sepporta, Fuccelli and Rosignoli108). AOM is known for inducing inflammation-driven CRC. Oleuropein action was specific to the tumour cells as it reduced AOM-driven tumour incidence from 57 % to 14 % in the medial segment of the colon and at the same time shown to protect peripheral leukocytes from AOM-induced DNA damage in the A/J mice(Reference Sepporta, Fuccelli and Rosignoli108). Pro-apoptotic effect of oleuropein in colon cancer cells also explored as oleuropein limits CRC cells’ growth by stimulating p53-dependent apoptosis(Reference Cárdeno, Sánchez-Hidalgo and Rosillo89). Hence, oleuropein could be effective against CRC by virtue of its anti-inflammatory properties and through regulating cellular signalling pathways. Significant findings of various studies with oleuropein are highlighted in Table 3.
Table 3. Summary of studies involving oleuropein as intervention in CRC

Oleocanthal
OC is a phenolic secoiridoid present in abundance in olive oil(Reference Cicerale, Conlan and Sinclair109). Mounting scientific evidences suggest that OC can be effective in different cancers like lung or breast cancer(Reference Elnagar, Sylvester and El Sayed110,Reference Siddique, Kilgore and Tajmim111) . In various type of cancer, inflammation plays crucial role in cancer development and progression. Therefore, tumour-associated inflammation has been a target for cancer therapy for decades. In this context, OC could play an important role in strategies to combat CRC development and progression as OC documented to have ibuprofen-like anti-inflammatory actions(Reference Beauchamp, Keast and Morel112). In in vitro study, OC shown to be more effective than ibuprofen (a non-steroidal anti-inflammatory drug) as an anti-inflammatory agent to inhibit COX-1 and COX-2, most common targets for anti-inflammatory drugs(Reference Parkinson and Cicerale113). In an interesting study conducted by Cusimano et al.(Reference Cusimano, Balasus and Azzolina114) OC was shown to be more effective than commonly used COX inhibitors such as nimesulide, indomethacin to reduce inflammation via COX suppression. Same study also reported that OC is capable of inducing apoptosis by inducing PARP cleavage as well by activating of caspases 3/7. However, anti-cancerous activities of OC in CRC cells might be independent of COX inhibition as OC is able to inhibit the cancer cell growth of both COX-2 positive (HT-29) and COX-2 negative (SW480) colonic adenocarcinoma cells with equal efficiency(Reference Cusimano, Balasus and Azzolina114).
A few studies also shed light on the anti-cancerous activities of OC in CRC through a wide variety of mechanisms. Exposure to a lower concentration of OC (2–5 µg/ml) induced apoptosis in HT-29 colon cancer cells by reducing anti-apoptotic protein Bcl-2. Cleavage of the poly-adenosine diphosphate-ribose polymerase (PARP) as well as caspase-3 related to the apoptosis cell death pathway observed under the influence of OC in HT-29 cells which consequently led to DNA fragmentation. The same study also shed light on the ability of OC to induce apoptosis and reduce cell viability through a different mechanism by means of suppressing COX-2 expression and activation of AMPK (adenosine monophosphate-activated protein kinase)(Reference Khanal, Oh and Yun115). On the flip side, higher concentration of OC (50 µM) induced apoptosis in CRC cells in a completely different mechanism by increasing intracellular reactive oxygen species level. Increased reactive oxygen species caused DNA damage and impairment of mitochondrial membrane integrity but fascinatingly normal cells remained unharmed after long-term exposure with even higher dosage of OC (100 µM)(Reference Cusimano, Balasus and Azzolina114). The crucial findings from the studies employed with OC are summarised in Table 4.
