Increased adiposity is an important factor in the development of insulin resistance. An increased fat mass with a reduced visceral and lean mass has been shown in the smaller, possibly nutritionally compromised monozygotic twin(Reference Loos, Beunen and Fagard1, Reference Loos, Beunen and Fagard2) and also in babies from underdeveloped nations(Reference Yajnik, Fall and Coyaji3). In offspring from animal models of intra-uterine growth retardation (IUGR) such as the low protein model of maternal undernutrition there is an increased risk for the development of increased adiposity(Reference Guan, Arany and van Beek4) in adult life. The metabolic pathways leading to this altered body composition could be important contributors to the early onset of metabolic disease that is linked with low birth weight, poor early growth and nutrition(Reference Hales, Barker and Clark5–Reference Hales, Desai and Ozanne7).
A possible candidate contributing to an altered body composition in those exposed to poor early nutrition may be the insulin-like growth factor (IGF)-I system because IGF-I regulates fetal and postnatal growth(Reference Baker, Liu and Robertson8, Reference Underwood, Thissen and Lemozy9). There is evidence to suggest that factors in this system are altered with IUGR. In humans, IGF-I is reduced in IUGR children in comparison with children of normal height and IUGR twins have lower IGF-I but higher IGF binding protein (IGFBP)-1 levels in comparison with appropriate-for-gestational-age co-twins(Reference Cutfield, Hofman and Vickers10, Reference Bajoria, Sooranna and Ward11). Low protein offspring have reduced plasma and liver IGF-I levels and lower hepatic IGF-I expression but do not show any changes in levels of IGFBP(Reference Muaku, Beauloye and Thissen12, Reference El-Khattabi, Gregoire and Remacle13). A similar reduction in plasma IGF-I levels in addition to an increase in plasma IGFBP-1 levels is seen in pups from energy-deprived dams at day 22 of gestation and at postnatal day 9 but the changes in IGF-1 are not present at later time points(Reference Woodall, Breier and Johnston14).
It is postulated that changes that may occur as a result of IUGR give a ‘predictive adaptive response’ where if nutrition remains poor the individual would be at a survival advantage but risk of disease increases once the predictive adaptive response is exceeded(Reference Armitage, Khan and Taylor15). In short-term studies low protein offspring maintained on a low-protein diet may have a reduced risk for glucose intolerance or a prolonged time of onset in comparison with low protein offspring maintained on a normal-protein diet(Reference Sugden and Holness16). Therefore because IGF-I is nutritionally regulated both pre- and postnatally(Reference Baker, Liu and Robertson8, Reference Underwood, Thissen and Lemozy9) the aim of the present study was to observe any changes in factors of the IGF-I system in low protein offspring that may be modulated by a change in diet from low protein to a normal-protein diet at weaning and be involved in a change in body composition, potentially increasing the risk of metabolic disease.
Experimental methods
Diets
The experimental diets used in the present study include a control (20 % protein) and a low protein (8 % protein) diet. The compositions of these diets are detailed elsewhere(Reference Maloney, Gosby and Phuyal17). The control and low-protein diets were made on-site, in a powdered form. Offspring were allowed up to 40 g (650 kJ)/d. Mated females were given 40 g/d during gestation and week 1 of lactation, and 60 and 80 g/d on weeks 2 and 3 of lactation respectively. These amounts allowed dams to feed ad libitum (observation). The increase during lactation was to account for increased scatter and food intake by pups.
Experimental animals
Approval for the present study was obtained from the University of Sydney Animal Ethics Committee. All animals were housed in the Biochemistry Animal House, University of Sydney at 22°C on a standard 12 h light–12 h dark cycle. Time-mated female Wistar rats (Animal Resources Centre, Perth, WA, Australia) were randomly assigned to a 20 % protein (CON; n 6) or 8 % protein (LP; n 9) diet. Dams remained on the assigned diet throughout gestation and lactation. At birth, litter sizes were culled to fifteen or less pups/dam where necessary by reducing female numbers. There was no effect of maternal diet on litter size before (CON, 13·8 (sem 1·0) (range 10–17); LP, 13·8 (sem 0·4) (range 12–16); P = 0·95) or after (CON, 13·3 (sem 0·8) (range 10–15); LP, 13·7 (sem 0·3) (range 12–15), P = 0·66) culling. At weaning, male offspring from each maternal group were randomly assigned to the 20 % protein (con) or 8 % protein (lp) diet to give four study groups (CON-con, CON-lp, LP-con and LP-lp). Body weights (g) of all offspring were measured weekly from birth.
