The gastrointestinal tract (GIT) has a key role in nutrient supply, and it has an impact on the functions of the entire organism, as the highest number of immune cells and the highest diversity in microbiota are present in the gut( Reference Martin, Nauta and Ben Amor 1 ). The immediate postnatal period is the most critical phase in the establishment of the intestinal microbial population, and accumulating evidence suggests that this early colonisation of the gut also determines the reactivity of the immune system in later phases of life( Reference Round and Mazmanian 2 ). For example, Sjögren et al. ( Reference Sjögren, Jenmalm and Böttcher 3 ) showed that a more diverse microbiome early in life seems to reduce the prevalence of allergies. During the initial maturation period, the intestinal tract undergoes profound growth, as well as morphological and functional differentiation( Reference Vael and Desager 4 ). Previous data have shown that breast-fed infants benefit from the special composition of colostral and early breast milk, as they have a lower risk of developing intestinal disorders and a lower incidence and severity of diarrhoea, inflammatory reactions and atopic diseases when compared with formula-fed infants( Reference Walker 5 ). The high concentration (12–15 g/l) and structural diversity of human milk oligosaccharides (HMO) are unique to humans, and HMO represent the first prebiotics in life. Hence, prebiotic oligosaccharides, including galacto-oligosaccharides (GOS) derived from bovine milk, are added to infant formulas, alone or together with other prebiotics( Reference Oozeer, van Limpt and Ludwig 6 , Reference Arslanoglu, Moro and Schmitt 7 ). Previous data have suggested that GOS have beneficial effects on the composition of the microbiota and the priming of the infant’s immune system( Reference Jeurink, van Esch and Rijnierse 8 , Reference Fanaro, Marten and Bagna 9 ). In adults, GOS are also effective in alleviating symptoms of irritable bowel syndrome( Reference Silk, Davis and Vulevic 10 ). To allow a closer understanding of the different mechanisms involved in these beneficial effects of GOS and related substances, various animal models have been applied( Reference Barnes, Hartmann and Holst 11 – Reference Sangild, Thymann and Schmidt 14 ). It appeared that, in contrast to rodents, the postnatal gut development and nutritional requirements of piglets more closely resemble the human infant in many aspects( Reference Heinritz, Mosenthin and Weiss 15 – Reference Calder, Krauss-etschmann and De 17 ). The purpose of this study was to apply such a piglet model using normally delivered piglets and supplementing their standard formula diet with a fully characterised GOS mixture, to identify physiological and morphological changes in the intestine of GOS-treated piglets. To this end, at different time points in the perinatal period the effects of GOS on the fermentation, microbiota composition, brush-border enzyme activity, histomorphology of the intestinal tract, the intestinal integrity and the intestinal defence mechanism were investigated.
Methods
Animals
All in vivo experimental protocols were approved by the Ethics Committee for Animal Experiments (reference number: DEC 2011.III.11.117) and were performed in compliance with governmental and international guidelines on animal experimentation.
The experiment was carried out with Landrace×Yorkshire piglets obtained from the faculty-owned farm. The piglets were naturally farrowed and stayed with the sow for 24–48 h postpartum to obtain maternal antibodies with the colostrum. Thereafter, forty piglets were selected from four litters and allocated to four experimental groups (ten piglets per group) with an equal distribution of littermates, weight and sex in each group. The selected piglets were transferred into a separate room with four individual pens, equipped with floor heating and additional IR lamps and nesting material. A 12 h light–12 h dark cycle was used.
Diets
After weaning, all piglets received a commercial milk replacer diet (Milkiwean babymilk Yoghurt; Trouw Nutrition) (online Supplementary Table S1). All piglets were offered the milk diets in large plates and started unassisted drinking within 6 h after weaning. Initially, the calculated daily ration (according to the product information) was presented in six feedings per d (approximately 600 ml/piglet per d) and increased gradually to 1600 ml/piglet per d offered at four feedings per d. Two groups received the milk replacer alone, and the other two groups received the same milk replacer supplemented with 0·8 % short-chain GOS, which is comparable to the amount of oligosaccharides added to infant formula( Reference Van Hoffen, Ruiter and Faber 18 ). GOS was obtained from FrieslandCampina Domo (Vivinal® GOS syrup, 75 % DM), which contain oligosaccharides with a degree of polymerisation (DP) of 2–8 with approximately 59 % (w/w) GOS, 21 % (w/w) lactose, 19 % (w/w) glucose and 1 % (w/w) galactose on DM. Drinking water was provided ad libitum.
Experimental design
From each group (controls and GOS-fed piglets), one subgroup of ten animals was killed at the age of approximately 4 d (3 d on the GOS-supplemented diet), whereas the other two subgroups (control and GOS-fed piglets) of ten animals were killed at the age of approximately 27 d (26 d on the GOS-supplemented diet). Weight of the piglets was registered at weaning (day 1) and twice a week during the entire experiment. At the end of the experimental periods, the piglets were individually anaesthetised with an intramuscular injection of 10 mg/kg ketamine (Narketan®; Vetoquinol) and 4 mg/kg azaperon (Stresnil®; Elanco Animal Health), followed by induction of euthanasia with an intra-cardiac injection of 200 mg/kg pentobarbital (Euthasol®; Virbac Animal Health). Blood was collected via cardiac puncture immediately after death.
Sampling procedures and analyses
Measurement of pH in the digesta
After euthanasia, the GIT was removed and the contents of the stomach, duodenum, jejunum, ileum, caecum and colon were collected by gently squeezing the digesta from the different parts. Immediately after sampling, the pH was recorded using pH-indicator strips (Merck), and the digesta samples were stored at −80°C.
Measurement of caecal SCFA and lactate
Caecum digesta was weighed and homogenised in PBS (100 mg digesta/ml) using a Precellys 24 tissue homogeniser (Bertin Technologies) five times for 10 s at 6000 rpm with a minimum of 5 min cooling period on ice in between. Samples were then centrifuged for 15 min at 14 000 rpm and the supernatant was collected and stored at −80°C.
