The rapid increase in the prevalence of diabetes mellitus is becoming a serious threat to public health all over the world. By the year 2030, diabetes mellitus is expected to affect almost 5 % of the world's population – an estimated 366 million individuals(Reference Gouda, Sagoo and Harding1). Diabetes imposes a considerable burden in terms of premature mortality, morbidity and healthcare costs. Life expectancy for individuals with diabetes is estimated to be up to 10 years shorter than for individuals without diabetes(Reference Clarke, Glasziou and Patel2). Diabetes mellitus is a metabolic disorder characterised by the presence of hyperglycaemia and relative or absolute insulin deficiencies. A prolonged uncontrolled hyperglycaemic state leads to complications of diabetes, such as vision loss, renal failure, nerve damage and cardiovascular system disease(Reference Ramachandra, Shetty and Salimath3). The prevention and control of complications associated with diabetes have become one of the key issues in biomedical research. Current management of diabetes relies mainly on the reduction of dietary carbohydrate intake and the use of hypoglycaemic agents to lower the level of circulating glucose. Limiting and/or delaying intestinal carbohydrate absorption would help to correct plasma glucose concentrations and reduce diabetic complications(Reference Dyer, Wood and Palejwala4). It has been observed that there is an increased glucose absorption in the small intestine(Reference Sheldl and Wilson5), which may be attributed to increased intestinal disaccharidase activities. Disaccharidases are essential for the terminal absorption of carbohydrate digestion, especially in the conversion of disaccharides to readily soluble monosaccharides. Studies have shown that diabetes mellitus causes structural and functional changes in the intestine, namely intestinal hyperplasia, increases in intestinal glucose absorption, and alterations in the activities of brush-border enzymes such as maltase, sucrase and lactase(Reference Clarke, Glasziou and Patel2, Reference Schedl, Al-Jurf and Wilson6–Reference Burant, Flink and DePaoli11). The increased intestinal monosaccharide absorption in diabetes can further complicate the pathophysiology of this disease(Reference Shetty, Kumar and Salimath9).
At present, the available therapies for diabetes mellitus include insulin and many oral hypoglycaemic agents, such as biguanides and sulfonylureas. However, treatment with sulfonylureas and biguanides is also associated with side effects and failing to significantly alter the course of diabetic complications(Reference Grover, Yadav and Vats12). The Diabetes Control and Complications Trial demonstrated that even an optimal control of blood glucose could not prevent complications, suggesting that alternative treatment strategies are needed(Reference Pareek, Sharma and Khajja13). As an alternative approach, herbal plants with medicinal value have been used for centuries in order to better manage diabetes and its complications(Reference Oubre, Carlson and Kiny14). Thus, herbal therapy has a bright future in the treatment of diabetes mellitus.
In China, Gynura divaricata (L.) DC. is mostly wild and is consumed as a vegetable in some areas. It is used for treating diabetes in certain regions of China. Investigations have shown that both the extract of G. divaricata and polysaccharide from G. divaricata, one of the active components of G. divaricata, possess anti-hyperglycaemic actions in diabetic rats and mice(Reference Li, Ren and Min-Zhuo15–Reference Jiang, Hu and Qiu17). However, the exact mode of action of polysaccharide from G. divaricata in the treatment of diabetes mellitus remains to be shown. In the present paper, the effects of polysaccharide from G. divaricata on the activities of intestinal disaccharidases were investigated in streptozotocin-induced diabetic rats and the potential mechanisms for its glucose-lowering effects were explored.
Materials and methods
Materials
Disaccharidase (include maltase, sucrase and lactase) kits were purchased from Jiancheng Bioengineering Company (Nanjing, China). A glucose kit was purchased from Rongsheng Biotech Co. Ltd (Beijing, China). An insulin ELISA kit was obtained from Adlitteram Diagnostic Laboratory (San Diego, CA, USA). Streptozotocin was purchased from Sigma-Aldrich (St Louis, MO, USA). Fresh aerial parts of G. divaricata (L.) DC. were procured from a local market and were authenticated by Doctor Lingli Mou (Department of Pharmacognosy, Hunan Normal University, Changsha, China). Fresh aerial parts of G. divaricata (L.) DC. was cleaned and cut into pieces, and then the G. divaricata pieces were air-dried at 40 ± 2°C. All other chemicals were of analytical grade.
