Phyto-oestrogens have been postulated to lower the risk of colorectal cancer on the basis of the fact that the incidence of colorectal cancer is lower in populations with a high intake of phyto-oestrogens( Reference Ferlay, Ervik and Dikshit 1 , Reference Pampaloni, Bartolini and Tonelli 2 ). Phyto-oestrogens are plant-derived heterocyclic phenols and have similar structural features as endogenous oestrogens, which enable them to bind oestrogen receptors( Reference Kuiper, Carlsson and Grandien 3 , Reference Kuiper, Lemmen and Carlsson 4 ). The activation of oestrogen receptors by phyto-oestrogens may induce the transcription of genes involved in angiogenesis, cellular adhesion, proliferation and apoptosis( Reference Barzi, Lenz and Labonte 5 ). These altered transcriptional activities are likely to be involved in the anticarcinogenic effects against colorectal cancer, but the exact protective mechanisms remain to be elucidated. There are three principal groups of phyto-oestrogens: isoflavones, lignans and coumestans( Reference Cornwell, Cohick and Raskin 6 , Reference Thompson, Boucher and Liu 7 ). Isoflavones, mainly found in soyabeans, are the most common phyto-oestrogens in Asian populations, as soya foods are regularly consumed in Asian countries. In Western populations, the predominant source of phyto-oestrogens are lignans, which are present in seeds, grains, vegetables, fruits and animal products( Reference Kuhnle, Dell’Aquila and Aspinall 8 ). Coumestans account for <5 % of total phyto-oestrogen intake( Reference Zamora-Ros, Knaze and Lujan-Barroso 9 ), and are mainly found in soyabean sprout and spinach. To become biologically active, the inactive plant-lignan precursors must be metabolised by the gut microflora into mammalian lignans – namely, enterodiol and enterolactone( Reference Valentin-Blasini, Sadowski and Walden 10 , Reference Rowland, Faughnan and Hoey 11 ). Isoflavones are more bioactive than lignans, especially in the form of aglycone from soyabean-fermented products, which can be absorbed directly by human intestines( Reference Zubik and Meydani 12 ).
Earlier epidemiological studies have predominantly studied soya consumption in relation to colorectal cancer( Reference Yan, Spitznagel and Bosland 13 , Reference Woo and Kim 14 ). More recent studies have specifically assessed the dietary intake of isoflavones, the bioactive constituent in soya. These studies reported inconsistent associations between isoflavone exposure and colorectal cancer risk, including inverse associations( Reference Akhter, Iwasaki and Yamaji 15 – Reference Shin, Lee and Lee 19 ), positive associations( Reference Zamora-Ros, Not and Guinó 20 ) as well as null associations( Reference Yang, Shu and Li 21 – Reference Hedelin, Lof and Sandin 28 ). A recent meta-analysis found that high isoflavone intake was associated with a 24 % lower risk of colorectal cancer( Reference Tse and Eslick 29 ). However, no published meta-analyses to date have assessed the association of colorectal cancer risk with other phyto-oestrogen subtypes and their potential dose–response relationships.
The aims of this meta-analysis were therefore to summarise the current evidence on exposure to phyto-oestrogens in relation to the risk of colorectal cancer and adenoma and to provide a comprehensive review of the associations found with the two main classes of phyto-oestrogens – isoflavones and lignans. We further conducted a dose–response analysis and explored sources of heterogeneity among studies.
Methods
Search strategy
This study was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement( Reference Moher, Liberati and Tetzlaff 30 ). We carried out a literature search in MEDLINE (from 1975), EMBASE (from 1984) and Cochrane Library (from 1993) databases up to June 2016 without language restrictions to identify observational studies (case–control and cohort studies) that examined the association between phyto-oestrogens and risk of colorectal, colon or rectal neoplasms (cancer or adenoma). The following terms were used as search strategy: ‘(colorectal OR colon OR rectum) AND (cancer OR tumor OR neoplasm OR malignancy OR adenoma) AND (phytoestrogen OR isoflavone OR lignan)’. The detailed search strategies are available in the online Supplementary Material. Furthermore, we manually reviewed references of relevant articles to identify additional articles. There are no clinical trials on this topic.
Study selection
After removal of duplicate articles across databases, two independent investigators (R. J. and A. B.) conducted an initial screening on titles and abstracts to remove clearly irrelevant publications such as editorials, reviews, experimental studies or those not reporting relevant exposures or outcomes. Full-texts of potential publications were then reviewed by the same investigators to identify eligible studies, which reported relative risks (RR) (hazard ratio, RR or odds ratio) for the association between risk of colorectal cancer or adenoma and phyto-oestrogens, measured as either dietary consumption or circulating biomarkers.
Data extraction
Two investigators (R. J. and A. B.) independently reviewed the eligible studies and extracted data, and accuracy was checked thereafter. The following data were extracted from the identified publications: author, year of publication, region of the study, study period, sex and age of participants, study type, number of cases and non-cases, method of exposure assessment, type of phyto-oestrogens, exposure level, outcome ascertainment, covariates in the fully adjusted models, and RR and corresponding 95 % CI for the most fully adjusted models. The quality of each study was assessed using the Newcastle–Ottawa scale (NOS) based on participant selection, exposure and outcome ascertainment, as well as potential confounding( Reference Wells, Shea, O’Connell and Peterson 31 ) (online Supplementary Material). The NOS ranged from 0 to a maximum of 9, and a higher score is indicative of higher study quality. For studies with missing information, attempts were made to contact the corresponding author of the original studies for further information.