The caecal SCFA levels of acetic acid, propionic acid, iso-butyric acid, butyric acid, iso-valeric acid and valeric acid were quantitatively determined as described previously( Reference Bakker-Zierikzee, Alles and Knol 19 ). The SCFA were captured using a Shimadzu GC2010 GC (Shimadzu Corporation) equipped with a flame ionisation detector. SCFA concentrations were determined using 2-ethylbutyric acid (Sigma Chemical Company) as an internal standard.
For lactate measurement, homogenised caecum digesta was thawed on ice and centrifuged for 5 min at 14 000 rpm, and 100 µl of the supernatant was heated for 10 min at 100°C to inactivate all enzymes. Lactate was determined enzymatically using an l-lactic acid detection kit with d- and l-lactate dehydrogenase (Boehringer Mannheim)( Reference Bakker-Zierikzee, Alles and Knol 19 ).
Characterisation of dietary oligosaccharides
Oligosaccharides were analysed in caecal digesta and faecal samples, of control- and GOS-fed piglets, after salt and monomer removal from the samples by solid-phase extraction. Capillary electrophoresis with laser-induced fluorescence detection was used to identify and quantify oligosaccharides, as described previously( Reference Albrecht, Schols and van Zoeren 20 ).
Microbiota analysis
Faecal samples were collected in sterile tubes at days 0, 12 and 26 of the intervention study, and stored directly at −80°C. As the animals were housed as a group to guarantee the social contact between the piglets, it was not possible to link all faecal samples to the corresponding piglet within a group. DNA extraction was performed as described previously( Reference Walton, van den Heuvel and Kosters 21 ). Briefly, 1 ml of a 10 % (w/v) faecal slurry in anaerobic PBS (0·1 mol/l, pH 7) was prepared, homogenised for 2 min and centrifuged for 5 min at 13 400 g . Bacterial DNA was isolated from the frozen pellet using a Qiagen stool kit according to the manufacturer’s instructions with an additional lysozyme (Sigma, 30 mg/ml Tris-EDTA)/metanolysin (Sigma, 1000 U/ml Tris-EDTA) step to aid breakdown of the cell walls. Quantitative PCR (qPCR) was conducted from an adapted method of Ritalahti et al. ( Reference Ritalahti, Amos and Sung 22 ). Briefly, 5 µl of DNA samples/standards was applied and 20 µl of mastermix solution (Applied Biosystems) was added including relevant primer sets and probes with 6-carboxyfluorescein as a reporter fluorophore on the 5' end, with dihydrocyclopyrroloindole tripeptide minor groove binder quencher on the 3' end( Reference Walton, van den Heuvel and Kosters 21 ). For total bacteria, SYBR Green mix (Applied Biosystems) was used. Samples were analysed using the AB 7700 sequence detector (Applied Biosystems) in conjunction with the Sequence Detector System software (Applied Biosystems). Total number of bacteria, bifidobacteria, lactobacilli, Clostridia, Bacteroides spp. and Escherichia coli (online Supplementary Table S1) were determined using qPCR( Reference Walton, van den Heuvel and Kosters 21 , Reference Nauta, van den Heuvel and Veurink 23 , Reference Nauta, van den Heuvel and Veurink 24 ). Results are expressed as log10 colony-forming unit (CFU)/g faeces.
Measurement of intestinal disaccharidase activities
After killing the animals on day 26, the entire intestine was removed and washed in saline after collecting the contents of the different segments (duodenum, jejunum, ileum, caecum and colon). The mucosa of each segment was scraped off with a microscope slide and frozen in liquid N2. After thawing, the mucosal scrapings were weighed, solubilised in cold PBS (200 mg/ml), homogenised with TissueLyser II (Qiagen) (homogeniser) and centrifuged for 10 min (3000 g ) to collect the supernatants. Protein concentrations of each supernatant were determined using a Pierce® BCA Protein Assay Kit (Thermo Scientific). The digestive enzymes lactase, sucrase and maltase were determined by the method of Dahlqvist( Reference Dahlqvist 25 ). Briefly, this procedure consists of measuring glucose levels of homogenised mucosal scrapings incubated with the specific substrate. The substrates lactose, sucrose and maltose were dissolved to prepare 56 mm solution in 0·1 m-sodium maleate buffer (pH 6·0). Glucose was used as a standard, and the glucose content released during the reaction is determined by the glucose oxidase method, which allows the calculation of specific enzyme activity expressed as units (U) of disaccharidase per mg protein. One unit is defined as the activity of a disaccharidase needed to hydrolyse 1 µmol of disaccharide/min.
Histomorphometric analysis of pig intestines
The small intestine parts (duodenum, jejunum and ileum) were fixed in 10 % neutral-buffered formalin, embedded in paraffin and 5-µm sections were cut and stained with haematoxylin–eosin according to standard methods. Photomicrographs were taken with an Olympus BX50 microscope (Olympus) equipped with a Leica DFC 320 digital camera (Leica). The morphometric analysis of the sections was performed on ten randomly selected, well-oriented villi and crypts per animal. A computerised microscope-based image analyser (Cell^D; Olympus) was used to determine histomorphometric parameters: villus height (measured from the tip of the villus to the villus–crypt junction), villus breadth top, villus breadth base, villus width (measured at the bottom of villi), crypt depth (measured from the crypt–villus junction to the base of the crypt), villus surface area (total surface of the villus) and epithelial cell area (villus surface area minus villus area without epithelial layer). These regions of interest were manually defined for each villi separately.