Table 4. Summary of oleocanthal mediated anti-CRC activities

Apigenin and luteolin
Apigenin and luteolin, two most important phenolic compounds belong to flavonoids group, have shown therapeutic potential in different cancers like melanoma and cervical cancer(Reference Tuorkey116). Apart from olive oil, these two compounds present at varying concentration in different other sources (pepper, carrot, celery, thyme, rosemary, oregano, etc.)(Reference Wang, Chen and Zhu117). Apigenin has been shown to reduce proliferation, migration and invasion of different CRC cells in a dose-dependent manner through down-regulation Wnt/β-catenin pathway. In particular, apigenin inhibited β-catenin activation and its nuclear entry, thereby downstream Wnt gene expression(Reference Xu, Wang and Song118). Wnt/β-catenin is particularly important in intestinal stem cell renewal during homoeostasis as well as played a significant role in intestinal diseases like CRC. Inhibitory action of apigenin on Wnt/β-catenin signalling further confirmed in CRC organoid model as in presence of apigenin intestinal organoid growth was significantly suppressed(Reference Xu, Wang and Song118). Apigenin possesses anti-metastatic properties as well, shown in BALB/c-nu mice where apigenin protected from metastasis in liver and lung(Reference Chunhua, Donglan and Xiuqiong119). Moreover, apigenin can accomplish pro-apoptotic role in CRC cells by boosting FADD (Fas-associated protein with death domain) expression and phosphorylation of FADD(Reference Wang, Yao and Wen120). It could synergistically augment the chemotherapeutic action of 5-Fluorouracil (5-FU), in a liposome formulation containing both apigenin and 5-FU. The combination therapy showed better efficacy than the drug alone in tumour xenograft model in nude mice(Reference Sen, Banerjee and Mandal121). It is also shown to regulate a range of cellular functions to combat CRC like NF-κB/Snail pathway(Reference Tong, Shen and Zhang122), PI3K/Akt/mTOR pathway, autophagy(Reference Chen, Xu and Yu123), STAT3 signalling(Reference Maeda, Takahashi and Nakai124), glycolysis(Reference Shan, Shi and Yang125) and gut microbiome(Reference Bian, Wan and Liao126). Apart from several health benefits of apigenin, at high concentration it may also act as a sedative(Reference Gazola, Costa and Castellanos127). So, there is a long road ahead before integrating apigenin in treatment protocol for CRC patients.
Another flavonoid, luteolin can inhibit colorectal carcinogenesis by activating Nrf2/ARE pathway through epigenetic modifications(Reference Zuo, Wu and Xiao128). It suppresses the expression of DNA methyltransferases whereas activated the expression of DNA demethylases to increase the Nrf2 expression. Nrf2 may then interact with p53 to direct CRC cell death via apoptosis(Reference Kang, Piao and Hyun129). Anti-tumour activities of luteolin may also depend on ERK1/2 as it ameliorated epithelial-to-mesenchymal transition in metastatic colon cancer cells, SW620 through activation of ERK1/2 and FOXO3a(Reference Potočnjak, Šimić and Gobin130). Luteolin can interfere in the cell cycle as well and can block cell cycle at the G2/M phase and induce apoptosis subsequently(Reference Chen, Zhang and Gao131). Furthermore, it is also shown to suppress CRC metastasis by regulating micro-RNA (miR-384) or CREB1 expression(Reference Yao, Rao and Zheng132,Reference Liu, Lang and Jin133) and also potent to reduce colon carcinogenesis by suppressing the matrix metalloproteinases in animal model(Reference Pandurangan, Dharmalingam and Sadagopan134). Significant studies with apigenin or luteolin in CRC are featured in Table 5.
Table 5. Summary of studies utilised apigenin and luteolin as intervention

Pharmacokinetics and toxicity profile of olive phenols
EVOO has several health benefits due to the presence of phenolic compounds. In this section, we have included a brief overview on the pharmacokinetics of the principal phenolics present in olive oil. Phenolic compounds are absorbed in a dose-dependent manner in the gut and go through intestinal/hepatic first-pass metabolism(Reference Visioli, Galli and Bornet135). Olive oil phenols are readily absorbed in the small intestine and colon by passive transport, though it depends on the vehicle employed(Reference Vissers, Zock and Roodenburg136). In that case, EVOO is considered as the best matrix for HTyr for its oily nature(Reference Alemán-Jiménez, Domínguez-Perles and Medina137). HTyr reaches maximum plasma concentration quickly (˜7 min) after intake. HTyr and its derivatives are well distributed in different tissues like muscle, liver, testis, brain and kidney(Reference Robles-Almazan, Pulido-Moran and Moreno-Fernandez138) and converted into both oxidised and methylated derivatives (like O-methylated derivative of HTyr, glucuronides of HTyr) revealed by HPLC analysis(Reference Mateos, Goya and Bravo139). Metabolites from HTyr and its derivatives are primarily excreted by the kidneys with a complete elimination time of approximately 6 h(Reference Rodríguez-Morató, Boronat and Kotronoulas140). However, one problem of hydroxytyrosol is its poor bioavailability as Covas et al.(Reference Covas, de la Torre and Farré-Albaladejo141) demonstrated that the maximum level of HTyr achieved in plasma was ˜15 µM when given diet of 40 ml of olive oil to healthy human volunteer (366 mg/kg). The reason behind this almost undetectable level (0·1–1 %) of free form of HTyr in body fluids is probably due to extensive first pass metabolism in both gut and liver(Reference de la Torre142,Reference Pastor, Rodríguez-Morató and Olesti143) . Hence, critical measurement of free HTyr in plasma possibly by novel methodologies would help to understand its dose-effect better.