Male pups were killed by lethal injection of Nembutal (sodium pentobarbitone; 100 mg/kg body weight) at 10 d (CON n 8; LP n 9), 21 d (weaning) (CON n 8; LP n 9) and 12 weeks (CON-con n 8; CON-lp n 9; LP-con n 9 and LP-lp n 7) for tissue and blood collection. Tissues were collected in the morning, 1 h after the removal of food and dams. Livers were immediately frozen in liquid N2 and stored at − 80°C for later gene expression analysis. Blood samples were collected by cardiac puncture and serum stored at − 20°C until subsequent analysis.
Serum measurements
A rat insulin RIA kit (Linco Research, St Louis, MO, USA) was used to measure serum insulin concentration. The intra- and inter-assay CV for the insulin assay are 4·6 and 9·4 % respectively. Rat acid-labile subunit (ALS), IGF-I and IGFBP-1 were measured by specific RIA as reported previously(Reference Baxter and Dai18–Reference Lewitt, Saunders and Baxter20).
Assessment of gene expression
The expressions of liver IGF-I, ALS and IGFBP-1 were determined using real-time PCR. RNA was extracted from frozen liver and cDNA generated using described methods(Reference Bryson, Phuyal and Proctor21). cDNA was stored at − 20°C until use. Primer sequences were designed using Primer Express software (Applied Biosystems, Foster City, CA, USA). The primers used for each gene were as follows: IGF-I, caggctatggctccagcatt (forward) and acatctccagcctcctcagatc (reverse); ALS, ggcctcgacctaagagacgtt (forward) and ggcca-gtaagtagccagtgtca (reverse); IGFBP-1, agctgccgctcaacagaaa (forward) and tgttgcagtttg-gcagataaaatt (reverse); 18S rRNA, gacggaccagagcgaaagc (forward) and aacctccgactttcgttcttgatt (reverse). Reactions took place in the ABI Prism 7000 Sequence Detection System (Applied Biosystems) using the SYBR Green two-step PCR protocol (Applied Biosystems). Each reaction mixture contained 25 ng cDNA, SYBR Green Universal Master Mix, forward and reverse primers (final primer concentration 300 nm) and sterile water. Tubes were incubated at 50°C for 2 min, 95°C for 10 min and then cycled at 95°C for 15 s and 60°C for 1 min for forty cycles. The amount of cDNA was calculated relative to the amount of endogenous control (18S rRNA) initially present.
Analysis of body composition
Dual-energy X-ray absorptiometry measures body composition by quantifying the differences in the X-ray attenuation characteristics of bone and soft tissue. Bone, lean and fat mass were determined using a narrow angle ( < 5°) fan-beam dual-energy X-ray bone densitometer (Lunar Prodigy; GE Medical Systems, Madison, WI, USA). This machine utilises an X-ray beam with two distinct energy peaks (38 and 70 keV). The data were analysed using ‘Small Animal’, version 6.10 on scans performed at a resolution of approximately 0·6 × 1·05 mm. Rats were sedated (15 mg pentobarbitone sodium/kg and 25 mg ketamine/kg) and placed in a prone position on the scan table of the densitometer. Limbs were stretched out and placed flat for easier delineation of body regions. Scans, each lasting approximately 1 min, were performed in duplicate. The CV for bone, fat and lean mass were 0·7, 4·0 and 0·6 % respectively.