Statistical analysis
Meta-analyses were conducted for overall phyto-oestrogens (isoflavones and lignans combined) and separately for isoflavones and lignans. We used the RR from the highest v. the lowest category of exposure in the meta-analysis, as exposure assessment methods and categorisation differed among studies. In the meta-analysis for overall phyto-oestrogens, when a study reported RR for the specific type(s) of phyto-oestrogens, but not for overall phyto-oestrogens, we used the RR for the only reported subtype (isoflavones or lignans) or the most commonly consumed subtype in that population. Therefore, for a Swedish study that reported estimates for dietary isoflavones and lignans separately, only the RR for lignans, the major consumed phyto-oestrogen, were included( Reference Hedelin, Lof and Sandin 28 ). Combined RR and 95 % CI were computed in the meta-analyses using a random-effect model when between-study heterogeneity was observed (P<0·1), whereas the fixed-effect model was used otherwise. All analyses were conducted both for colorectal cancer and for colorectal neoplasms and separately for cohort (including nested case–control and case–cohort studies) and case–control studies.
Dose–response analysis was carried out for dietary isoflavones intake, lignans intake and circulating enterolactone concentrations only, because of limited studies on circulating isoflavone concentrations( Reference Ward, Chapelais and Kuhnle 27 ). We used the method proposed by Greenland & Longnecker( Reference Greenland and Longnecker 32 ) under random-effect model.
For the dose–response analysis of isoflavones and lignans, units were transformed to mg/d for all eligible studies. For the Spanish study( Reference Zamora-Ros, Not and Guinó 20 ), reported intakes (mg/4184 kJ (mg/1000 kcal)) were multiplied by 2 to account for the average energy intake of 8368 kJ/d (2000 kcal/d) in the study population. For the Swedish study( Reference Hedelin, Lof and Sandin 28 ), reported intakes µg/(d×MJ) were multiplied by 6·5 to account for the average energy intake of 6500 kJ/d in the study population. To derive a dose–response trend for each study, the distribution of cases and non-cases, RR and corresponding CI as well as cut-off values for at least three categories were required. If RR were available only for male or female separately, separate dose–response curves were derived and combined using fixed-effect models. Median or mean levels of isoflavones intake for each category were assigned to the corresponding RR. When these were not reported, the midpoint of the upper and lower boundaries was used. For studies with an unbounded highest category, we assumed the width of that category to be the same as its closest adjacent category. For studies that used an unbounded lowest category, the lower boundary was set to 0 mg/d. As isoflavones intake varies greatly between Asian and Western populations, dose–response analyses were conducted separately for Asian and Western studies. A potential, non-linear dose–response relationship was examined by using restricted cubic splines with three fixed percentiles (10, 50 and 90 %) and testing whether the coefficient of the second spline equals 0( Reference Orsini, Li and Wolk 33 , Reference Harrell, Lee and Pollock 34 ). Given the scarce number of studies available, case–control and cohort studies were combined for the dose–response analysis of dietary isoflavones as well as lignans. However, analysis according to study design was performed, where possible.
For the dose–response analysis of the biomarker enterolactone, we converted all RR corresponding to per doubling in enterolactone concentration after applying a log2 transformation to the exposure level. For the two Dutch studies( Reference Kuijsten, Arts and Hollman 35 , Reference Kuijsten, Hollman and Boshuizen 36 ), which used log10 transformation and an increment of log10 14·61 and log10 39·1, we consequently adjusted the RR and corresponding CI to the power of 0·26 (log102/log1014·61) and 0·19 (log102/log1039·1).
Heterogeneity was evaluated by Cochran’s Q test, and the proportion of total variation due to heterogeneity was quantified by I 2 ( Reference Higgins and Thompson 37 ). For the Cochran’s Q test, we defined statistical significance as P<0·1 rather than the conventional level of P<0·05, because of the low power of this test( Reference Hedges and Pigott 38 ). To explore heterogeneity across the studies, we performed a sensitivity analysis for overall phyto-oestrogen meta-analysis by leaving one study out at a time and calculated the pooled RR in the remaining studies. We further conducted subgroup analysis by phyto-oestrogen subtype, study design, ethnicity, cancer site and sex to explore the sources of heterogeneity. To assess possible bias due to confounding, we conducted pooled analyses stratified by adjustment factors, including BMI, physical activity, total energy intake, family history of colorectal cancer and non-steroidal anti-inflammatory drug (NSAID) use. Egger’s regression test and funnel plots were used to evaluate small study effects( Reference Egger, Davey Smith and Schneider 39 ). Apart from what was specified, the significance level was set to 0·05 in all the analyses. All analyses were carried out using the metaphor( Reference Viechtbauer 40 ) and dosresmeta( Reference Crippa and Orsini 41 ) packages in R environment (version 3.1.2).