On the other hand, the metabolism of oleuropein goes through the rapid degradation by colonic microflora to form HTyr, which significantly increases the amount of free HTyr. So, it should come into consideration while consuming crude extract containing both oleuropein and hydroxytyrosol, could increase the free HTyr level in plasma. Sulphated and glucuronidated metabolites of HTyr are the primary metabolites of oleuropein in plasma and urine in humans(Reference de Bock, Thorstensen and Derraik144).
In the case of OC, it is believed that passive diffusion of OC in small intestine is possible(Reference Lozano-Castellón, López-Yerena and Rinaldi de Alvarenga145) and it is rapidly hydrolysed through the gastrointestinal tract(Reference Mehmood, Usman and Patil146). OC is mainly metabolised by phase I reactions (hydration, hydrogenation and hydroxylation) and mainly happens in the liver and small intestine. The hydrogenated and hydrated metabolites of OC are further glucuronidated through phase II reactions(Reference López-Yerena, Vallverdú-Queralt and Mols147). However, oral bioavailability of OC is compromised due to the high intestinal metabolism. Despite the current surge of research with OC due to its anti-inflammatory properties, its absorption, distribution, metabolism and excretion properties are not well characterised. Therefore, extensive in vivo analysis with OC is crucial to develop it as a therapeutic intervention.
Comparatively, flavones (apigenin and luteolin) are less absorbed with < 1 µmol/l plasma concentration in human compared with other polyphenols(Reference Hostetler, Ralston and Schwartz148). Apigenin is also well distributed into the tissues after administration in rat or mice(Reference Wang, Firrman and Liu149). After absorption, apigenin remains in blood circulation or tissues in the form of glucuronide, sulphate conjugates or luteolin as these are the major metabolites of apigenin(Reference Tang, Chen and Huang150). Apigenin has a slow elimination rate and possibly accumulates in the body(Reference Gradolatto, Basly and Berges151). Despite the numerous favourable effects of apigenin, in vivo studies involving animal model as well as human studies are considerably less in number which is probably because of apigenin’s low water solubility (1·35 μg/ml) and high permeability(Reference Salehi, Venditti and Sharifi-Rad152). Therefore, different methodologies such as liposome, nanosuspension and micelle have been explored by different groups to improve the solubility and bioavailability of apigenin(Reference Ding, Chen and Wang153,Reference Zhai, Guo and Liu154) . On the other hand, glucuronidation and methylation are major metabolic pathways of luteolin in humans which are mediated by UDP-glucuronosyltransferases and catechol-O-methyltransferases, respectively (Reference Wang, Chen and Zhu117). Monoglucuronide form of luteolin is predominant in human serum(Reference Shimoi, Okada and Furugori155). Apigenin and luteolin are mainly excreted in bile, urine or faeces(Reference Tang, Chen and Huang150,Reference Simons, Renouf and Murphy156) .
Toxicological studies along with the in-vitro genotoxicity studies revealed HTyr as a non-mutagenic, non-genotoxic compound and advocate for its long-term consumption(Reference Bertelli, Kiani and Paolacci157). Even at very high dose (500 mg/kg/d), HTyr exerts no adverse effects in rats(Reference Auñon-Calles, Canut and Visioli158). Since 2011, European Food Safety Authority authorised health claim on olive oil containing at least 250 mg/kg of hydroxytyrosol and its derivatives(Reference López-Huertas, Lozano-Sánchez and Segura-Carretero159). Ames test results ascertain that neither apigenin nor luteolin is mutagenic or toxic(Reference Czeczot, Tudek and Kusztelak160). Overall, olive oil phenolics are considered safe(Reference Romani, Ieri and Urciuoli161,Reference Ashrafizadeh, Bakhoda and Bahmanpour162) although recently Kouka et al.(Reference Kouka, Tekos and Papoutsaki163) revealed that protective action of olive oil may be tissue specific and it can act as both antioxidant (in brain or muscle tissues) and pro-oxidants in tissues such as spleen or pancreas as shown in male Wistar rats(Reference Kouka, Tekos and Papoutsaki163). Therefore, effect of olive oil on different human organs should be exploited critically before developing the dosing protocol.