Animals
All animal studies were performed in accordance with the Principles for Biomedical Research Involving Animals developed by the Council for International Organizations of Medical Sciences as well as institutional guidelines. Male Sprague–Dawley rats (body weight 160–180 g) were supplied by the Laboratory Animal Center of Hunan Normal University (grade II, certificate no. XK 2009-0012). The experimental protocol was approved by the Hunan Normal University Ethics Committee for the use of experimental animals (approval number HNNU-AE0168) and conformed to the Guide for the Care and Use of Laboratory Animals. Rats were maintained at 22 ± 2°C and 55 ± 5 % relative humidity on a 12 h light–12 h dark cycle. Animals had free access to standard rodent pellet food (Laboratory Animal Center of Hunan Normal University), except when fasted before experiments, and water ad libitum. After randomisation into two groups (a diabetic group and a normal control group), the rats were acclimatised for a period of 3 d in the new environment before the initiation of the experiment. During the experiments the rats were kept fasting for 16 h before drug administrations and for 3 h after dosings, with free access to water.
Preparation of the polysaccharide from Gynura divaricata
According to the method of Jiang et al. (Reference Jiang, Qiu and Liu18) with a minor modification, pieces of G. divaricata were ground to powder and a nine-fold mass of water was added. The mixture was heated to 90°C and maintained for 2 h, and then was filtered. Another nine-fold mass of water was added again, and the extraction process was the same as above. Then the two filtrates were mixed, concentrated, precipitated with ethanol and deproteinised by the Sevag method. The product then was decolourised with activated carbon and washed three times with dehydrated alcohol. With freeze dehydration, the grey polysaccharide from G. divaricata was obtained.
Streptozotocin-induced diabetic rats
Diabetes was induced by streptozotocin (Sigma-Aldrich, St Louis, MO, USA). After rats were fasted for at least 12 h, stable diabetes was induced by a single intraperitoneal injection of streptozotocin (55 mg/kg body weight) dissolved in 0·1 m-sodium citrate buffer (pH 4·5) to male Sprague–Dawley rats, and control group rats received only the citrate buffer. On day 7 after the injection of streptozotocin, blood samples of the rats in the fasting state were collected from the oculi chorioideae vein under mild anaesthesia to check the diabetic status. Thereafter, blood samples were collected into heparinised Eppendorf tubes, and centrifuged. Plasma glucose concentrations were measured using commercial kit reagents (Rongsheng Biotech Co. Ltd, Beijing, China) based on the glucose oxidase method(Reference Trinder19). Only animals in the diabetes group with hyperglycaemia ( ≥ 14 mmol/l) were used in the study.
Experimental protocol
Rats used in the experiment were divided into two groups of age-matched normal rats (normal control group (NCG) and normal group treated with polysaccharide from G. divaricata (NGP); eight rats in each group) and two groups of diabetic rats (diabetic model group (DMG) and diabetic group treated with polysaccharide from G. divaricata (DGP); eight rats in each group). Rats in the G. divaricata polysaccharide-treated normal group (NGP group) and diabetic group (DGP group) were given the polysaccharide from G. divaricata (400 mg/kg body weight, once daily) suspended in 0·5 % carboxymethyl cellulose sodium salt aqueous solution by intragastric administration for 33 d, respectively. Rats in the NCG and DMG groups were given an equal volume of 0·5 % carboxymethyl cellulose sodium salt aqueous solution. Food intake and the body weights of all animals were monitored throughout the experiment.
On day 31 of dosing, the urine of all rats was collected, under a layer of toluene, by keeping the rats in metabolism cages for a period of 24 h. The content of reducing sugars present in the urine was measured by the dinitrosalicylic acid method(Reference Miller20).
On day 32 of dosing, the rats of the four groups were fasted overnight, and blood was drawn by the retro-orbital plexus and collected in tubes containing heparin to measure the fasting plasma glucose and fasting plasma insulin levels. Plasma was separated from the blood and used for analysis. The plasma glucose level in rats fasted overnight was measured by the glucose oxidase method(Reference Trinder19) using a commercially available kit. The assays of plasma insulin levels were accomplished using the ELISA kit, according to the manufacturer's protocol.
At the end of the experiment, 4 h before killing, an oral sucrose tolerance test (OSTT) was performed according to a standard protocol (see below) on all the animals and then they were killed under light diethyl ether anaesthesia for the collection of blood and the small intestines.