Quantitative RT-PCR analysis of intestinal tissue samples
For mRNA studies, the pig intestine was flushed with cold PBS and separated into different segments. These segments were defined as follows: duodenum, jejunum, ileum, caecum and colon. These whole intestinal wall samples (approximately 2–3 cm) were snap-frozen in liquid N2 and stored at −80°C for RNA isolation. A quantity of 50mg of each sample was suspended into 350 μl of RNA lysis buffer with β-mercaptoethanol and homogenised using a TissueLyser (Qiagen) for 1 min/25 Hz. Total RNA was isolated using spin columns according to the manufacturer’s instructions (Promega). Subsequently, 1 µg of extracted total RNA was reverse-transcribed with the iScript TM cDNA Synthesis kit (Bio-Rad Laboratories Inc.). The iQSYBR Green Supermix (Bio-Rad Laboratories Inc.) was used for the qRT-PCR according to the manufacturer’s instructions. qRT-PCR was performed using the MyIQ single-colour real-time PCR detection system (Bio-Rad Laboratories Inc.) and MyIQ System software version 1.0.410 (Bio-Rad Laboratories Inc.). The PCR cycle parameters were as follows: general denaturation at 95°C for 3 min, one cycle, followed by forty cycles of 95°C for 20 s, annealing temperature (AT) for 30 s and elongation at 72°C for 30 s. Primer sequences for claudin 1–4 (CLDN1–4), occludin (OCLN), zona occludens protein-1–2 (ZO-1–2), porcine β-defensin-1–3 (pBD-1–3), porcine epididymis protein 2 splicing variant C (pEP2C) and protegrins 1–5 (PG1–5) with corresponding AT are listed in online Supplementary Table S2 and were derived from the National Center for Biotechnology Information GenBank and were manufactured commercially (Eurogentec). Hypoxanthine phosphoribosyltransferase 1 (HPRT1) was used as the reference gene, as HPRT1 is a good reference gene for transcripts in different pig tissues( Reference Nygard, Jørgensen and Cirera 26 ). No significant effect of GOS treatment on the C t values of HPRT compared with the control animals was observed (data not shown).
Western blot analysis
Approximately 50 mg of intestinal samples (duodenum and colon) were lysed using 500 μl of RIPA lysis buffer (Thermo Scientific) with protease inhibitors (Roche Applied Science), and the total protein concentration was measured by the BCA protein assay kit (Thermo Scientific). Standardised protein amounts of boiled samples were isolated by electrophoresis (CriterionTM Gel, 4–20 % Tris-HCL; Bio-Rad Laboratories Inc.) and electro-transferred onto polyvinylidene difluoride membranes (Bio-Rad). Membranes were blocked with PBS supplemented with 0·05 % Tween-20 (PBST) and 5 % milk proteins and incubated overnight at 4°C with antibodies for OCLN (1:1000; Abcam), ZO-1 (1:1000; Invitrogen) or CLDN1 (1:1000; Invitrogen). After washing in PBST, the membranes were incubated with an appropriate horseradish peroxidase-conjugated secondary antibody (1:5000; Dako) for 2 h at room temperature. Finally, blots were washed in PBST, incubated with ECL Prime Western Blotting Detection Reagent (Amersham Biosciences) and digital images were obtained with the ChemiDoc MP imager (Bio-Rad Laboratories Inc.). Subsequently, the membranes were re-probed with a β-actin antibody (1:4000; Cell Signaling) to assess the equality of loading. Signal intensities were quantified using the ImageJ version 1.47 software (National institutes of Health), and the protein expression was normalised with β-actin and expressed as the mean fold change in relation to the control group.
Measurement of zona occludens protein-1 in serum samples
Serum was derived from blood (approximately 10 ml), harvested by centrifugation (15 min at 1500 g ) in BD vacutainer tubes (BD) and stored at –20°C. The ZO-1 levels in serum samples were measured by ELISA according to the manufacturer’s instructions (Elabscience).
Measurement of secretory IgA in saliva
Saliva was collected using Salivette tubes (Sarstedt) containing a synthetic swab. The sampled pigs were allowed to chew the swab before feeding time at days 0, 12, 15, 19, 22 and 26, until thoroughly moist. The swabs were then placed in test tubes and centrifuged at 3000 g for 10 min. The saliva samples were removed and stored at −20°C until analysis. The secretory IgA (sIgA) levels in the saliva samples were measured by ELISA according to the manufacturer’s instructions (MyBioSource.com).
Statistical analyses
Experimental results are expressed as means with their standard errors. Analyses were performed by using GraphPad Prism (version 6.01) (GraphPad Prism). Differences between groups were statistically determined by using an unpaired two-tailed Student’s t test or a two-way ANOVA with Bonferroni’s post hoc test. Microbiota data are displayed on a logarithmic scale. Results were considered statistically significant when P<0·05. Changes in relative mRNA expression between groups are described when an expression ratio of 2-fold or higher is observed( Reference Karlen, McNair and Perseguers 27 ).
Results
No effect of galacto-oligosaccharides on animal health and body weight
The piglets remained healthy during the experimental period, and no diarrhoea was observed in response to the dietary treatment. No significant increase in body weight was observed after 3 d of GOS diet (control: 10·4 (sem 2·0) % increase v. GOS: 11·1 (sem 1·3) % increase) or after 26 d of GOS diet (control: 341·8 (sem 16·5) % increase v. GOS: 345·4 (sem 11·9) % increase).
Galacto-oligosaccharides modulate the intestinal microbiota
The total microbial population in the faeces was quantified at days 0, 12 and 26 of the experiment (26-d experiment) using qPCR and converting the results into log10 CFU/g faeces (Fig. 1). Total bacterial counts remained almost constant, and no significant differences were observed between controls and GOS-supplemented diets (Fig. 1(a)). Next to total faecal counts, several bacterial groups including Bacteroides, lactobacilli, bifidobacteria, E. coli and Clostridia were quantified in the faeces. Bacteroides counts were increased on day 0 before the onset of GOS feeding and seem to reflect inter-individual variability (Fig. 1(b)). Lactobacilli were significantly increased in the GOS-diet group at day 26 (Fig. 1(c)), and a similar increase on day 26 was observed for Bifidobacterium spp. (Fig. 1(d)). In addition, the increase in bifidobacteria was not significantly different when using a primer set for bifidobacteria analysis in multiple species (data not shown). E. coli and Clostridia counts decreased over time (Fig. 1(e) and (f)), and Clostridia could not be detected at days 12 and 26. Although the number of E. coli bacteria tended to decrease in the GOS-treated animals at day 12, no significant differences between treatment groups could be detected.