Results
Literature search
We identified twenty-three potential publications of twenty studies for full-text assessment( Reference Akhter, Iwasaki and Yamaji 15 – Reference Hedelin, Lof and Sandin 28 , Reference Kuijsten, Arts and Hollman 35 , Reference Kuijsten, Hollman and Boshuizen 36 , Reference Cutler, Nettleton and Ross 42 – Reference Butler, Koh and Wang 48 ), of which four( Reference Honma, Yamamoto and Ohnaka 43 , Reference Li, Zhang and Holman 45 , Reference Rossi, Bosetti and Negri 46 , Reference Butler, Koh and Wang 48 ) were excluded and two could only be included for narrative review( Reference Ward, Kuhnle and Mulligan 22 , Reference Cutler, Nettleton and Ross 42 ) (Fig. 1). Two of the excluded studies did not provide RR for relevant exposures( Reference Honma, Yamamoto and Ohnaka 43 , Reference Li, Zhang and Holman 45 ), one was a duplicate report( Reference Rossi, Bosetti and Negri 46 ) of an included study( Reference Rossi, Negri and Talamini 18 ) and the other one was a recent conference abstract( Reference Butler, Koh and Wang 48 ) of an included study( Reference Butler, Wang and Koh 24 ). The two studies( Reference Ward, Kuhnle and Mulligan 22 , Reference Ward, Chapelais and Kuhnle 27 , Reference Cutler, Nettleton and Ross 42 ) that could be reviewed narratively found null associations between phyto-oestrogens intake( Reference Ward, Kuhnle and Mulligan 22 ), isoflavones intake( Reference Ward, Kuhnle and Mulligan 22 , Reference Cutler, Nettleton and Ross 42 ) or isoflavone concentration( Reference Ward, Chapelais and Kuhnle 27 ) and colorectal cancer risk. Enterolignans intake was found to be associated with a reduced risk of colorectal cancer in females but not in males( Reference Ward, Kuhnle and Mulligan 22 ). Among these two studies that could not be included, one study did not provide a RR( Reference Cutler, Nettleton and Ross 42 ) and the other study reported a RR per doubling of phyto-oestrogen, isoflavones and enterolignans intake( Reference Ward, Kuhnle and Mulligan 22 ) and isoflavones concentration( Reference Ward, Chapelais and Kuhnle 27 ), which could not be transformed to highest v. lowest RR because the distribution of phyto-oestrogen intake or isoflavones concentration was skewed. Therefore, seventeen publications were selected for the meta-analysis.

Fig. 1 Flow chart of publication selection.
Study characteristics
The characteristics of the seventeen studies included in the meta-analysis are shown in Table 1, among which there are eight prospective studies (including five cohort studies( Reference Yang, Shu and Li 21 , Reference Oba, Nagata and Shimizu 23 , Reference Butler, Wang and Koh 24 , Reference Akhter, Inoue and Kurahashi 26 , Reference Hedelin, Lof and Sandin 28 ), two nested case–control studies( Reference Ward, Chapelais and Kuhnle 27 , Reference Kuijsten, Hollman and Boshuizen 36 ) and one case–cohort study( Reference Johnsen, Olsen and Thomsen 44 )) and nine case–control studies( Reference Akhter, Iwasaki and Yamaji 15 – Reference Zamora-Ros, Not and Guinó 20 , Reference Budhathoki, Joshi and Ohnaka 25 , Reference Kuijsten, Arts and Hollman 35 , Reference Theodoratou, Kyle and Cetnarskyj 47 ). RR were reported for colorectal or colon cancer in fifteen studies and colorectal adenomas in two studies( Reference Akhter, Iwasaki and Yamaji 15 , Reference Kuijsten, Arts and Hollman 35 ). Nine studies were conducted in Europe( Reference Ravasco, Monteiro-Grillo and Marques Vidal 17 , Reference Rossi, Negri and Talamini 18 , Reference Zamora-Ros, Not and Guinó 20 , Reference Ward, Chapelais and Kuhnle 27 , Reference Hedelin, Lof and Sandin 28 , Reference Kuijsten, Arts and Hollman 35 , Reference Kuijsten, Hollman and Boshuizen 36 , Reference Johnsen, Olsen and Thomsen 44 , Reference Theodoratou, Kyle and Cetnarskyj 47 ), seven in Asia( Reference Akhter, Iwasaki and Yamaji 15 , Reference Shin, Lee and Lee 19 , Reference Yang, Shu and Li 21 , Reference Oba, Nagata and Shimizu 23 – Reference Akhter, Inoue and Kurahashi 26 ) and one in Canada( Reference Cotterchio, Boucher and Manno 16 ). Among the four cohort studies conducted in Asian populations( Reference Yang, Shu and Li 21 , Reference Oba, Nagata and Shimizu 23 , Reference Butler, Wang and Koh 24 , Reference Akhter, Inoue and Kurahashi 26 ), one focused only on colon cancer risk( Reference Oba, Nagata and Shimizu 23 ) and one included only women( Reference Yang, Shu and Li 21 ). A Swedish cohort also included solely women( Reference Hedelin, Lof and Sandin 28 ). The NOS quality score ranged from 5 to 9. One study had a score of 5( Reference Ravasco, Monteiro-Grillo and Marques Vidal 17 ), one had a score of 6( Reference Shin, Lee and Lee 19 ), three had a score of 7( Reference Akhter, Iwasaki and Yamaji 15 , Reference Rossi, Negri and Talamini 18 , Reference Zamora-Ros, Not and Guinó 20 ), four had a score of 8( Reference Cotterchio, Boucher and Manno 16 , Reference Budhathoki, Joshi and Ohnaka 25 , Reference Kuijsten, Arts and Hollman 35 , Reference Theodoratou, Kyle and Cetnarskyj 47 ) and the remaining eight studies had a score of 9( Reference Yang, Shu and Li 21 , Reference Oba, Nagata and Shimizu 23 , Reference Butler, Wang and Koh 24 , Reference Akhter, Inoue and Kurahashi 26 , Reference Ward, Chapelais and Kuhnle 27 , Reference Hedelin, Lof and Sandin 28 , Reference Kuijsten, Hollman and Boshuizen 36 , Reference Johnsen, Olsen and Thomsen 44 ).