Oral sucrose tolerance test
After overnight fasting, on the day of killing, an oral dose of vehicle (0·5 % carboxymethyl cellulose sodium salt) or polysaccharide from G. divaricata (2·5 mg/kg) was given 30 min before oral sucrose administration. In the OSTT, sucrose solution at a dose of 2·5 g/kg body weight was orally administered to overnight-fasted rats. Blood samples were collected from the tail vein at 0, 30, 60 and 120 min after the oral sucrose load and treated as before for plasma glucose analysis.
Intestinal homogenate
Rats were killed under diethyl ether anaesthesia and the small intestines were harvested. The intestinal lumen was flushed with ice-cold saline to free it from food particles and then cut open. The mucosa was scraped using a glass slide and homogenised in 0·9 % saline. Then it was centrifuged at 3000 rpm for 10 min at 4°C. The supernatant fraction obtained was used for the assay of disaccharidases.
Disaccharidase assay
With a commercially available kit, the assay of disaccharidases was carried out by determining the specific activities of sucrase, maltase and lactase, which were correlated to the amount of glucose released from sucrose, maltose and lactose, respectively, at 37°C in maleate buffer (0·2 m; pH 6·0) at different time intervals as described by Dahlquist(Reference Dahlqvist21). The amount of protein in the samples was determined by the method of Lowry et al. (Reference Lowry, Rosebrough and Farr22).
Statistical analysis
Results are expressed as mean values and standard deviations. Differences in mean values were analysed using Student's t test and ANOVA. Significance is defined as P < 0·05. In the analysis of the results of the OSTT, the statistical comparison of area under the curve was performed after logarithmic processing.
Results
Diabetes was induced in male Sprague–Dawley rats by injecting streptozotocin in a citrate buffer. According to the measured fasting plasma glucose, diabetic rats were grouped by their average diabetic status (plasma glucose ≥ 14·0 mmol/l) after excluding the rats whose fasting plasma glucose was not high enough (plasma glucose < 14 mmol/l). The experiment was terminated when rats had been treated with polysaccharide from G. divaricata for 33 d and the OSTT investigation had been accomplished. Rats were fasted overnight and blood was drawn from the retro-orbital plexus for determining plasma glucose. At the end of experimental period, rats were killed under diethyl ether anaesthesia. Diabetic status was evaluated by measuring urine volume, urine sugar, fasting plasma glucose and plasma insulin levels at the end of the experiment.
Effect of polysaccharide from Gynura divaricata on body weight, food intake, urine volume and urine sugar in control and diabetic rats
The body weight and food consumption of the rats were monitored. Animals of both normal groups (NCG and NGP) had body weights more than 300 g. Body weight was the lowest in the DMG group, and the status of body-weight loss in the DGP group was remarkably improved.
Food intake in both normal groups (NCG and NGP) was under 15 g/d per rat. Higher food consumption was found in the DMG group rats (25·8 g/d per rat), and food consumption was lower for the DGP group rats (Table 1).
Table 1 Effects of polysaccharide from Gynura divaricata on the food intake and body weight in control and diabetic rats
(Mean values and standard deviations)
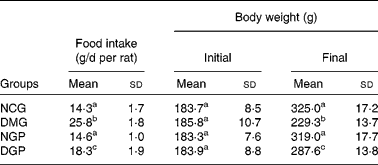
NCG, normal control group; DMG, diabetic model group; NGP, normal group treated with polysaccharide from G. divaricata; DGP, diabetic group treated with polysaccharide from G. divaricata.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·01).
Furthermore, the excretion of urine was assayed. Both the NCG and NGP groups excreted 14 ml/d during the experiment. However, the DMG group of rats excreted 83 ml/d. However, when treated with polysaccharide from G. divaricata, the polyuria status of diabetic rats (DGP group) was significantly improved with an excretion of 47 ml/d (Table 2).
Table 2 Effects of polysaccharide from Gynura divaricata on the urine volume and urine sugar in control and diabetic rats
(Mean values and standard deviations)

NCG, normal control group; DMG, diabetic model group; NGP, normal group treated with polysaccharide from G. divaricata; DGP, diabetic group treated with polysaccharide from G. divaricata.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·01).