Fig. 1 Galacto-oligosaccharides (GOS) modulate the intestinal microbiota. Faeces of control (![]() ) and GOS-fed piglets (
) and GOS-fed piglets (![]() ) were collected at days 0, 12 and 26, and the composition of the intestinal microbiota was determined by quantitative PCR as described in the Methods section. Animals per group (n 3–9). Bacterial numbers are expressed as log10 colony-forming units (CFU)/g faeces with their standard errors (two-way ANOVA with Bonferroni’s post hoc test; statistically significantly different from the corresponding control group: *P<0·05, **P<0·01, ***P<0·001).
) were collected at days 0, 12 and 26, and the composition of the intestinal microbiota was determined by quantitative PCR as described in the Methods section. Animals per group (n 3–9). Bacterial numbers are expressed as log10 colony-forming units (CFU)/g faeces with their standard errors (two-way ANOVA with Bonferroni’s post hoc test; statistically significantly different from the corresponding control group: *P<0·05, **P<0·01, ***P<0·001).
The pH in caecal digesta is significantly decreased after 26 d galacto-oligosaccharides in the diet
A decrease in pH value of the caecum digesta was observed in piglets fed the GOS diet for 3 d compared with the control (pH 6·1 (sem 0·27) v. pH 6·7 (sem 0·26), respectively) (Fig. 2(a)); however, this effect was not significant and less pronounced than the effect for the GOS diet after 26 d. Significantly lower pH values were observed in the caecum digesta of piglets fed the GOS diet for 26 d (pH 5·9 (sem 0·11)) as compared with piglets fed the control diet (pH 7·1 (sem 0·32)) (Fig. 2(b)). The pH in the content of the stomach, duodenum, jejunum, ileum and colon was not significantly affected by the experimental diet (Fig. 2(a) and (b)).

Fig. 2 Dietary galacto-oligosaccharides (GOS) lead to a pH decrease and an increase in butyric acid in caecum digesta. On days 3 (a, c) and 26 (b, d), the control (![]() ) and GOS (
) and GOS (![]() )-fed piglets were killed and the content of the stomach, duodenum, jejunum, ileum, caecum and colon was collected for pH analysis (a, c) and butyric acid measurement (b, d), as described in the Methods section. Animals per group (n 10). Values are expressed in pH value (pH analysis) or mmol/l (butyric acid measurement) as means, with their standard errors (unpaired two-tailed Student’s t test; statistically significantly different from the control group: *P<0·05, **P<0·01).
)-fed piglets were killed and the content of the stomach, duodenum, jejunum, ileum, caecum and colon was collected for pH analysis (a, c) and butyric acid measurement (b, d), as described in the Methods section. Animals per group (n 10). Values are expressed in pH value (pH analysis) or mmol/l (butyric acid measurement) as means, with their standard errors (unpaired two-tailed Student’s t test; statistically significantly different from the control group: *P<0·05, **P<0·01).
Dietary galacto-oligosaccharides lead to an increase in butyric acid in caecum digesta
Although the SCFA concentrations were not significantly affected by GOS after the 3-d exposure period, butyric acid tended to increase in caecum digesta (control: 0·90 (sem 0·13) mmol/l v. GOS: 1·22 (sem 0·25) mmol/l) (Fig. 2(c)). At 26 d, a significantly higher molar concentration of butyric acid was measured (control: 0·95 (sem 0·08 mmol/l) v. GOS: 1·28 (sem 0·10) mmol/l) (Fig. 2(d)), whereas no significant differences were observed in the concentrations of acetic acid, propionic acid and valeric acid in the caecum digesta after GOS diet for 26 d. Iso-butyric acid and iso-valeric acid were not detectable in the caecum digesta, and d- and l-lactate levels were below the detection limit (0·2 mmol/l) after 26 d of control or GOS diet, and no significant differences could be observed after 3 d of GOS (d-lactate; control: 0·579 (sem 0·136) mmol/l v. GOS: 0·523 (sem 0·135) mmol/l and l-lactate; control: 1·025 (sem 0·267) mmol/l v. GOS: 0·838 (sem 0·214) mmol/l).
No intact galacto-oligosaccharide structures are present in faecal samples from piglets fed a galacto-oligosaccharide diet
The presence of oligosaccharides derived from the GOS diets was investigated in the piglet caecal and faecal samples. A low abundance of oligosaccharides was detected in faecal samples of piglets fed a GOS diet for 3 d (Fig. 3). These oligosaccharides predominantly represent DP2 and DP3 and were not recognised as intact GOS structures. After 26 d of GOS diet, in caecal digesta samples a low abundance of oligosaccharides was detected as well, whereas in faecal samples hardly any oligosaccharides were present (Fig. 3). As expected, in the faecal samples of piglets fed the control diet for 3 or 26 d no quantifiable oligosaccharides were present (data not shown).

Fig. 3 No intact galacto-oligosaccharides (GOS) structures are present in faecal samples from piglets fed a GOS diet. Capillary electrophoresis with laser-induced fluorescence (LIF) detection profiles of vivinal GOS, and of oligosaccharides as detected in faecal samples of piglets fed a GOS diet for 3 and 26 d (f-day 3, and f-day 26), and in caecal digesta samples of piglet fed a GOS diet for 26 d (d-day 26). dp, Degree of polymerisation.
Galacto-oligosaccharides induce histomorphological changes in the intestine of piglets
Histomorphometric analysis of the small intestine (duodenum, jejunum and ileum) of piglets was performed after 3 and 26 d of GOS diet. Tables 1 and 2 show the results on the small intestinal histology (villus height, villus breadth top, villus breadth base, crypt depth, villus area, villus area without epithelial cell area, epithelial cell area) in relation to the type of diet and small intestinal segment. Already after 3 d, histomorphological differences were observed in the duodenum of the piglets fed a GOS diet as the villi were increased in height and the mucosa area was enlarged (Table 1).
Table 1 Intestinal morphology of piglets fed a control (CON) or galacto-oligosaccharides (GOS) diet for 3 dFootnote † (Mean values with their standard errors)

Statistically significant difference between the means of control and GOS-fed groups: *P<0·05, **P<0·01.
† Analysed by using an unpaired two-tailed Student’s t test.
