Healthy ageing is defined by the WHO as ‘the process of developing and maintaining the functional ability that enables well-being in older age’(1). Intrinsic and functional capacities tend to decrease with ageing, but it is recognised that lifestyle choices and interventions can contribute to the trajectory of each individual(Reference Hung, Ross and Boockvar2). For some of them, ageing might be accompanied by a period of chronic illnesses, frailty and disability(Reference Crimmins and Beltrán-Sánchez3,Reference Seeman, Merkin and Crimmins4) .
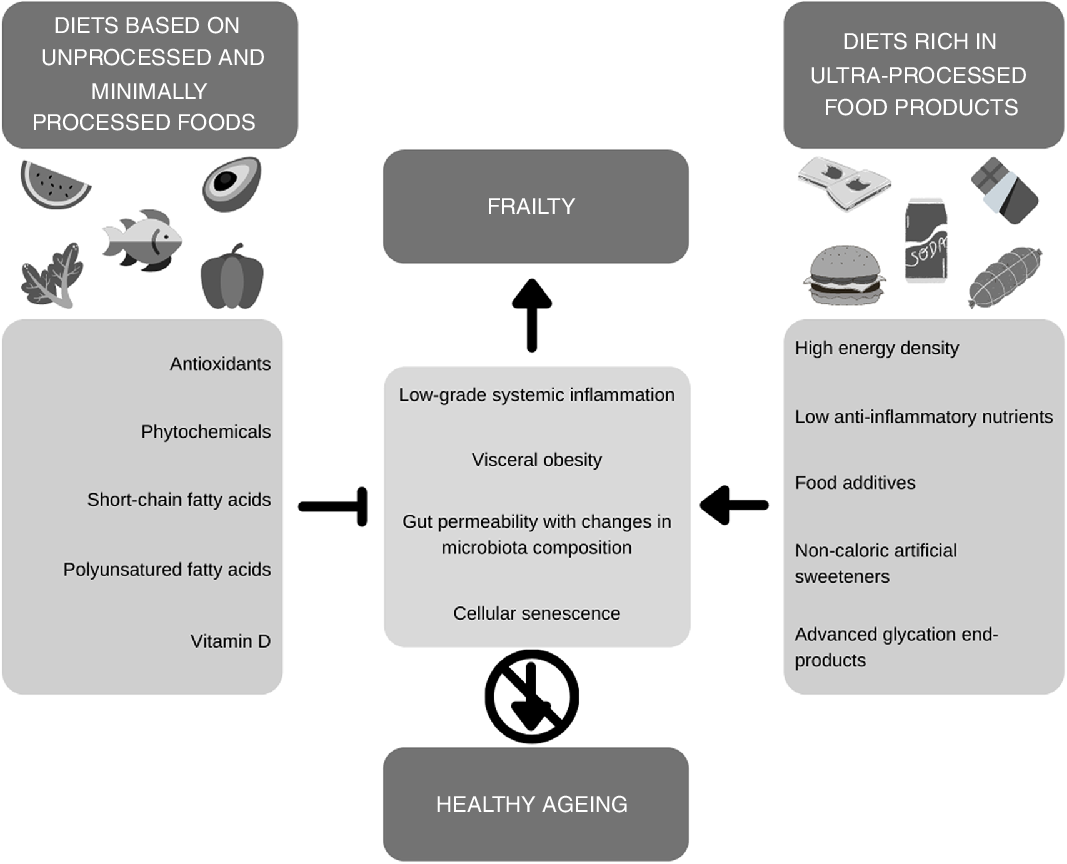
Frailty is a multifactorial ageing-related syndrome characterised by a decline in functional reserve and resistance to stressors, resulting in negative health outcomes(Reference Morley, Vellas and van Kan5). Although there has been no consensus on a definition nor a standard measurement for frailty, this syndrome is widely recognised to have multiple causes, which include genetic, biological, physical, psychological, social and environmental factors(Reference Clegg, Young and Iliffe6). There is a growing body of evidence on the development of frailty in older adults and its association with low-grade systemic inflammation and how different dietary habits influence this association.