The complete metabolic profile of OOP is yet to be elucidated. To develop OOP as clinical intervention, biological relevance of phenolic metabolites should be characterised. Further efforts are needed to increase the bioavailability of HTyr or apigenin possibly by changing the solubility. Novel formulation strategies are crucial in this sense for better absorption of phenolic compounds, especially for flavonoids.
Discussion
Olive oil is full of beneficial components which may turn useful for the prevention and possible therapeutic intervention in CRC. Mounting evidence advocates the chemotherapeutic potentiality of olive oil phenolic compounds, particularly in CRC. The phenolic components of olive oil can act on different stages of carcinogenesis process, such as oxidative stress, inflammation, cell cycle, immune regulation, apoptosis as well as an epigenetic alteration. Waste products produced during olive oil extraction may also be used as a cheap alternative of olive oil to develop food supplement to combat CRC. Altering the gut microbiome could hold the key to amend several intestinal disorders including CRC. On that background, a few studies have already provided evidences to link imbalance of the intestinal microbiota and occurrence of CRC. On the other hand, EVOO is capable of altering the gut microbial population by stimulating the growth of beneficial bacteria, for example, lactic acid bacteria(Reference Luisi, Lucarini and Biffi164) and at the same time reducing the abundance of pathogenic bacteria (e.g. Enterococcus, Staphylococcus)(Reference Rodríguez-García, Sánchez-Quesada and Algarra75). EVOO also possesses anti-inflammatory effects in the gut by producing SCFA(Reference Millman, Okamoto and Teruya165). Because of the significant role played by gut microbiome for maintaining cellular integrity and protecting against pathogenic organisms, any changes in the gut microbial community can exert adverse effects. For example, during the intestinal dysbiosis, disruption of the homoeostasis between the host and the intestinal microbiota occurs(Reference Zhang, Li and Gan166,Reference Iebba, Totino and Gagliardi167) , which turns out to be one of the major causes of inflammatory bowel disease(Reference Schippa and Conte168,Reference Nagao-Kitamoto, Kitamoto and Kuffa169) and eventual progression to CRC(Reference Wu, Yang and Zhang170,Reference Sheflin, Whitney and Weir171) . Therefore, maintenance or restoration of homoeostasis of intestinal microbiota could be a substantial treatment or prevention strategy against the CRC. In this context, olive oil and its phenolic compounds could be useful to restore/modify gut microbiome for good and prevent carcinogenesis.
Conclusion and future direction
Most of the potential benefits olive oil discussed in the current review have emerged mainly from in vitro studies and animal studies. Therefore, additional efforts are need of the hour to mechanistically characterise biological activities of EVOO or individual phenolic components in human. Pharmacokinetics and pharmacodynamics must be studied extensively to develop the effective dose of these compounds. The relation between the structure and activity of these olive oil phenolics should be deciphered to engineer new drugs based on the molecular scaffold of these olive oil components. Further, clinical trial with hydroxytyrosol or oleuropein or the combination of different components from olive oil must be started immediately to develop a chemopreventive strategy or therapeutic intervention. This current review critically assessed the potential of olive oil phenolic constitutes as a preventive or possible therapeutic agent in CRC by studying the molecular mechanism of the each of the olive oil phenolic compounds and the olive oil phenolic extract as a whole (summarised in Fig. 4). As the exploration to find the novel and cheap therapeutic strategy against CRC lingers, interventions by means of various olive oil-derived phenolic compounds may ultimately turn out to be a precise management system to control or prevent CRC.

Fig. 4. Summary of the key anti-colorectal cancer activities of olive oil and its phenolic compounds. The major activities of each phenolic compound are shown here. For each activity, molecular pathways/signalling molecules targeted by olive oil phenolic compounds demonstrated here by indicating upward arrowhead (↑ = up-regulating), downward arrowhead (↓ = down-regulating) and cross sign (× = blocking).
Acknowledgements
The authors greatly acknowledge the help of authority of MAKAUT, WB for providing the necessary support.
This study was supported by DST Inspire faculty research grant (DST/INSPIRE/04/2017/000675; India) and MAKAUT, WB research seed grant to D. N. A. S. was supported by Junior research fellowship from Council of Scientific and Industrial research (Award no: 09/1213(0002)/2019-EMR-I; India). S. S. was supported by the scholarship from Department of Biotechnology, Govt. of India.
All authors participated in data curation, developing the methodology and writing the original draft of the paper. A. S. and D. N. were responsible for conceptualisation, formal analysis, investigation and reviewing and editing subsequent versions of the paper. A. S. was solely responsible for visualisation, while D. N. was solely responsible for funding acquisition, project administration, handling of resources, and supervision and validation of the work
There are no conflicts of interest.