Table 2 Intestinal morphology of piglets fed a control (CON) or galacto-oligosaccharides (GOS) diet for 26 dFootnote † (Mean values with their standard errors)

Statistically significant difference between the means of control and GOS-fed groups: *P<0·05, **P<0·01.
† Analysed by using an unpaired two-tailed Student’s t test.
The villi in the duodenum of piglets given the GOS diet for 26 d were proportionally thicker, as the villus width and the villus breadth base were significantly increased in the GOS-fed piglets (Table 2). In addition, the jejunum of piglets fed a GOS diet for 26 d showed thicker and larger villi, as an increase in villus height, villus breadth top and villus breadth base was measured (Table 2). No differences in ileum were observed after 3 or 26 d of GOS diet.
Galacto-oligosaccharides modulate the activity of disaccharidases
The disaccharidase activity increased with time in all segments of the intestines (Tables 3 and 4). GOS in the diet for 3 d did not significantly affect the low lactase and maltase activity levels in the intestinal mucosa, except for a slight increase in maltase activity in the colon of the GOS-fed group compared with the control piglets (Table 3). The jejunal mucosa of piglets at day 26 was associated with the highest lactase, sucrase and maltase activities, whereas the colon and caecum contained the lowest brush-border enzyme activity (Table 4). Duodenal and jejunal disaccharidase activities were similar between control piglets and piglets fed a GOS diet for 26 d, but ileal lactase, sucrase and maltase activity were lower in piglets fed a GOS diet compared with the controls (Table 4). Lactase and sucrase activity were not detectable in caecal and colonic mucosa after 26 d. The maltase activity in caecal mucosa was significantly increased in piglets fed a GOS diet for 26 d compared with control animals (Table 4).
Table 3 Intestinal disaccharidase activities at 3 dFootnote † (Mean values with their standard errors)
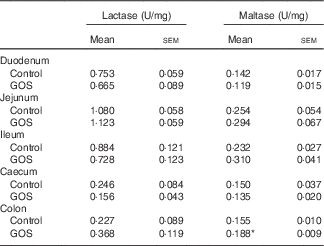
GOS, galacto-oligosaccharides.
*Statistically significant difference between the means of control and GOS-fed groups: P<0·05.
† Analysed by using an unpaired two-tailed Student’s t test.
Table 4 Intestinal disaccharidase activities at 26 dFootnote † (Mean values with their standard errors)
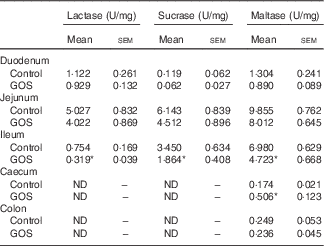
GOS, galacto-oligosaccharides; ND, not detectable.
*Statistically significant difference between the means of control and GOS-fed groups: P<0·05.
† Analysed by using an unpaired two-tailed Student’s t test.
The mRNA and protein expression of different tight junction proteins are increased by dietary galacto-oligosaccharides
The potential effect of GOS on the mRNA expression and protein levels of tight junction (TJ) proteins were measured by qRT-PCR, as well as by Western blot analysis. Interestingly, already after 3 d of GOS, different TJ proteins were up-regulated in different parts of the intestine (Fig. 4(a, c, e, g, i)). In the duodenum, jejunum, ileum and caecum of piglets fed a GOS diet enhanced mRNA levels of CLDN1 were detected, and ZO-1 and ZO-2 mRNA expression levels were increased in the caecum and colon after 3 d of GOS diet. No significant differences were detected at TJ protein level after 3 d of control or GOS diet (Fig. 5 and online Supplementary Fig. S1).

Fig. 4 The mRNA expression levels of different tight junction proteins are up-regulated by dietary galacto-oligosaccharides (GOS). Piglets received a control or GOS diet for 3 or 26 d, and samples from different parts of the intestine (duodenum (a, b), jejunum (c, d), ileum (e, f), caecum (g, h) and colon (i, j)) were collected and mRNA levels of tight junction proteins (claudin 1–4 (CLDN1–4), occludin (OCLN), zona occludens proteins 1–2 (ZO-1–2) were measured by quantitative RT-PCR. Animals per group (n 10). Results are expressed as relative mRNA expression (fold of control, normalised to hypoxanthine phosphoribosyltransferase 1; HPRT) as means, with their standard errors. The horizontal line represents the control group.

Fig. 5 The protein levels of different tight junction proteins are increased by dietary galacto-oligosaccharides (GOS). Piglets received a control (![]() ) or GOS (
) or GOS (![]() ) diet for 3 or 26 d, and samples from different parts of the intestine (duodenum (a, b, c) and colon (d, e, f)) were collected and tight junction proteins levels (claudin (CLDN1), occludin (OCLN) and zona occludens protein (ZO-1)) were measured by Western blot analysis. Animals per group (n 4). Results are expressed as relative protein expression (optical density normalised with β-actin) as means, with their standard errors (two-way ANOVA with Bonferroni’s post hoc test; statistically significant difference: *P<0·05).
) diet for 3 or 26 d, and samples from different parts of the intestine (duodenum (a, b, c) and colon (d, e, f)) were collected and tight junction proteins levels (claudin (CLDN1), occludin (OCLN) and zona occludens protein (ZO-1)) were measured by Western blot analysis. Animals per group (n 4). Results are expressed as relative protein expression (optical density normalised with β-actin) as means, with their standard errors (two-way ANOVA with Bonferroni’s post hoc test; statistically significant difference: *P<0·05).
GOS supplemented to the diet for 26 d enhanced the mRNA levels for OCLN, ZO-1 and ZO-2 in the duodenum, whereas in the jejunum, caecum and colon the mRNA levels of only CLDN1 were up-regulated (Fig. 4(b, d, f, h, j)). Furthermore, the OCLN protein expression was significantly increased in the colon of GOS-fed piglets compared with control piglets at 26 d (Fig. 5(e)), but the CLDN1 and ZO-1 protein expression did not significantly differ between control and GOS-fed piglets (Fig. 5(a, d, c, f) and online Supplementary Fig. S1). GOS did not remarkably affect the mRNA expression of CLDN2–4 in different parts of the intestine. Besides diet effects, an increase in OCLN and ZO-1 protein expression was observed in the duodenum of GOS-fed animals at 26 d compared with the GOS-fed piglets at 3 d (Fig. 5(b) and (c)). In addition, no differences were detected in ZO-1 serum levels between the control and GOS-fed piglets (online Supplementary Fig. S2). However, the ZO-1 serum levels were significantly decreased in the piglets from the 26-d study compared with the piglets used in the 3-d study.