Table 1 Characteristics of studies on phyto-oestrogens included in the meta-analysis (Pooled relative risks (RR) and 95 % confidence intervals)
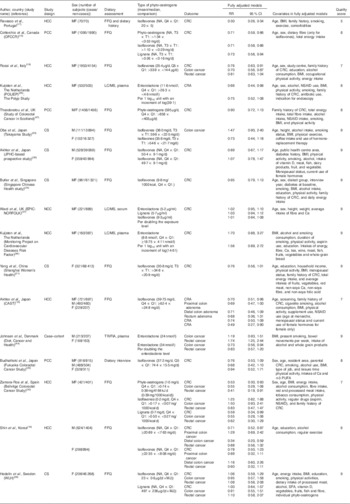
HCC, hospital-based case–control study; MF, male and female; CRC, colorectal cancer; OFCCR, Ontario Familial Colon Cancer Registry; LC, liquid chromatography; CRA, colorectal adenoma; NSAID, non-steroidal anti-inflammatory drug; PCC, population-based case–control study; CS, cohort study; JHPC, The Japan Public Health Center; EPIC-NORFOLK, European Prospective Investigation into Cancer and Nutrition-Norfolk; NCC, nested case–control study; CAST, the Colorectal Adenoma Study in Tokyo; TR/FIA, time-resolved fluoroimmuno-assay; WLH, Women’s Lifestyle and Health cohort study.
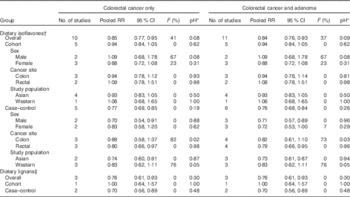
Overall phyto-oestrogens
A total of six cohort( Reference Yang, Shu and Li 21 , Reference Oba, Nagata and Shimizu 23 , Reference Butler, Wang and Koh 24 , Reference Akhter, Inoue and Kurahashi 26 , Reference Hedelin, Lof and Sandin 28 , Reference Kuijsten, Hollman and Boshuizen 36 ) and seven case–control studies( Reference Cotterchio, Boucher and Manno 16 – Reference Zamora-Ros, Not and Guinó 20 , Reference Budhathoki, Joshi and Ohnaka 25 , Reference Theodoratou, Kyle and Cetnarskyj 47 ) were used in the meta-analysis of overall phyto-oestrogen intake and colorectal cancer risk. Two case–control studies of colorectal adenoma were added to the meta-analysis for colorectal neoplasm risk( Reference Akhter, Iwasaki and Yamaji 15 , Reference Kuijsten, Arts and Hollman 35 ). The pooled RR of colorectal cancer and colorectal neoplasms for the highest v. lowest category of overall phyto-oestrogens intake was 0·78 (95 % CI 0·63, 0·96) and 0·76 (95 % CI 0·63, 0·92), respectively, under a random-effect model. There was significant heterogeneity among studies for both colorectal cancer risk (I 2=90 %, pH<0·01) and colorectal neoplasms risk (I 2=87 %, pH<0·01). By omitting one study at a time, we identified the Portuguese study by Ravasco et al. ( Reference Ravasco, Monteiro-Grillo and Marques Vidal 17 ) as the main source of heterogeneity, which was given a low-quality score of 5. This study was presented as a cross-sectional study with a case–control design, but the data analysis was performed using a proportional hazards model. Because of the inappropriate data analysis, this study was excluded from further analyses. After excluding the study by Ravasco et al.( Reference Ravasco, Monteiro-Grillo and Marques Vidal 17 ), the pooled RR were 0·84 (95 % CI 0·76, 0·92) and 0·82 (95 % CI 0·75, 0·90) for colorectal cancer and colorectal neoplasms, respectively (Table 2, Fig. 2). The heterogeneity statistic I 2 dropped to 32 % (pH=0·06) for colorectal cancer and 29 % (pH=0·07) for colorectal neoplasms.

Fig. 2 Pooled relative risk of colorectal cancer for highest v. lowest phyto-oestrogens; results from a random-effect meta-analysis.
Table 2 Meta-analyses of the association (highest v. lowest categories) between exposure to overall phyto-oestrogens and colorectal cancer, overall and by selected subgroups and adjustment (Pooled relative risks (RR) and 95 % confidence intervals)

* Heterogeneity within subgroup.