An important issue regarding dietary habits is the increased intake of ultra-processed products (UPP) worldwide. UPP are industrial formulations made with highly processed ingredients, previously submitted to processes such as hydrolysis, hydrogenation, interesterification or any other chemical modifications, which are then combined with food additives like colourings, flavourings and emulsifiers. This results in hyper-palatable and convenient products with long shelf life and low cost, but also low nutritional value that frequently replace unprocessed and minimally processed foods in traditional diets(Reference Monteiro, Levy and Claro7,Reference Monteiro, Cannon and Levy8) . Due to these characteristics, long-term consumption of UPP can negatively impact health by promoting changes in the gut microbiota, weight gain, as well as the activation of the inflammatory pathways(Reference Christ, Lauterbach and Latz9). As such, in this article, we intend to (i) discuss the role that UPP might have on the development of frailty considering the inflammatory potential of this type of food and (ii) to raise awareness on deleterious effects of excess UPP intake in development of adverse health outcomes, in particular, frailty and compromised healthy ageing.
Low-Grade systemic inflammation, frailty and resilience
Ageing is associated with a number of changes to the organism; in this context, an important contribution of low-grade systemic inflammation has been identified(Reference Franceschi and Campisi10), which is also termed as inflammaging (Reference Franceschi, Garagnani and Parini11). Studies have shown the role of systemic inflammation in different health outcomes associated with frailty, which in the long term can be linked to disability and, ultimately, anticipation of death(Reference Franceschi, Capri and Monti12). There is still no consensus about the factors leading to the development of this inflammatory process, although several hypotheses have been described. Its potential mechanisms include cellular senescence, genetic susceptibility, visceral obesity, increased gut permeability with changes in microbiota composition, impaired recycling and elimination of cell debris, intrinsic defects in immune cells, and recurrent acute infections throughout life(Reference Ferrucci and Fabbri13).
Another important concept related to inflammaging is the body’s ability to counteract the pro-inflammatory molecules such as IL-6, C-reactive protein (CRP) and TNF-α (Reference Franceschi, Capri and Monti12,Reference Ferrucci and Fabbri13) . A healthy immune system is capable of secreting anti-inflammatory molecules (for instance, IL-10 and transforming growth factor-beta (TGF-β)) in response to the increased pro-inflammatory ones(Reference Franceschi, Santoro and Capri14). As such, health consequences of inflammaging rely on the balance between pro- and anti-inflammatory processes and involve mechanisms such as sensing the Toll-like receptors with activation of the transcription factor NF-κB and the assembly of the NLRP3 inflammasome.
Drawing a parallel between inflammaging and frailty, both are conditions resulting from the loss of body resilience, which refers to the capacity to respond to or recover from stressors(Reference Franceschi, Capri and Monti12). During life, stressors like pathogens or danger-associated molecular patterns, as well as environmental, socio-economic, personal and interpersonal aspects, are responsible for challenges to immune resilience. Consequences of these challenges include T-cells immunosenescence, reduced expression of clusters of differentiation in innate immune cells and gene expression of cellular senescence(Reference Franceschi, Garagnani and Parini11,Reference Reed15) .
Studies with centenarians have advanced the understanding of the relationship between inflammaging and resilience. It has been hypothesised that centenarians postpone the onset of age-related conditions or diseases(Reference Franceschi, Garagnani and Parini11). Borras et al. (Reference Borras, Ingles and Mas-Bargues16) attribute this compressed morbidity to a higher resilience that overcomes inflammaging; resilient centenarians develop anti-inflammaging mechanisms against age-related molecular damage.
In a literature review, Pansarasa et al. (Reference Pansarasa, Pistono and Davin17) have described the important relationships between the ageing of the immune system and the manifestations of frailty. Chronic inflammation leads to mitochondrial dysfunction and oxidative stress and ends up creating a vicious cycle, which is associated with or could be a risk factor for reductions in physical and functional capacities. According to the authors, the inflammatory mediators IL-6, CRP and TNF-α are likely to be the main biomarkers related to the model that explains the physiological processes leading to frailty.
Additionally, a recent systematic review by Marcos-Pérez et al. (Reference Marcos-Pérez, Sánchez-Flores and Proietti18) has shown increased levels of IL-6 and CRP among pre-frail and frail individuals. The authors highlight that further studies are still needed to establish whether other inflammation biomarkers such as TNF-α, IL-1β, soluble TNF receptors, intercellular adhesion molecule-1 (ICAM-1) and monocyte chemoattractant protein-1 (MCP-1) are also related to the development of frailty. However, there is still a lack of longitudinal studies investigating whether inflammation could contribute to the development of frailty or could be a feature of this condition and other phenotypes related to ageing; the majority of the current evidence comes from cross-sectional studies.