Defensin porcine β-defensin-2 mRNA expression levels are increased by dietary galacto-oligosaccharides
mRNA levels of defensins, antimicrobial peptides secreted by the colonic epithelium, were measured in the colon of piglets fed the GOS diet for 3 and 26 d compared with piglets fed the control diet (Fig. 6). The mRNA expression of pBD-2 was increased in the colon of piglets fed a GOS diet for 3 d (Fig. 6(a)) compared with the control piglets, but this effect was absent in the piglets fed a GOS diet for 26 d (Fig. 6(b)). No differences were detected in the mRNA expression of the other defensins, pBD-1, pBD-3, pEP2C and PG1–5 between GOS-treated and control animals.

Fig. 6 Defensin porcine β-defensin (pBD)-2 mRNA expression levels are increased by dietary galacto-oligosaccharides (GOS). Piglets received a control or GOS diet for 3 or 26 d, and samples from the colon were collected and mRNA levels of defensins (pBD-1, pBD-2, pBD-3, porcine epididymis protein 2 splicing variant C (pEP2C) and protegrins 1–5 (PG1–5)) were measured by quantitative RT-PCR. Animals per group (n 10). Results are expressed as relative mRNA expression (fold of control, normalised to hypoxanthine phosphoribosyltransferase 1; HPRT) as means, with their standard errors. The horizontal line represents the control group.
Secretory IgA levels in saliva are increased by dietary galacto-oligosaccharides
The effects of GOS on the mucosal immune system were investigated by measuring sIgA in saliva. At days 19, 22 and 26, the addition of GOS in the diet significantly increased the salivary IgA levels (39·0, 20·1 and 25·2 % increase, respectively) compared with the piglets fed a control diet (Fig. 7). At the earlier time points (0, 12 and 15 d), the salivary concentrations of IgA remained unaffected by GOS (Fig. 7).

Fig. 7 Secretory IgA (sIgA) levels in saliva are increased by dietary galacto-oligosaccharides (GOS). Saliva of control (![]() ) and GOS (
) and GOS (![]() )-fed piglets was collected at different time points at days 0, 12, 15, 19, 22 and 26, and the sIgA levels were measured by ELISA. Animals per group (n 8−10). Values are expressed in µg/ml as means, with their standard errors (two-way ANOVA with Bonferroni’s post hoc test; statistically significantly different from the control group: **P<0·01, ***P<0·001).
)-fed piglets was collected at different time points at days 0, 12, 15, 19, 22 and 26, and the sIgA levels were measured by ELISA. Animals per group (n 8−10). Values are expressed in µg/ml as means, with their standard errors (two-way ANOVA with Bonferroni’s post hoc test; statistically significantly different from the control group: **P<0·01, ***P<0·001).
Discussion
It is known that piglets share many characteristics with human infants in the perinatal development of their intestinal tract( Reference Calder, Krauss-etschmann and De 17 ). Hence, one of the main objectives of this study was to establish a model with neonatal piglets suitable for studying the effects of early life dietary interventions on intestinal development and function. In the current study, two different diets were compared: a commercial milk formula for piglets with or without GOS. The selection of GOS as model compounds was based on the fact that these oligosaccharides are currently the most widely used oligosaccharides in infant formulas and are known to have gut-modulating properties( Reference Tzortzis and Vulevic 28 ).
A major difference between piglets and human newborns is the level of protection by maternal antibodies at birth. Human newborns are protected in the first period of life by placentally transferred maternal antibodies( Reference Palmeira, Quinello and Silveira-Lessa 29 ), whereas newborn piglets are fully dependent on acquiring maternal antibodies from passive immunisation via colostrum because of the poor passage rate of antibodies through the placenta( Reference Sangild 30 ). Therefore, in this in vivo model, the piglets stayed with the sow to obtain colostrum during the first 24–48 h. This implies, however, that the primary colonisation of the intestinal flora followed a natural pattern for piglets, and differences in composition of the microflora because of dietary oligosaccharides might be less pronounced when compared with infants.
Related to the early suckling period, the composition of the intestinal microbiota varied already between animals after 24–48 h of life, before the onset of the formula feeding (day 0). This was expected as the piglets originated from different sows and therefore the micro-environment during the immediate postnatal period was slightly different. Data by Thompson et al. ( Reference Thompson, Wang and Holmes 31 ) showed a high level of individuality in 1- and 2-week-old piglets, suggesting a considerable randomness to the process of acquiring microbes. This rapid colonisation of the intestinal tract is also reflected by the finding that bacterial quantities in the faeces at day 0 were already comparable with the numbers at days 12 and 26. Previous investigations already indicated that within 12 h after birth bacterial densities could stabilise at 109−1010 bacteria/g colonic content of the pig and within 48 h 90 % of the microflora can be constituted( Reference Swords, Wu and Champlin 32 ). It is also known that the bacterial colonisation in the intestine of breast-fed infants is stabilised within the first few days of life( Reference Fanaro, Chierici and Guerrini 33 ). Differences between the two diets, composed of a standard pig formula and the same formula supplemented with GOS, showed particularly differences in the prevalence of lactobacilli and bifidobacteria, which is in line with clinical trials including human infants( Reference Fanaro, Marten and Bagna 9 ).
The high bacteria density present at the first stages of life, in addition to increased bifidobacterial numbers in the intestine of the GOS-fed piglets, may explain the low abundance of intact GOS structures in caecal samples, as well as their absence in the faeces of GOS-fed piglets. GOS might have a high stability in the small intestines, as the presence of GOS in serum and urine demonstrates their selective adsorption in the porcine intestinal tract (E Difilippo, M Bettonvil, HAM Willems, S Braber, J Fink-Gremmels, PV Jeurink, MHC Schoterman, H Gruppen and HA Schols, unpublished results).