Stratified by study design, a significant inverse association with colorectal cancer was observed in case–control studies (pooled RR 0·76; 95 % CI 0·69, 0·84) but not in cohort studies (pooled RR 0·95; 95 % CI 0·85, 1·06) (Table 2, Fig. 2). The results for colorectal cancer were comparable with those for colorectal neoplasms. We did not find substantial differences in associations for colorectal cancer by subgroups in case–control studies. For cohort studies, there were differences between females (pooled RR 0·88; 95 % CI 0·72, 1·08) and males (pooled RR 1·10; 95 % CI 0·68, 1·78), between colon cancer (pooled RR 0·94; 95 % CI 0·78, 1·14) and rectal cancer (pooled RR 1·09; 95 % CI 0·79, 1·52), and between Asian (pooled RR 0·93; 95 % CI 0·83, 1·05) and Western studies (pooled RR 1·23; 95 % CI 0·74, 2·05), which were non-significant and based on a limited number of studies. All studies except for two case–control studies included BMI( Reference Cotterchio, Boucher and Manno 16 , Reference Shin, Lee and Lee 19 ) and one included physical activity( Reference Cotterchio, Boucher and Manno 16 ) as adjustment variables. Only two cohort studies accounted for family history of colorectal cancer( Reference Yang, Shu and Li 21 , Reference Butler, Wang and Koh 24 ) and one accounted for NSAID use( Reference Kuijsten, Hollman and Boshuizen 36 ) whereas four case–control studies adjusted for family history( Reference Rossi, Negri and Talamini 18 , Reference Zamora-Ros, Not and Guinó 20 , Reference Budhathoki, Joshi and Ohnaka 25 , Reference Theodoratou, Kyle and Cetnarskyj 47 ) and two adjusted for NSAID use( Reference Zamora-Ros, Not and Guinó 20 , Reference Theodoratou, Kyle and Cetnarskyj 47 ). In the analyses of colorectal cancer stratified by adjustment factors, the RR was found to be attenuated after adjustment for BMI and physical activity (online Supplementary Table S1). The funnel plot was symmetric and showed no evidence of publication bias (P=0·61, online Supplementary Fig. S1).
Isoflavones
The meta-analysis of the association between isoflavones intake and colorectal cancer risk was based on five prospective cohort( Reference Yang, Shu and Li 21 , Reference Oba, Nagata and Shimizu 23 , Reference Butler, Wang and Koh 24 , Reference Akhter, Inoue and Kurahashi 26 , Reference Hedelin, Lof and Sandin 28 ) and five case–control studies( Reference Cotterchio, Boucher and Manno 16 , Reference Rossi, Negri and Talamini 18 – Reference Zamora-Ros, Not and Guinó 20 , Reference Budhathoki, Joshi and Ohnaka 25 ). One additional case–control study( Reference Akhter, Iwasaki and Yamaji 15 ) was included in the analysis for colorectal neoplasms( Reference Akhter, Iwasaki and Yamaji 15 , Reference Cotterchio, Boucher and Manno 16 , Reference Rossi, Negri and Talamini 18 – Reference Yang, Shu and Li 21 , Reference Oba, Nagata and Shimizu 23 – Reference Akhter, Inoue and Kurahashi 26 , Reference Hedelin, Lof and Sandin 28 ). The pooled RR for the highest v. lowest isoflavones intake were 0·85 (95 % CI 0·77, 0·95) and 0·84 (95 % CI 0·76, 0·93) for colorectal cancer and colorectal neoplasms, respectively (Table 3, online Supplementary Fig. S2). There was evidence of moderate heterogeneity among studies (I 2=40 %, pH=0·08).The inverse associations observed for colorectal cancer were statistically significant in case–control studies (pooled RR 0·77; 95 % CI 0·69, 0·85) but not in cohort studies (pooled RR 0·94; 95 % CI 0·84, 1·05). We did not observe significant heterogeneity by sex, cancer site or study population in both cohort studies and case–control studies.
Table 3 Meta-analyses of the association (highest v. lowest categories) of dietary isoflavones and dietary lignans with colorectal cancer by selected subgroups (Pooled relative risks (RR) and 95 % confidence intervals)
* Heterogeneity within subgroup.
† Under random-effect models.
‡ Under fixed-effect model.
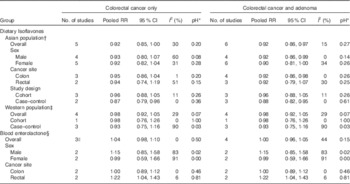
In the dose–response analyses of dietary isoflavones intake with colorectal cancer risk, five Asian studies( Reference Shin, Lee and Lee 19 , Reference Yang, Shu and Li 21 , Reference Oba, Nagata and Shimizu 23 , Reference Budhathoki, Joshi and Ohnaka 25 , Reference Akhter, Inoue and Kurahashi 26 ) and four Western studies( Reference Zamora-Ros, Knaze and Lujan-Barroso 9 , Reference Cotterchio, Boucher and Manno 16 , Reference Rossi, Negri and Talamini 18 , Reference Hedelin, Lof and Sandin 28 ) were included. No evidence of departure from linearity was found for both Asian (P=0·55) and Western studies (P=0·39). Inverse associations were identified in both populations, with a pooled RR of 0·92 (95 % CI 0·85, 1·00) for each 20 mg/d increase in isoflavones intake in Asian populations and 0·98 (95 % CI 0·92, 1·05) for each 0·1 mg/d increase in Western populations (Table 4, Fig. 3). Among Asian populations, the inverse association for risk of colorectal neoplasms was statistically significant (pooled RR 0·92; 95 % CI 0·86, 0·97). No evidence of study heterogeneity was observed within both Western studies (I 2=29 %, pH=0·07) and Asian studies (I 2=30 %, pH=0·20). In Asian studies, the inverse association was significant in case–control (pooled RR 0·87; 95 % CI 0·79, 0·96) but not cohort studies (pooled RR 0·96; 95 % CI 0·88, 1·05). No substantial differences were observed according to sex and cancer site.