If a causal relationship between inflammation and frailty is actually present, it is likely that lifestyle factors – especially dietary habits – might be implicated in the development of this adverse condition, as we explain in the next section.
Diet as a mediator between systemic inflammation and frailty
Diet plays an important role in the prevention and treatment of several non-communicable diseases, as well as promoting health and well-being throughout life cycles, favouring healthy ageing. Evidence suggests that healthy dietary habits have a positive impact on frailty. The overall quality of the diet, with adequate energy and protein intake and consumption of antioxidants and a number of phytochemicals, is an important factor to prevent or delay the development of frailty in older adults(Reference Kelaiditi, Guyonnet and Cesari19,Reference Lorenzo-López, Maseda and de Labra20) .
Moreover, diet is an important modulator of the gut microbiota, which is related to systemic inflammation. Fermentation of dietary fibres in the large intestine involves metabolic cross-feeding wherein the fermentation product of one or more species of bacteria provides a substrate for other species, producing SCFA, particularly acetate, propionate and butyrate(Reference Sarbini and Rastall21,Reference Flint, Scott and Louis22) . These SCFA have several metabolic functions, such as reducing inflammation, increasing barrier function and down-regulating pro-inflammatory mediators that induce lipopolysaccharides(Reference Mohajeri, Brummer and Rastall23). Increasing the concentration of acetate and butyrate can reduce the production of microbial-derived metabolites associated with atherogenesis and the development of CVD(Reference Tang, Wang and Levison24–Reference Li, Obeid and Klingenberg26). Besides, it has been demonstrated that SCFA can inhibit osteoclast differentiation, contributing to bone health(Reference Iwami and Moriyama27,Reference Lucas, Omata and Hofmann28) .
On the other hand, some dietary components can be deleterious and exert pro-inflammatory effects, which is the case of UPP. Their nutritional profile, characterised by high energy density and low anti-inflammatory nutrients, is an attribute of pro-inflammatory diets, which in turn are associated with the development of low-grade systemic inflammation(Reference Shivappa, Stubbs and Hébert29). Two different mechanisms could explain the effect of UPP intake on systemic inflammation. The first relates to the modulation of intestinal hormones that regulate satiety and, consequently, body weight. In a 2-week randomised clinical trial, Hall et al. (Reference Hall, Ayuketah and Brychta30) found that participants who consumed an ultra-processed diet presented higher energy intake and weight gain compared with those who went on an unprocessed diet. Participants on an ultra-processed diet had lower levels of the anorectic hormones active GLP-1 and PYY, while participants in the unprocessed diet group had lower levels of the orexigenic hormone ghrelin. These results may indicate a deleterious effect of UPP intake on satiety mechanisms, leading to increased energy intake and, therefore, oxidative stress and inflammation(Reference Boden, Homko and Barrero31,Reference Bloomer, Trepanowski and Kabir32) . Furthermore, GLP-1 is an incretin with a substantial role in satiety and glucose regulation(Reference Krieger33), and hyperglycemia is another factor that can cause oxidative stress and inflammation(Reference Nadkarni, Chepurny and Holz34). The second mechanism relates to negative changes promoted by Western diet, characterised by a high intake of UPP, to the gut microbiota, contributing to increased inflammation. It has been suggested that the presence of acellular nutrients, food additives, non-energetic artificial sweeteners and possibly advanced glycation end products, as well as the lack of fermentable fibres and phytochemicals, could be responsible for the altered composition and metabolism of gut microbes(Reference Zinöcker and Lindseth35). Finally, it is also possible that pro-inflammatory states are not a direct consequence of UPP intake but derive from the presence of higher body adiposity, or obesity and its metabolic consequences, which indeed are promoted by both pro-inflammatory diets and UPP intake(Reference Lopes, Araújo and Levy36–Reference Pagliai, Dinu and Madarena40).
Is there a link between ultra-processed products intake and frailty?