Results from clinical studies indicated that GOS is fermented by the bacterial flora in the human colon( Reference Sako, Matsumoto and Tanaka 34 ). De Leoz et al.( Reference De Leoz, Wu and Strum 35 ) suggested that in premature human infants at least a portion of the GOS passes through the intestinal tract undigested by the intestinal microbiota. On the other hand, an in vitro study conducted in a fermentation screening platform showed that adult human inocula were able to ferment a broader variety of dietary fibres than pig inocula( Reference Jonathan, Van Den Borne and Van Wiechen 36 ). In vitro, GOS was fermented by adult human microbiota, thereby producing acetate, propionate, butyrate, succinate and lactate( Reference Ladirat, Schuren and Schoterman 37 ). Clinical studies with humans showed elevated levels of acetate, butyrate and lactate in faecal samples after oligosaccharides diets, which was associated with acidic faecal pH values( Reference Bakker-Zierikzee, Alles and Knol 38 – Reference Van Dokkum, Wezendonk and Srikumar 40 ). Walton et al. ( Reference Walton, van den Heuvel and Kosters 21 ) concluded that the elevated bifidobacteria numbers account for the increased butyrate production observed after GOS supplementation. Higher butyric acid production was also observed in the faeces of formula (including GOS and fructo-oligosaccharides (FOS))-fed infants compared with breast-fed infants( Reference Holscher, Faust and Czerkies 39 ). In the current study, caecal acetic acid, propionic acid, valeric acid and lactate concentrations did not differ significantly between the control and GOS-fed piglets, but the concentration of butyric acid was significantly increased. As the overall levels of butyric acid remained low, butyric acid might not be the only factor that contributes to lower the caecal pH values in the GOS-fed piglets. Butyric acid has been shown to prevent the colonisation of various pathogens such as E. coli ( Reference Campbell, Fahey and Wolf 41 ), and dietary butyrates are described to inhibit inflammation and to improve the gut barrier function( Reference Hamer, Jonkers and Venema 42 ).
Besides effects on the large intestine, GOS can also exert positive effects on the gut morphology in the small intestine. The pattern of porcine small intestinal development is comparable to that reported for the human small intestine, in contrast to for example rodents, and the villus development in pigs occurs at similar relative times in gestation when compared with humans( Reference Calder, Krauss-etschmann and De 17 , Reference Dekaney, Bazer and Jaeger 43 ). The increased villus surface area and villus:crypt ratio in the duodenum induced by a GOS diet already within 3 d and the increased villus surface area in duodenum and jejunum after 26 d GOS can be related to improved utilisation of nutrients( Reference Pluske, Thompson and Atwood 44 ). In humans and pigs, very little is known about the effects of oligosaccharides on gut morphology early in life, and the exact mechanism for the changes in villus surface is still unclear. Previous investigations on ileal histomorphology demonstrated that villus height was increased in neonatal piglets receiving formula+GOS via enteral feeding compared with parenterally fed piglets, but no differences were found when compared with enterally fed piglets given formula alone( Reference Monaco, Kashtanov and Wang 45 ). FOS treatment increased ileal villus length compared with control in a neonatal intestinal failure piglet model( Reference Barnes, Hartmann and Holst 11 ), whereas dietary supplementation of chito- and cello-oligosaccharide increased the villus height in the small intestine of weaning pigs( Reference Liu, Piao and Kim 46 , Reference Jiao, Song and Ke 47 ). The presence of GOS might prevent villus shortening occuring after early weaning in pigs( Reference Lallès, Bosi and Smidt 48 , Reference Walthall, Cappon and Hurtt 49 ), rather than increasing the villus surface area. Tsukahara et al.( Reference Tsukahara, Inoue and Nakatani 50 ) found a positive correlation between villus height and disaccharidase activity in the small intestine of piglets. These digestive enzymes are located in the brush-border membrane and facilitate nutrient utilisation. Both in newborn humans and piglets lactase activity is elevated during birth( Reference Walthall, Cappon and Hurtt 49 ), but pigs differ from humans by being born with low activities of disaccharidases that are necessary for hydrolysis of non-lactose carbohydrates( Reference Buddington, Sangild and Hance 51 ). Indeed, we observed low maltase activity levels, whereas the sucrase activity was not even measurable at day 3. In humans, the absolute disaccharidase activity levels tend to be higher in human jejunum and ileum compared with the duodenum( Reference Townley 52 ), which was also observed in our piglet study at 26 d. Although the maltase levels were low in the caecum at day 26, the increased maltase levels in the GOS group might convey an advantage for the post-weaning adaptation to diets for growing piglets, as these diets are mainly composed of starch. As lactose was mainly present in the commercial milk replacer, the reduction in ileal brush-border enzyme activity observed in piglets fed a GOS diet for 26 d may result in an increased passage and availability of lactose to the large intestine, resulting in an increased risk for osmotic diarrhoea( Reference Lackeyram, Mine and Widowski 53 ). Diarrhoea was not observed in our study, in which a dosage of 0·8 % GOS was used. These undesirable effects are likely to be dose-dependent, as diarrhoea and flatulence were observed in adults only after consumption of more than 15 g of prebiotics/d( Reference Macfarlane, Steed and Macfarlane 54 ). In addition to nutrient digestion and absorption, the gastrointestinal epithelium also serves as a physical barrier against potentially harmful stressors that enter the intestinal tract, including bacteria, toxins and viruses, as well as undesirable substances in nutrients. Therefore, intestinal epithelial cells are connected by TJ, which seal the apical intercellular space( Reference Suzuki 55 ). To our knowledge, no information about TJ expression patterns in human neonates is available, as clinical studies mainly focus on increased intestinal permeability and abnormal expression of TJ proteins related to the pathogenesis of various gastrointestinal diseases, such as inflammatory bowel disease( Reference Odenwald and Turner 56 ). The intestinal barrier function and the TJ network in the pig intestine is mainly investigated around weaning time, when the intestinal barrier is challenged by rapid feed changes and infections( Reference Wang, Zhang and Wu 57 ). We studied here the intestinal TJ patterns in healthy piglets and showed a slight up-regulation of the mRNA expression of different TJ proteins in the intestines of GOS-fed piglets, which was not accompanied by an increase in protein expression, except an increased OCLN protein expression in the colon by dietary GOS given for 26 d. In neonatal mammals, the gastrointestinal permeability is known to be enhanced during the first few days( Reference Skrzypek, Valverde Piedra and Skrzypek 58 , Reference Beach, Menzies and Clayden 59 ), which is in line with our data, as OCLN and ZO-1 protein levels were lower in the duodenum from GOS-fed piglets at day 3 compared with day 26. The higher serum levels of ZO-1 after 3 d in piglets fed a control or GOS diet may be considered as a marker of the incomplete barrier functions. This hypothesis is enforced by lower ZO-1 serum levels at the 26-d measurements, where a higher degree of maturity is achieved. Previously, we could demonstrate in an in vitro model with Caco-2 cells that GOS also directly protect the intestinal barrier integrity by stimulating the TJ assembly( Reference Akbari, Braber and Alizadeh 60 ), and in addition another in vitro study suggests that fermentation products of prebiotics, such as butyrate, are able to prevent disruption of the intestinal epithelial barrier( Reference Peng, He and Chen 61 ). Cani et al. ( Reference Cani, Possemiers and Van de Wiele 62 ) proposed that a selective gut microbiota modulation by prebiotics consequently improves gut barrier function, including improved TJ, by a glucagon-like peptide-2-dependent mechanism.