Fig. 3 Pooled relative risk of colorectal cancer for isoflavones intake; results from a random-effect dose–response meta-analysis.
Table 4 Dose–response analysis of the association of dietary isoflavones and circulating enterolactone with colorectal cancer risk under random-effect models (Pooled relative risks (RR) and 95 % confidence intervals)
* Heterogeneity within subgroup.
† RR presented for per 20 mg/d increase in isoflavones intake.
‡ RR presented for per 0·1 mg/d increase in isoflavones intake.
§ RR presented for per doubling the enterolactone concentration.
|| RR based on three cohort studies.
Lignans
Three studies including two case–control studies( Reference Cotterchio, Boucher and Manno 16 , Reference Zamora-Ros, Not and Guinó 20 ) and one cohort study( Reference Hedelin, Lof and Sandin 28 ) carried out in Western populations evaluated the association of lignans intake with colorectal cancer risk. The pooled RR for case–control studies indicated a significant association with reduced colorectal cancer risk for the highest v. lowest category (pooled RR 0·70; 95 % CI 0·56, 0·89). However, no association was found in the cohort study. No study heterogeneity was detected (I 2=0, pH=0·30) (Table 3, online Supplementary Fig. S3). In the dose–response analyses of these three studies, significant departure from linearity was detected (P<0·01). A non-linear relationship was observed, with an inverse association between dietary lignans intake and colorectal cancer at low-to-moderate intakes and no further reduction in risk at higher intakes >2·5 mg/d (Fig. 4).

Fig. 4 Pooled dose–response association between dietary lignans and colorectal cancer risk; results from a random-effect dose–response meta-analysis.
The dose–response analysis for circulating enterolactone concentrations was based on three prospective studies( Reference Ward, Chapelais and Kuhnle 27 , Reference Kuijsten, Hollman and Boshuizen 36 , Reference Johnsen, Olsen and Thomsen 44 ) for colorectal cancer risk and one additional case–control study for colorectal neoplasms risk( Reference Kuijsten, Arts and Hollman 35 ), also solely in Western populations. No association was observed of the risk of either colorectal cancer (pooled RR 1·04; 95 % CI 0·98, 1·10) or colorectal neoplasms (pooled RR 1·00; 95 % CI 0·96, 1·05) with per doubling of blood enterolactone concentration (Table 4, online Supplementary Fig. S4). However, a 22 % increased risk of rectal cancer (95 % CI 1·04, 1·43) was observed, whereas no association was seen for colon cancer (pooled RR 1·00; 95 % CI 0·89, 1·12).
Discussion
The meta-analysis suggests that a greater phyto-oestrogen intake may be associated with a reduced risk of colorectal cancer and colorectal neoplasms. The inverse association was, however, statistically significant in case–control studies and not in cohort studies. Dose–response analysis of isoflavones indicated an 8 % lower risk of colorectal neoplasms per 20 mg/d increase in Asian populations. For lignans, a non-linear inverse association with colorectal cancer risk was found for dietary lignans intake, whereas there was no association for circulating enterolactone concentrations.
For overall phyto-oestrogens, there was evidence of moderate heterogeneity in the association with colorectal cancer. First, the study design could be a potential source of heterogeneity, as heterogeneity was detected between subgroups but not within subgroups by study design. Case–control studies are considered prone to recall bias, that is, cancer patients are more likely to recall a potential cancer-related diet. In pooled analyses, four of six Western studies were case–control studies, whereas four of the six Asian studies were cohort studies. Therefore, recall bias may partly explain the more prominent effects in case–control studies and Western populations. Heterogeneity could also be partly due to the fact that the pooled RR was derived from estimates of different phyto-oestrogen subgroups, including dietary phyto-oestrogens, dietary isoflavones, dietary lignans and blood enterolactone concentrations. Considering the different results for isoflavones and enterolactone, it appears warranted to discuss the association with colorectal cancer for isoflavones and lignans separately. The symmetric funnel plot suggested no evidence of small study effects, such as publication bias.