Currently, a lot of attention has been drawn to the role of UPP in non-communicable diseases. However, this specific question regarding frailty has only recently been investigated. The only published longitudinal study that tried to address this relationship was the one carried out by Sandoval-Insausti et al. (Reference Sandoval-Insausti, Blanco-Rojo and Graciani41), using data from Seniors-ENRICA (Study on Nutrition and Cardiovascular risk factors in Spain) cohort. In this prospective study conducted in community-dwelling older adults, the authors have found that the intake of UPP was strongly associated with frailty. UPP contributed to an average of 19·3 % of the energy content of diet. In the highest quartile of UPP consumption, represented as a percentage of total energy intake, older adults had an OR of 3·67 in being identified as frail compared with those in the lowest quartile. This association was independent of socio-demographic, lifestyle and morbidity factors.
On the other hand, a systematic review of prospective cohort studies has shown a protective effect on frailty of the Mediterranean dietary pattern, which is characterised by a high intake of plant foods (fruits, vegetables, whole-grain cereals, herbs, nuts and seeds), olive oil as the principal source of fat, low-fat dairy products consumed in moderate amounts, red meat and processed meat consumed in low amounts, and preference for seasonal, fresh and minimally processed foods(Reference Bach-Faig, Berry and Lairon42). The effect measures combined in the meta-analysis were adjusted for socio-demographic, lifestyle and morbidity factors(Reference Kojima, Avgerinou and Iliffe43). Such an effect is mediated by the high intake of antioxidant-rich foods, which contribute to decreasing oxidative stress(Reference Soysal, Isik and Carvalho44) reducing the expression of inflammatory markers(Reference Casas, Sacanella and Estruch45) and modulating gut microbiota through an increase in SCFA production(Reference Ghosh, Rampelli and Jeffery46).
Although evidence from studies addressing dietary patterns is still scarce, promising results have been produced by research assessing the Dietary Inflammatory Index (DII). A recent study examining data from the National Health and Nutrition Examination Survey (2007–2014) found that older adults in the highest quintile of DII (a more inflammatory diet) were more likely to be pre-frail and frail, regardless of age, sex, race, education, smoking status and co-morbidities(Reference Resciniti, Lohman and Wirth47). Another longitudinal study using data from the Seniors-ENRICA cohort found that individuals in the highest tertile of DII showed a significantly higher risk of frailty when compared with those in the lowest tertile, even after adjustment for confounders(Reference Laclaustra, Rodriguez-Artalejo and Guallar-Castillon48). Additionally, a systematic review conducted by Vicente et al. (Reference Vicente, Quaresma and de Melo49) has shown a significant association and predictive value of DII regarding frailty. This higher risk of frailty is assumed to be due to increased inflammatory markers, including CRP, IL-6 and TNF-α (Reference Hébert, Shivappa and Wirth50).
Furthermore, it has been suggested that specific components and nutrients lacking in diets rich in UPP could be related to the development of frailty in older adults. Examples are PUFA – especially n-3 – and vitamin D, which have been reported to be related to lower CRP and IL-6 levels. Yet, cross-sectional and prospective studies conducted so far have been insufficient to clarify this particular association(Reference Pansarasa, Pistono and Davin17). Likewise, dietary fibre intake is associated with an increased prevalence of bacteria species with beneficial health effects, such as Bifidobacterium spp. and F. prausnitzii (Reference Ramirez-Farias, Slezak and Fuller51,Reference Dewulf, Cani and Claus52) . Concerning the gut microbiota, a higher intake of refined sugars and the presence of obesity have been associated with the proliferation of pathogenic bacteria and reduced diversity of microbes(Reference Payne, Chassard and Lacroix53–Reference Fu, Bonder and Cenit55).
Moreover, we highlight the publication of four systematic reviews addressing the relationship between UPP intake and the risk of non-communicable diseases. These reviews have some limitations, as most of the accumulated evidence is from cross-sectional studies. Besides, there are differences in the classification of foods concerning processing, adjustment for confounding variables and the methods of dietary assessment. Despite this, UPP intake has been related to an increased risk of all-cause mortality, CVD, cerebrovascular disease, depression, metabolic syndrome, low HDL-cholesterol levels, overweight, obesity, high waist circumference and cancer (all types and breast cancer in postmenopausal women)(Reference Chen, Zhang and Yang37–Reference Pagliai, Dinu and Madarena40). It is important to note that all these diseases contribute to a lower quality of life and life expectancy, and some are also related to an increased risk of frailty in older adults.