The urinary sugar excretion in normal rats (NCG and NGP groups) was very low (for example, in milligram ranges) (Table 2). The DMG group excreted 8·57 g reducing sugars/d, which was much higher than normal values. At the same time, rats in the DGP group excreted about 3·12 g reducing sugars/d, which was statistically significant compared with diabetic rats in the DMG group.
The data above show that the polysaccharide from G. divaricata has beneficial effects on the food intake and urine excretion of diabetic rats.
Effect of polysaccharide from Gynura divaricata on fasting plasma glucose and insulin levels in control and diabetic rats
Fasting plasma glucose in the blood drawn by the retro-orbital plexus at the end of the experiment was measured. Rats in both the NCG and NGP groups had fasting plasma glucose of about 6 mmol/l (Table 3). A significantly higher fasting plasma glucose level was found in the DMG rats, which was about 25 mmol/l. For rats in the DGP group, the fasting plasma glucose level was remarkably reduced by 42 %, indicating the hypoglycaemic effect of the polysaccharide from G. divaricata in diabetic rats. There were no significant differences in the fasting plasma insulin levels in the rats of the NCG and NGP groups. However, the fasting plasma insulin level in DMG rats was found to be 44 % lower when compared with NCG rats (P < 0·01). However, when the diabetic rats were treated with the polysaccharide from G. divaricata, the fasting plasma insulin level was higher (P < 0·01) (Table 3).
Table 3 Effects of polysaccharide from Gynura divaricata on the levels of fasting plasma glucose (FPG) and fasting plasma insulin (FPI) in diabetic rats
(Mean values and standard deviations)

NCG, normal control group; DMG, diabetic model group; NGP, normal group treated with polysaccharide from G. divaricata; DGP, diabetic group treated with polysaccharide from G. divaricata.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·01).
Effect of the polysaccharide from Gynura divaricata on the oral sucrose tolerance test in control and diabetic rats
The OSTT test showed that plasma glucose levels in the DMG group were higher compared with those of the other diabetic group at 30, 60 and 120 min. Sucrose tolerance significantly improved in DGP rats, compared with DMG rats (P < 0·01). For DGP rats, the area under the curve of plasma glucose decreased by approximately 38 % compared with that of DMG rats. In the OSTT, no significant difference of the area under the curve of blood glucose was found between the NCG and NGP rats (Table 4).
Table 4 Effect of polysaccharide from Gynura divaricata on the oral sucrose tolerance test in control and diabetic rats
(Mean values and standard deviations)
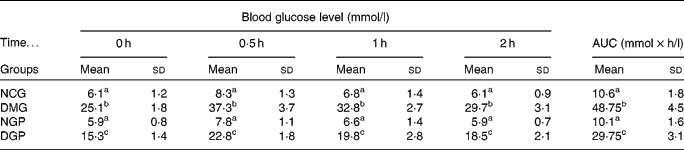
AUC, area under the curve; NCG, normal control group; DMG, diabetic model group; NGP, normal group treated with polysaccharide from G. divaricata; DGP, diabetic group treated with polysaccharide from G. divaricata.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·01).
Effect of the polysaccharide from Gynura divaricata on activities of intestinal disaccharidases in control and diabetic rats
The specific activities of intestinal disaccharidases, namely maltase, sucrase and lactase, were measured in control and diabetic rats (Tables 5–7). For maltase activity, a two-fold increase was observed in the duodenum, jejunum and ileum in the DMG group when compared with the NCG group. Treatment with polysaccharide from G. divaricata led to a significant decrease in the maltase activity of the three intestine segments during diabetes (DGP group) when compared with the DMG rats. Similarly, there was a significant increase in sucrase activity of the three intestine segments in DMG rats when compared with NCG rats and a significant reduction in the sucrase activity of the three intestine segments was also observed by administrating the polysaccharide from G. divaricata. During diabetes (DMG group), a remarkable increase in lactase activity of the three intestine segments was observed when compared with its normal control (NCG group), and a significant decrease in lactase activity was observed only in the duodenum by administrating the polysaccharide from G. divaricata during diabetes (DGP group). However, administration of the polysaccharide from G. divaricata did not lead to a remarkable change in lactase activity of the jejunum and ileum during diabetes mellitus. In the NGP group, the activities of disaccharidases were not obviously changed when compared with the NCG group.