The association of isoflavones intake with a 16 % reduced risk of colorectal cancer is consistent with a recent meta-analysis( Reference Tse and Eslick 29 ), which found an 24 % reduced colorectal cancer risk for highest v. lowest categories based on eight studies. However, Tse & Eslick( Reference Tse and Eslick 29 ) did not include three recent studies( Reference Shin, Lee and Lee 19 , Reference Zamora-Ros, Not and Guinó 20 , Reference Hedelin, Lof and Sandin 28 ) and did not trim the cross-sectional study that caused asymmetry of the funnel plot( Reference Ravasco, Monteiro-Grillo and Marques Vidal 17 ), which may have led to overestimation of risk reduction. We found significant heterogeneity by study design. On the basis of cohort studies, the inverse association for dietary isoflavones was weaker and not statistically significant. The lack of significance could be due to insufficient power considering the weak association, but this could also indicate that there is no association. Any real association is likely to be closer in magnitude to that based on the cohort studies. An earlier meta-analysis already discussed the absence of association for three Asian cohort studies( Reference Yang, Shu and Li 21 , Reference Oba, Nagata and Shimizu 23 , Reference Akhter, Inoue and Kurahashi 26 ) in contrast to inverse associations found in case–control studies in Western countries( Reference Yan, Spitznagel and Bosland 13 ).
Isoflavones consumption varies greatly between Asian and Western populations. Asian populations have an average isoflavones consumption of >30 mg/d, whereas Western populations consume <1 mg/d( Reference Zamora-Ros, Knaze and Lujan-Barroso 9 ). Apart from differences in the amount of isoflavones intake, processing of isoflavone-containing foods could also differ between Asian and Western populations and in turn influence the bioavailability of isoflavones( Reference Zubik and Meydani 12 ). In Asia, the major source of isoflavones is soyabean-fermented products such as tofu and tempeh, which contain the aglycones form of isoflavones. However, in Western diets, isoflavones come from cooked soyabeans, soya milk and texturised vegetable proteins mainly in the form of glucosides. The aglycones form of isoflavones can be absorbed by the intestine directly, whereas glucoside forms need to be hydrolysed by β-glucosidases to aglycone forms in the jejunum. This large geographic difference in isoflavones consumption levels and processing approach justified the stratification of the dose–response analysis by ethnicity of the study population. Given lower levels and less variability of isoflavones intake across Western populations, the dose–response analysis did not yield a significant inverse relationship as in the Asian population. However, there were only four Western studies( Reference Cotterchio, Boucher and Manno 16 , Reference Rossi, Negri and Talamini 18 , Reference Zamora-Ros, Not and Guinó 20 , Reference Hedelin, Lof and Sandin 28 ) that could be included in the meta-analysis, of which one was a prospective study( Reference Hedelin, Lof and Sandin 28 ) that found no association. In a further prospective study in the UK that could not be included, neither dietary intake( Reference Ward, Kuhnle and Mulligan 22 ) nor serum levels of isoflavones( Reference Ward, Chapelais and Kuhnle 27 ) were found to be associated with colorectal cancer risk. Thus, large-scale, prospective investigations, particularly in Western populations, are warranted to understand the possible protective role of isoflavones in the development of colorectal cancer.
Inconsistent findings for dietary lignans intake and blood enterolactone concentrations with respect to colorectal cancer risk were observed. A non-linear, dose–response association between dietary lignans and colorectal cancer risk was detected in our study, whereas no significant association was detected for per doubling increase of blood enterolactone levels. It may be because of several reasons. First, this could be explained by the study design, as the results regarding dietary lignans were based mainly on case–control studies and that regarding enterolactone were based solely on cohort studies. Second, lignans intake was estimated from FFQ representing long-term consumption, whereas circulating enterolactone concentration captures recent short-term status. Although FFQ measurement may be subject to recall bias, long-term status might be more relevant for cancer development. Moreover, dietary lignans only account for a moderate amount of variation in circulating enterolactone concentrations( Reference Milder, Kuijsten and Arts 49 ). Plant lignans are converted by gut microflora into the bioactive metabolites enterolactone and enterodiol. As the binding sites for bioactive lignan metabolites are limited, the protective effect of lignans might reach a plateau when the level of lignan metabolites achieves a certain threshold. Thus, a non-linear association between dietary lignans and colorectal cancer risk as observed in our study seems biologically plausible. Enterolactone is the predominant metabolite, and therefore is the preferred biomarker to measure exposure to the bioactive component of lignans( Reference Kilkkinen, Stumpf and Pietinen 50 , Reference Horner, Kristal and Prunty 51 ). However, only one( Reference Johnsen, Olsen and Thomsen 44 ) of the four included studies accounted for the effect of antibiotics, which influences the activity of gut microflora, and therefore is a strong determinant of enterolactone and enterodiol concentration, and found that this strengthened the association with colorectal cancer risk. Because of the small number of studies, which are all from European countries, including only one cohort study on dietary lignans intake, the current evidence is too limited to draw conclusions regarding lignans.
Overall, the associations of isoflavones and lignans with colorectal neoplasms are consistent with those for colorectal cancer. Colorectal adenoma is an early step in the development of colorectal cancer, and therefore is an informative end point for studying colorectal carcinogenesis. Studies have shown that isoflavones and lignans are associated with a reduced risk of both colorectal adenoma and its recurrence( Reference Akhter, Iwasaki and Yamaji 15 , Reference Kuijsten, Arts and Hollman 35 , Reference Bobe, Sansbury and Albert 52 , Reference Bobe, Murphy and Albert 53 ). These consistent findings suggest that phyto-oestrogens might have a chemo-preventive effect already in the initial stages of colorectal carcinogenesis. This is in line with results from animal studies, where phyto-oestrogens are associated with a reduction in a number of early carcinogenesis-related markers such as formation of aberrant crypt foci, size and number of adenomas( Reference Danbara, Yuri and Tsujita-Kyutoku 54 – Reference Javid, Moran and Carothers 57 ).