Ultra-Processed products intake from a public health perspective
Assuming that the possible relationship between the consumption of UPP and frailty outlined in the previous section is confirmed, implications arise not only at the individual level but also from a public health perspective.
UPP are becoming increasingly more prevalent in food systems, notably because of the influence of transnational food and drink corporations. There is still a wide variation between regions and countries on the share of such products in populations’ diets. In countries such as the USA, Canada and the UK, the consumption of UPP has reached approximately 60 % of total energy intake, while in Brazil they represent around 20 % and in Colombia only 16 %(Reference Monteiro, Moubarac and Cannon56–Reference Baker, Machado and Santos62).
Several studies assessing secular trends of food intake have shown increases in the consumption of UPP throughout the years, resulting in important changes in dietary patterns. This is the case, for instance, of Canada, Brazil and Mexico(Reference Moubarac, Batal and Martins63–Reference Marrón-Ponce, Tolentino-Mayo and Hernández-F65). Moreover, global sales of UPP are on the rise, and their consumption is markedly growing in middle-income countries(66,67) .
There is now a wealth of evidence from nationally representative population-based cross-sectional studies confirming that the intake of UPP negatively affects the overall quality of population diets, resulting in a pro-inflammatory dietary pattern. The presence of these products contributes to higher energy density and higher carbohydrate, free sugars, fatty acids (total, saturated and trans) and Na content of diets. At the same time, higher shares of UPP in diets are related to lower protein, dietary fibre, vitamin (A, C, D and E) and mineral (such as Zn, Fe, Ca, K and Mg) intakes(Reference Steele, Baraldi and Louzada57,Reference Moubarac, Batal and Louzada58,Reference Rauber, Louzada and Steele61,Reference Louzada, Martins and Canella68–Reference Cediel, Reyes and Corvalán76) .
Indeed, cross-sectional studies usually point to higher intakes of UPP among younger individuals, especially in children and adolescents, the lowest intake primarily being identified among older adults(Reference Parra, Costa-Louzada and Moubarac60,Reference Marrón-Ponce, Flores and Cediel71,Reference Cediel, Reyes and Louzada73,Reference Vandevijvere, Ridder and Fiolet75,Reference Adams and White77–Reference Machado, Steele and Louzada80) . Strategies to prevent the burden of disease in old age and delay the onset of disability should begin in childhood and continue throughout life(Reference Michel, Newton and Kirkwood81).
Conclusion
In the past few years, a lot of attention has been devoted to the study of the relationship between the consumption of UPP and the development of chronic diseases – especially obesity, CVD, diabetes and cancer. In this paper, we go further by showing a scientific rationale that an increased consumption of UPP could also have negative implications on healthy ageing.
Research on the association between dietary patterns and the development of frailty is still incipient. Nonetheless, there is currently no doubt about the association between unhealthy diets and the occurrence of diet-related non-communicable diseases, especially those related to low-grade systemic inflammation. Because inflammation is also believed to contribute to the development of frailty, it is likely that the nutrient profile of UPP contributes to the onset of this condition. Therefore, more research is needed in order to establish this possible causal relationship between the consumption of UPP, inflammation and frailty, as well as the underlying biological mechanisms involved. In addition to that, we suggest prospective studies on frailty to address the influence of food processing on biomarkers of inflammation and the microbiota of older adults. Also, intervention studies could investigate whether improving diets (by reducing the share of UPP and increasing the share of anti-inflammatory foods and nutrients) is effective for preventing or reducing the risk of frailty in older adults.
Finally, from a public health perspective, we must raise awareness among the younger populations on adverse consequences of unhealthy dietary habits such as increased consumption of UPP. In the long term, excess UPP intake may lead not only to diet-related non-communicable cardiometabolic diseases but also frailty and compromised healthy ageing.
Acknowledgements
None.
This manuscript was designed by A. B. M. A. B. M., A. D. M. and L. do N. M. F. equally contributed to the draft of the manuscript. S.M.L.R supervised this work and critically reviewed the text, providing significant inputs. All authors have approved the final version of this manuscript.
The authors have no conflicts of interest to disclose.