Table 5 Effects of polysaccharide from Gynura divaricata on the activity of maltase (U/mg protein) in different intestinal sections of control and diabetic rats
(Mean values and standard deviations)
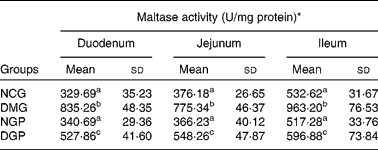
NCG, normal control group; DMG, diabetic model group; NGP, normal group treated with polysaccharide from G. divaricata; DGP, diabetic group treated with polysaccharide from G. divaricata.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·01).
* 1 U = 1 nmol glucose formed per min.
Table 6 Effects of polysaccharide from Gynura divaricata on the activity of sucrase (U/mg protein) in different intestinal sections of control and diabetic rats
(Mean values and standard deviations)
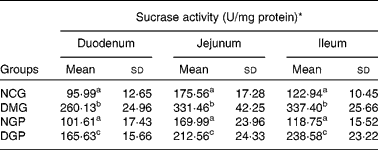
NCG, normal control group; DMG, diabetic model group; NGP, normal group treated with polysaccharide from G. divaricata; DGP, diabetic group treated with polysaccharide from G. divaricata.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·01).
* 1 U = 1 nmol glucose formed per min.
Table 7 Effects of polysaccharide from Gynura divaricata on the activity of lactase (U/mg protein) in different intestinal sections of control and diabetic rats
(Mean values and standard deviations)
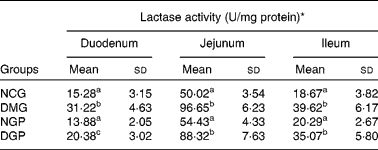
NCG, normal control group; DMG, diabetic model group; NGP, normal group treated with polysaccharide from G. divaricata; DGP, diabetic group treated with polysaccharide from G. divaricata.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·01).
* 1 U = 1 nmol glucose formed per min.
Discussion
Appropriate nutritional management is essential for restoring and maintaining a good metabolic state. In this context, the diet remains a cornerstone in diabetic management. Dietary carbohydrate in humans is a major nutrient and disaccharidases (mainly sucrase and maltase) play an important role in carbohydrate digestion. It is generally recognised that carbohydrates are digested into oligosaccharides and then into disaccharides by enzymes secreted by the digestive tract. Disaccharides are then converted into monosaccharides by disaccharidases located in the small-intestinal mucosa(Reference Lebovitz23, Reference Levin24). Postprandial hyperglycaemia is considered to be a major risk factor for patients with diabetes. It has been reported that many polysaccharides could reduce the postprandial plasma glucose level. Chen et al. reported the hypoglycaemic effects of polysaccharides from the tuberous root of Liriope spicata in type 2 diabetic mice(Reference Chen, Liu and Bai25). Wu et al. and Mao et al. demonstrated the hypoglycaemic activity of Astragalus polysaccharide in diabetic rats and mice(Reference Wu, Ou-Yang and Wu26, Reference Mao, Yu and Wang27). Zhang et al. found that Artemisia sphaerocephala Krasch seed polysaccharide could lower the plasma glucose level in diabetic rats induced by alloxan(Reference Zhang, Huang and Hou28). Zhao et al. reported the anti-hyperglycaemic effect of polysaccharide isolated from Dendrobium chrysotoxum Lindl in diabetic mice(Reference Zhao, Son and Kim29). Li et al. reported that polysaccharide isolated from pumpkin can remarkably reduce the plasma glucose in diabetic rats induced by alloxan(Reference Li, Fu and Rui30). Hannan et al. demonstrated that the soluble dietary fibre fraction of Trigonella foenum-graecum (fenugreek) seed remarkably lowered the level of blood glucose in model rats of type 1 and type 2 diabetes mellitus(Reference Hannan, Ali and Rokeya31). In the present study, it was shown that the polysaccharide from G. divaricata significantly ameliorated the increases in urine volume, urine sugar and fasting plasma glucose during diabetes mellitus. Some investigations have indicated that polysaccharides exert their hypoglycaemic activities via inhibiting the expressing and activity of protein tyrosine phosphatase 1B or by stimulating the secretion of insulin(Reference Wu, Ou-Yang and Wu26, Reference Mao, Yu and Wang27, Reference Wang, Zhang and Mao32, Reference Hwang, Lee and Baek33). However, according to Lipinski's rule, polysaccharides can hardly be absorbed into the circulatory system when orally administrated because the molecular weights of polysaccharides are often more than 500 Da(Reference Lipinski, Lombardo and Dominy34). Therefore, it could be speculated that the target by which polysaccharides exert their hypoglycaemic action might be located in the gastrointestinal tract.