Statistical analyses
Results were analysed by two-way ANOVA testing for maternal and post-weaning diet effects. Fisher's protected least-significance differences post hoc test was used to test for differences between individual groups. Statistical significance was achieved when P < 0·05. All data are expressed as mean values with their standard errors.
Results
Body weight
At birth, pups from LP dams were significantly lighter than pups from CON dams (CON, 6·15 (sem 0·06); LP: 5·90 (sem 0·06) g; P < 0·05) and this difference persisted throughout lactation and into weaning (CON, 44·8 (sem 0·5); LP, 24·1 (sem 0·4) g; P < 0·05). Fig. 1 presents the growth curves of each study group until age 12 weeks. LP offspring maintained reduced body weights through to 12 weeks in comparison with CON offspring independent of post-weaning diet (P < 0·05). CON and LP offspring maintained on the lp diet from weaning demonstrated reduced body weights at 12 weeks when compared with their littermates allocated the con diet at weaning (P < 0·05).

Fig. 1 Body weight growth curves of offspring of dams fed a 20 % protein (control) diet (●, ○) or an 8 % protein (low-protein) diet (▲, △) maintained on a control (●, ▲) or low-protein (○, △) diet from weaning until age 12 weeks. ↓ , Onset of postnatal diets. Data are means. As the standard errors are smaller than the symbols they have been excluded from the figure.
Body composition
Table 1 presents body composition data at age 10 weeks. Bone mineral density was reduced in animals exposed to a low-protein diet in utero (P < 0·0001; ANOVA) and post-weaning (P < 0·0001; ANOVA); the effects were additive with a further reduction seen in LP-lp animals. Bone mass and density were reduced in LP-con offspring in comparison with CON-con offspring and low protein feeding from weaning led to significant reductions in bone mass and bone mineral density in CON and LP offspring. LP offspring had a reduced lean mass (P < 0·0001; ANOVA). Lean mass was also reduced in CON and LP offspring as a result of feeding of the lp diet from weaning (P < 0·0001; ANOVA). Unlike bone and lean mass, fat mass was not affected by maternal (P = 0·3; ANOVA) or post-weaning (P = 0·07; ANOVA) diet. There was a significant interaction between maternal and post-weaning diet (P = 0·04; ANOVA) where CON-lp animals had a reduced fat mass in comparison with CON-con, an effect of postnatal feeding not seen in LP animals. Once expressed as a percentage of body mass, bone and lean mass were not reduced (data not shown). There was a significant interaction between maternal and post-weaning diet (P = 0·04; ANOVA) where LP-lp animals had increased percentage fat in comparison with LP-con, an effect not seen in CON animals.
Table 1 Body composition‡
(Mean values with their standard errors)
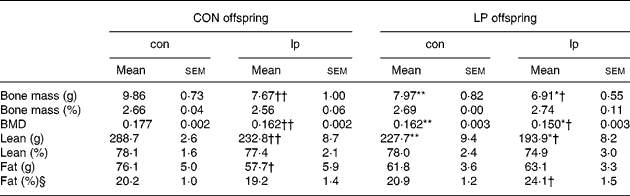
CON, maternal 20 % protein diet (control); LP, maternal 8 % protein diet (low protein); con, post-weaning 20 % protein diet (control); lp, post-weaning 8 % protein diet (low protein); BMD, bone mineral density.
Mean value was significantly different from that of the CON offspring (maternal diet effect): *P < 0·05, **P ≤ 0·0005.
Mean value was significantly different from that of the con-fed offspring (post-weaning diet effect): † P ≤ 0·02, †† P < 0·0005.
‡ Dual energy X-ray absorptiometry was used to measure bone surface area (cm2), bone mass (g), lean mass (g) and fat mass (g) in 10-week CON and LP offspring maintained on either a con or lp diet from weaning (21 d).
§ Fat (%) is fat mass expressed as a percentage of body weight.