Next to the mechanical barrier, endogenous antimicrobial peptides, such as defensis, protect the animal from invading pathogens. Defensins are already expressed during pregnancy in the fetal gut, which indicates their important role in the innate immune competence during early life( Reference Kai-Larsen, Gudmundsson and Agerberth 63 ). Lactobacilli improve gut barrier function in vitro via induction of defensins( Reference Schlee, Harder and Köten 64 ).The increase in pBD-2 as measured in the colon of piglets fed a GOS diet for 3 d might therefore have a protective effect as well, contributing to the suppression of microbial infections or bacterial outgrowth, as suggested in previous experiments showing a concentration-dependent inhibition of the growth of bacteria following the application of synthetic pBD-2 in pigs( Reference Veldhuizen, Rijnders and Claassen 65 ). Another component of the innate immune system in the intestine is sIgA, which binds harmful antigens at mucosal surfaces and neutralises toxins and virulence factors( Reference Brandtzaeg 66 ). Salivary IgA is mainly produced by the mucosa associated lymphoid tissue (MALT), and its concentration reflects MALT activity( Reference Erickson and Hubbard 67 ). Different studies indicate a strong link between the colonisation of the intestines, the development of the mucosal immune system and the production of endogenous sIgA( Reference Inoue and Ushida 68 , Reference Inoue, Tsukahara and Nakanishi 69 ). In infants, especially bifidobacteria seems to be important for the synthesis of endogenous sIgA( Reference Moreau and Gaboriau-Routhiau 70 ), probably by affecting the development of the IgA-producing plasmablasts( Reference Cebra 71 ). Monitoring of salivary IgA showed increased levels in GOS-fed piglets on days 19, 22 and 26, which can be considered as an improvement of the mucosal immune system. Promoting the abundance of specific intestinal bifidobacteria by dietary GOS as observed in our study might contribute to this elevated sIgA concentration in the saliva. However, a direct effect on the microbiome in the oral cavity could not be entirely excluded. Higher faecal sIgA concentrations were also observed in infants fed formula containing the prebiotic mixture GOS/FOS( Reference Scholtens, Alliet and Raes 72 ). As formula-fed infants lack the transfer of protective maternal sIgA and antibacterial peptides from breast milk( Reference Walker and Iyengar 73 ), they would potentially benefit from dietary ingredients that support the production of endogenous sIgA and β-defensins.
In conclusion, although there are some physiological differences in the perinatal development of their intestinal tract between neonatal piglets and human newborns, the results of this study qualify the piglet as a valid model in paediatric research for the assessment of dietary intervention strategies. Assessment of a number of key parameters shows that readily fermentable GOS stimulates the development of the intestinal microbiota, improves the intestinal architecture and seems to modulate barrier integrity and parameters of the innate immune system. The current experiments were conducted with healthy animals, and no challenge models were included. Further studies should aim to demonstrate differences in the intestinal integrity and immune competence of GOS-fed animals in the presence of infectious or chemical stressors relevant in the early phase of life in animals and humans.
Acknowledgements
The authors are grateful to all CCC members for the discussions and feedback during the meetings. The authors thank Gerrit Witte (Nutricia Research) for technical assistance during SCFA measurement, and Helmie van Dijck and Arjen Nauta (FrieslandCampina) for microbiota analyses and discussions on results. The authors thank M. A. M. Oosterveer-van der Doelen and J. L. de Nijs-Tjon for their technical assistance.
This project is jointly financed by the European Union, European Regional Development Fund and The Ministry of Economic Affairs, Agriculture and Innovation, Peaks in the Delta, the Municipality of Groningen, the Provinces of Groningen, Fryslân and Drenthe, the Dutch Carbohydrate Competence Center (CCC WP25; www.cccresearch.nl), Nutricia Research and FrieslandCampina. E. D. and S. B. were granted by the CCC programme, as indicated in acknowledgements; J. G. is associated with Nutricia Research and L. H. U. and M. H. C. S. with FrieslandCampina, respectively, which are both industrial partners in the Dutch CCC.
The authors’ contributions are as follows: A. A., P. A., E. D. and S. B. conducted the research, the acquisition and analyses of the data. S. B. wrote the manuscript and designed the experiments. J. G., H. A. S., L. H. U. and M. H. C. S. interpreted the data and critically revised the manuscript. J. F.-G. conceived the idea and revised the manuscript critically. All authors read and approved the final manuscript.
A. A., P. A., H. A. S. and J. F.-G. have no conflicts of interest to declare.
Supplementary material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114515004997