Diabetes frequently results in severe metabolic imbalances and pathological changes in various tissues. In the small intestine, diabetes mellitus may bring significant changes in the morphology and functions of the mucosa(Reference Zhao, Son and Kim29, Reference Li, Fu and Rui30). Hyperglycaemia in diabetes results from the up-regulation of the expression and activities of glucose carriers and disaccharidases in the intestinal mucosa(Reference Younoszai and Schedl10, Reference Fedorak, Cheeseman and Thomson35). Increased intestinal disaccharidase activities have been reported in human diabetics(Reference Schedl, Al-Jurf and Wilson6, Reference Younoszai and Schedl10). The changes in the specific activities of intestinal maltase, sucrase and lactase in the diabetic state observed in the present study were similar to those previously reported(Reference Shetty, Kumar and Salimath9, Reference Hannan, Ali and Rokeya31). Increased activities of disaccharidases (sucrase and maltase) were alleviated by administrating the polysaccharide from G. divaricata. However, in our present study the polysaccharide from G. divaricata was effective in significantly inhibiting the increased lactase activity of diabetic rats only in the duodenum. Moreover, in the OSTT, the presence of a strong postprandial anti-hyperglycaemic effect of the polysaccharide from G. divaricata revealed by the sucrose tolerance curve postulates that the main anti-diabetic activity of the polysaccharide from G. divaricata may result from inhibiting the intestinal disaccharidase activities of diabetic rats. It is suggested that the polysaccharide from G. divaricata exerted its anti-diabetic effect partly via inhibiting the increased activities of intestinal disaccharidases in the diabetic rats. Although the mechanism of the inhibitory effects of the polysaccharide from G. divaricata on the intestinal disaccharidases has not been investigated in the present study, we will explain it in further investigations. At present, it is supposed that the mechanism of the inhibitory effects of the polysaccharide from G. divaricata on the intestinal disaccharidases may be a competitive inhibition because the polysaccharide is also composed of many monosaccharides. Probably the polysaccharide exerts its inhibitory effect by competitively binding the intestinal disaccharidases.
There are two limitations to the study. First, the comparison of anti-diabetic effects between the polysaccharide isolated from G. divaricata and other non-digestible polysaccharides has not been performed. In our next study, we will compare the anti-diabetic effects of the polysaccharide isolated from G. divaricata with those of other non-digestible polysaccharides. Second, the values measured in the non-diabetic rats were not normalised. This may lead to the question: up to what degree of diabetes would it be useful to administer this polysaccharide to diabetic patients? It may be worth mentioning that in another clinical investigation completed by our group (Y-X Deng et al., unpublished results), we found that the polysaccharide isolated from G. divaricata had good hypoglycaemic effects when it was administrated to diabetic patents who presented hyperglycaemia from a mild to a moderate extent.
In summary, the present study confirmed the hypoglycaemic effect of the polysaccharide from G. divaricata and demonstrated its target and pharmacological mechanism. Amelioration of the diabetic state as indicated by urine sugar, urine volume and fasting plasma glucose in diabetic rats treated with the polysaccharide from G. divaricata appears to be due to the alleviation of increased activities of intestinal disaccharidases, thus making diabetic animals more tolerant to hyperglycaemia.
Acknowledgements
This investigation was supported by the Opening Fund of Key Laboratory of Chemical Biology and Traditional Chinese Medicine Research (Ministry of Education of China), Hunan Normal University (no. KLCBTCMR2009-03) and by research grants of Hunan Administration of Traditional Chinese Medicine (no. 2008083).
The animal study was designed by Y.-X. D., Y.-S. C., W.-R. Z., B. C. and L.-H. H. The animal study was performed by Y.-X. D., Y.-S. C. and L.-L. M. The preparation of the polysaccharide from G. divaricata was performed by X.-M. Q. and R. C. The biochemical assay was performed by C.-H. Y. and Y.-X. D. The manuscript was drafted and reviewed by Y. X. D. and B. C.
The authors have declared no conflict of interest.