Maternal diet influences on the liver insulin-like growth factor–insulin-like growth factor binding protein expression profile in 10 and 21 d offspring
Table 2 shows the IGF–IGFBP gene expression profile in 10 and 21 d LP and CON offspring. IGF-I expression was not different between the CON and LP offspring at 10 d but by 21 d a significant reduction was evident in LP offspring (P < 0·05). ALS gene expression was reduced in the livers of LP offspring at age 10 d (P < 0·005) and 21 d (P < 0·005) in comparison with CON offspring. IGFBP-1 gene expression was significantly elevated in LP offspring at 10 d (P < 0·001) but not at 21 d.
Table 2 Insulin-like growth factor (IGF)-I–IGF binding protein-1 (IGFBP-1) gene expression profile (arbitrary units) in the livers of 10 d and 21 d offspring
(Mean values with their standard errors)
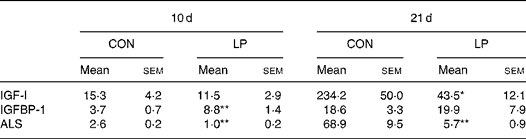
CON, maternal 20 % protein diet (control); LP, maternal 8 % protein diet (low protein); ALS, acid-labile subunit.
Mean value was significantly different from that of the CON offspring (maternal diet effect): *P < 0·05, **P ≤ 0·005.
Maternal diet influences on the serum insulin, insulin-like growth factor-I and acid-labile subunit levels in young offspring
Maternal influences on serum insulin, IGF-I and ALS levels at 10 and 21 d are shown in Fig. 2. Serum insulin levels have previously been shown to be reduced in 10 d and 26 d LP offspring(Reference Gosby, Maloney and Phuyal22) and in the present study were significantly reduced in LP offspring in comparison with CON offspring at 21 d (P < 0·005). Circulating IGF-I levels were significantly reduced in LP offspring at 10 d (P < 0·001) and 21 d (P < 0·001) in comparison with CON offspring. Between 10 and 21 d CON offspring experienced a significant increase in serum IGF-I concentrations (P < 0·001); this increase did not occur in LP offspring. Circulating ALS levels were also significantly reduced in LP offspring at 10 d (P < 0·0001) and 21 d (P < 0·0001) in comparison with CON offspring. Between 10 and 21 d there was a significant increase in ALS concentrations in CON (P < 0·05) but not in LP (P = 0·1) offspring.

Fig. 2 Maternal diet influences on serum insulin-like growth factor (IGF)-I profile at 10 d and 21 d. Serum insulin (a), IGF-I (b) and acid-labile subunit (ALS) (c) concentrations in 10 d and 21 d offspring of dams fed a 20 % protein (control) diet (■; CON) or an 8 % protein (low-protein) diet (□; LP). Data are means, with standard errors represented by vertical bars. Mean value was significantly different from that of the CON offspring (maternal diet effect): *P < 0·005, **P < 0·001, ***P < 0·0001. Mean value was significantly different from that of the 10 d old rats of the same maternal diet group (age effect): ‡ P < 0·05, ‡‡ P < 0·001.
Maternal and post-weaning diet influences on serum insulin and liver insulin-like growth factor–insulin-like growth factor binding protein profile in 12-week offspring
Maternal diet did not have significant effects on serum insulin, IGF-I or ALS concentrations at 12 weeks but did lead to increased IGFBP-1 levels in LP offspring (P = 0·02; ANOVA). This increase was only significant in those maintained on the lp diet (P = 0·03) and not in those maintained on the con diet (P = 0·3) from weaning.
Post-weaning diet did not affect serum IGF-1 (P = 0·5; ANOVA) or ALS (P = 0·1; ANOVA) levels but did have significant effects on serum insulin (P < 0·05; ANOVA) and IGFBP-1 (P < 0·001; ANOVA) levels (Figs. 3 (a)–(d)). Maintenance on the low-protein diet from weaning resulted in reduced insulin in CON offspring but not in LP offspring. This is possibly because LP offspring tended to maintain reduced insulin independent of postnatal diet, a maternal diet effect that was not significant. Post-weaning feeding of a low-protein diet resulted in an increase in IGFBP-1 levels in LP (P = 0·01) but not in CON offspring (P = 0·2).