Our study is the first to quantify the dose–response relationship of the association of colorectal cancer risk with dietary isoflavones and lignans, and to systematically evaluate the association of colorectal cancer risk with the lignans intake, lignan biomarkers and overall phyto-oestrogens. Previous meta-analyses solely compared the highest v. lowest category to evaluate the association between isoflavones and colorectal cancer risk. With the dose–response meta-analysis, it was possible to assess linearity of the relationships and visualise the non-linear associations for different exposure levels. To include all available studies for more accurate estimates, we converted differing exposure measurements into the same unit and pooled the trend estimate for colorectal cancer in each study. This allows a comprehensive view on the association between phyto-oestrogen and colorectal cancer risk based on current available evidence.
Observational studies have their inherent limitation of residual confounding, which will remain in the pooled analysis. A higher intake of phyto-oestrogens may be associated with a healthier lifestyle( Reference Zamora-Ros, Knaze and Lujan-Barroso 9 ), which may influence colorectal cancer risk independently. In the stratified analyses by adjusted factors, these factors were not found to influence the overall association substantially. Although some residual confounding may exist, the inverse associations observed are unlikely to be explained solely by healthy lifestyle, as almost all studies adjusted for the most relevant lifestyle factors. Another possible limitation is that different phyto-oestrogen subgroups were investigated, and different measurement methods were used in the included studies, which may lead to inconsistent results. Although some of this variation may be real, the use of various nutrient databases may have led to differences in estimated dietary phyto-oestrogen intake between the studies. As discussed above, FFQ is subject to recall bias whereas blood biomarkers could only reflect short-term exposure and are influenced by gut microflora, BMI, smoking and constipation( Reference Kilkkinen, Stumpf and Pietinen 50 , Reference Horner, Kristal and Prunty 51 ). Therefore, different determinants of various phyto-oestrogen subgroups might result in varying associations between phyto-oestrogens and colorectal cancer. We used the random-effect model to account for the variation and heterogeneity between studies. Applying RR for the highest v. lowest category in the overall meta-analysis made it possible to pool studies with different assessment methods and different categorisations of exposure. There was effect heterogeneity according to the study design for the association between phyto-oestrogens and colorectal cancer risk. Inverse associations for phyto-oestrogens overall and by subgroup were significant in case–controls studies but not in cohort studies. Furthermore, there was limited power for precise risk estimation of the association of different phyto-oestrogen subgroups with colorectal cancer because of the overall small number of cohort studies, especially for lignans, for which there was only one cohort study evaluating this relationship.
Several mechanisms may explain the potential anti-carcinogenic effects of phyto-oestrogens( Reference Pampaloni, Bartolini and Tonelli 2 ). Similar to oestrogen, phyto-oestrogens can bind to oestrogen receptors and possess a higher binding affinity to oestrogen receptor β (ESR2). ESR2 is the predominantly expressed oestrogen receptor in normal colon mucosa, and progressively decreases in the pathological mucosa paralleling the grade and the stage of colorectal cancer( Reference Caiazza, Ryan and Doherty 58 ). In the presence of ESR2, phyto-oestrogens exhibit anti-proliferative effects at a lower and more physiological concentration (0·1–10 µmol/l)( Reference Totta, Acconcia and Virgili 59 ). In addition, both animal studies and a randomised controlled trial in human subjects showed that dietary phyto-oestrogens can increase the expression levels of ESR2 in the colonic mucosa( Reference Principi, Di Leo and Pricci 60 – Reference Barone, Tanzi and Lofano 62 ). ESR2 expression could also inhibit colonic tumour growth in the xenograft mouse model( Reference Hartman, Edvardsson and Lindberg 63 ).
In summary, the currently available epidemiological studies did not provide sufficient evidence to draw a rigorous conclusion regarding an effect of phyto-oestrogens on colorectal cancer risk. The association of isoflavones with reduced risk of colorectal cancer was statistically significant only in case–control studies but not in cohort studies, which are less prone to recall and selection bias. Evidence of an association between dietary lignans or their active metabolites and colorectal cancer risk is limited, and cohort studies evaluating dietary lignans intake are lacking. It is presently unknown whether an effect of phyto-oestrogen could differ by ESR2 expression of the tumour. In view of the importance of colorectal cancer in cancer incidence and mortality in the general population, further prospective studies are warranted to clarify the association between different phyto-oestrogens and colorectal cancer risk and assess possible differential effects by ESR2 expression.
Acknowledgements
The work was supported by the German Federal Ministry of Education and Research (grant numbers 01ER0814 and 01ER0815) and the National Cancer Institute, National Institutes of Health, US Department of Health and Human Services (grant numbers U01 CA137088 and R01 CA164930).
R. J., A. B. performed the literature search; R. J., A. H. analysed the data; R. J. drafted the article; A. B., A. R. and J. C.-C. critically reviewed the manuscript; all the authors read and approved the final manuscript.
The authors disclose no potential conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114516004360