Fig. 3 Maternal and post-weaning dietary influences on serum insulin-like growth factor (IGF)–IGF binding protein (IGFBP) profiles at 12 weeks. Serum insulin (a), IGF-I (b), IGFBP-1 (c) and acid-labile subunit (ALS) (d) concentrations in offspring of dams fed a 20 % protein (control) diet (■; CON) or an 8 % protein (low-protein) diet (□; LP) maintained on a control (con) or low-protein (lp) diet from weaning (21 d). Data are means, with standard errors represented by vertical bars. *Mean value was significantly different from that of the CON offspring (maternal diet effect) (P < 0·05). † Mean value was significantly different from that of the con-fed offspring of the same maternal diet group (post-weaning diet effect) (P < 0·05).
Discussion
The study aimed to observe the effects of continued feeding of a low-protein diet on the IGF system and body composition because manipulation of the postnatal diet can prolong or accelerate the time of onset of glucose intolerance in low protein offspring(Reference Gosby, Maloney and Phuyal22, Reference Wilson and Hughes23). Although there were no persistent changes in IGF-1 and ALS with age in the low protein model, the present study demonstrated that low protein animals fed a low-protein diet from weaning had increased IGFBP-1 levels and percentage fat. These changes were not seen in the LP-con or CON-lp animals, suggesting that postnatal nutrition is important in the long-term effects of maternal protein restriction on body composition and that IGFBP-1 may play an important role. This could have important implications in the prevention of metabolic disease through dietary treatment in IUGR individuals.
The early reductions in circulating IGF-I and ALS levels with corresponding changes in the hepatic expression of IGF-I, IGFBP-1 and ALS in young LP offspring are consistent with the reduced growth in this model and are in agreement with other studies(Reference Muaku, Beauloye and Thissen12). Because undernutrition (via insulin deficiency) leads only to post-translational reductions in ALS, the presence of reductions in both mRNA and protein may indicate delayed maturation of the IGF system in the young LP offspring; this may also contribute to the early reductions in IGF-I(Reference Boisclair, Rhoads and Ueki24). IGFBP-1 levels decline in response to a rise in insulin. Young male LP offspring have reduced insulin secretion; therefore, insulin may play an important role in the transient change in IGFBP-1 seen at an early age in the present study. Insulin has been shown to be important in the alterations of IGF and IGFBP secretion and hepatocyte proliferation seen in low protein offspring at an early age(Reference El-Khattabi, Gregoire and Remacle13).
The early changes in the IGF–IGFBP profile as a result of maternal protein restriction were associated with persistent reductions in body weight. However, if LP offspring were maintained on the control diet (20 % protein) from weaning, relative body composition and circulating levels of IGF-I, IGFBP-1 and ALS were similar to CON offspring by the age of 3 months; this may be representative of the maturation of the IGF system in LP-con animals. Maintenance of CON and LP offspring on the low-protein diet from weaning did not have effects on IGF-I and ALS but did lead to reductions in bone mass and lean mass. The reduced lean and bone mass as a result of a postnatal low-protein diet may be partly attributed to reduced insulin found in CON-lp animals in comparison with CON-con animals. The reduced insulin in the CON-lp animals may be due to inadequate amino acid supply in the diet, leading to reduced levels of insulin, protein synthesis for lean mass and reduced stimulation of bone growth. In contrast, in comparison with all groups at the age of 3 months LP-lp animals had increased IGFBP-1 levels and increased percentage fat, the latter possibly resulting from a reduced lean mass accumulation. These changes are indicative of an interaction between maternal and postnatal diet. A proposed function of IGFBP-1 is to regulate the bioavailability of IGF-I(Reference Underwood, Thissen and Lemozy9). High IGFBP-1 levels could contribute to a reduction in lean mass accretion(Reference Lang, Vary and Frost25) and reduced body weight, possibly contributing to the increased percentage fat and changed body composition seen in the present study.
IGFBP-1 is regulated by many factors including nutrition, insulin and anthropometry(Reference Wolk, Larsson and Vessby26, Reference Hong, Brismar and Hall27). The absence of increased IGFBP-1 levels in the CON-lp animals suggests that acute nutrition is not a primary cause of the increase in IGFBP-1 found in the LP-lp animals. In the present study basal insulin levels tended to remain lower in all LP animals compared with CON-con animals at 3 months and this may be representative of impaired pancreatic function/insulin secretion in the low protein model(Reference Wilson and Hughes23) where young male LP offspring maintained on a normal-protein diet have reduced insulin secretion(Reference Gosby, Maloney and Phuyal22) but an increased glucose tolerance(Reference Shepherd, Crowther and Desai28) and improved insulin sensitivity (AK Gosby, CA Maloney and ID Caterson, unpublished results). Although there was not a significant reduction in basal insulin levels in LP-lp animals in comparison with LP-con or CON-lp animals, insulin secretion in response to a glucose load is influenced by postnatal diet in low protein offspring. Young male LP offspring fed a low-protein diet from weaning show a reduced insulin response but maintain glucose tolerance in comparison with CON-lp animals(Reference Gosby29). This reduced insulin secretion in comparison with CON animals is also seen in response to a high-fat, energy-dense diet (AK Gosby, CA Maloney and ID Caterson, unpublished results). Therefore, the increased basal IGFBP-1 levels seen in the present study could result from reduced insulin excursions.
High IGFBP-1 levels may be protective against vascular disease and low IGFBP-1 levels are associated with the development of glucose intolerance(Reference Heald, Siddals and Fraser30–Reference Sandhu, Heald and Gibson32). Moreover, reduced levels of IGFBP-1 have been shown in elderly individuals who were of low birth weight and may be associated with the effects of fetal programming on insulin resistance(Reference Kajantie, Dunkel and Rutanen33). Therefore the high IGFBP-1 levels seen in the present study in the LP-lp animals may be either protective against or be an indicator that these animals are at a lower risk for the development of metabolic disease. This is consistent with other studies that show LP animals maintained on an 8 % protein diet from weaning have an improved glucose tolerance in comparison with LP animals maintained on a 20 % protein diet from weaning(Reference Sugden and Holness16). This does not confirm that continued low protein feeding would prevent metabolic disease. All offspring in the present study were allowed up to 650 kJ/d. Because dietary protein may be associated with energy intake(Reference Sorensen, Mayntz and Raubenheimer34) and satiety(Reference Veldhorst, Smeets and Soenen35), an ad libitum feeding protocol could be associated with an increased food intake. In turn, these factors could lead to weight gain, possibly in the form of fat in preference to lean and bone mass and this could contribute to an earlier development of metabolic disease.
The results of the present study suggest that early disruptions in the IGF–IGFBP profile are not permanent but may be sensitive to postnatal nutrition with effects on body composition. The IGF–IGFBP system may be protective of glucose intolerance and CVD; therefore, manipulation of this system with postnatal diet is promising for the prevention of these diseases.
Acknowledgements
I. D. C., J. M. B., G. S. D., L. M. L. S., A. K. G. and C. A. M. designed the research. L. M. L. S., A. K. G. and C. A. M. performed the research. M. T., J. B. and A. K. G. carried out and analysed dual-energy X-ray absorptiometry scans. A. K. G. and L. M. L. S. analysed the data and wrote the paper. R. C. B. provided expertise and facilities for the IGF and IGFBP assays. I. D. C., C. A. M. and R. C. B. edited the manuscript.
The authors declare no conflict of interest.
A. K G. and L. M. L. S. contributed equally to the study.
This research was supported by a project grant from the National Health and Medical Research Council of Australia. We thank Sri Devi Meka and Kevin Hardman at the Kolling Institute for their assistance with the IGF and IGFBP assays. We thank Penfords Australia PL (formerly Starch Australasia) for their kind donation of maize starch and dextrose monohydrate.







