Type 2 diabetes is a critical disease clearly linked to obesity and physical inactivity. Appropriate nutritional advice is an important way to control and manage all the metabolic disorders associated with excessive fat storage1. It has been proposed that some carbohydrates, which are fermented in the caeco-colon, might be of particular interest in the field of obesity. Fructans are non-digestible and fermentable carbohydrates, which have interesting metabolic effects (decrease in fat mass development, steatosis and glycaemia), by acting through a mechanism different from the common dietary fibres prone to act on lipid metabolism, since they exhibit no gel-forming properties. Interestingly, the fermentation of fructans in the colon promotes incretin productionReference Cani, Daubioul, Reusens, Remacle, Catillon and Delzenne2–Reference Kok, Morgan, Williams, Roberfroid, Thissen and Delzenne4. Glucagon-like peptide (GLP)-1 is an incretin secreted by endocrine L cells after post-translational modification of the peptide derived from proglucagon gene expression; it is an important regulator of the pancreatic β-cell, known to promote insulin secretion, proinsulin biosynthesis and islet cell growth and neogenesis. Moreover, it is also considered as a key satietogenic peptideReference Hay, Sinclair, Bermano, Durward, Tadayyon and Docherty5, Reference Drucker, Philippe, Mojsov, Chick and Habener6. We have previously shown that Raftilose (RAF), a short-chain fructan derived from chicory roots inulin, increased portal and colonic GLP-1 (7-36) amide levels and that mice lacking GLP1 receptor functionality did not respond to RAF in terms of regulation of food intake, glycaemia and fat mass developmentReference Cani, Daubioul, Reusens, Remacle, Catillon and Delzenne2, Reference Cani, Knauf, Iglesias, Drucker, Delzenne and Burcelin7.
On the other hand, López et al. determined the molecular structure of fructans from Agave tequilana Weber var. azul, using different techniques. These fructans consist of a complex mixture containing principally β(2-1) linkages, but also some β(2-6), with branches, and with terminal or internal glucoseReference López, Mancilla-Margalli and Mendoza-Díaz8. Mancilla-Margalli & López reported the structural differences among agave fructans as well as within the same Agave species but grown in different environmental regionsReference Mancilla-Margalli and López9. The observed structural heterogeneity could be attributed to the plant adaptation mechanisms to survive in very inhospitable areas. These authors classified agave fructans in three major groups with two different structures, graminans and agavins. No physiological effect of agave-derived fructans has been reported until now. Gibson & Wang evaluated the properties of different types of fructo-oligosaccharides and found a variable growth of each of the different bacterial species – responsible for a specific fermentation pattern – which was dependent of the type of oligosaccharide usedReference Gibson and Wang10. Interestingly an in vitro assessed the prebiotic effect of fructans and proved an efficient stimulation of growth of Bifidobacteria and Lactobacilli by several agave fructans – Dasylirion spp. (DAS) and A. tequilana Gto (TEQ)Reference López, Urías-Silvas, Shiami, Benkeblia and Ondera11. This tremendous prebiotic potential opens new and excited alternatives for agave fructans as food ingredients and/or health-promoting ingredients.
Cani et al. compared the effect of the degree of polymerization (DP) of three fructans derived from inulin on GLP-1 (7-36) amide synthesis and showed that the most important increase was observed with short-chain fructans used in the present study, which is mostly fermented in the upper part of the caecum colonReference Cani, Dewever and Delzenne12. As mentioned previously, fructans from TEQ and DAS exhibit a similar bifidogenic potential in vitro as compared with Raftilose®Synergy1; the profile of fermentation and the extent of bacterial growth were dependent on the bacterial strain and on the Agave species or fructan typeReference López, Urías-Silvas, Shiami, Benkeblia and Ondera11. Matrix assistant laser desorption/ionization–time of flight (MALDI-TOF)-MS analysis (data not shown) of fructans from TEQ revealed the presence of a larger proportion of low DP fructo-oligosaccharides than in DAS, thus suggesting an effect prone to occur mostly in the caecum and in the proximal colon. DAS would be expected to be fermented mostly in the medial and distal colon. The difference in behaviour of TEQ and DAS compared with RAF, which is lineal, could be attributed to the structure of this kind of fructans assuming similarity with that previously reported by Lopez et al. for A. tequilana Weber var. azul, which present linkages of the type β(2-1) principally, but also some β(2-6) and branched of the neo typeReference López, Mancilla-Margalli and Mendoza-Díaz8.
Therefore, due to fructans structural diversity and their putative benefits on health, the aim of the present work was to evaluate the potential of TEQ v. inulin type fructans to modulate glucose and lipid metabolism and GLP-1 secretion in mice. In this work, DAS was included, which possesses similar characteristics with agave, such as plant morphology, geographical distribution and pollen characteristics. Fructans-like storage of carbohydrate has been found in this plant, in addition to its prebiotic properties.
Materials and methods
Animals and diets
Thirty-two male C57Bl/6J mice from Charles River Laboratories (12 weeks old at the beginning of the experiment) were housed in a temperature- and humidity-controlled room with a 12 h light–dark cycle. They were divided into four groups (eight mice per group, four mice per cage) according to diet. After an acclimatization period of 6 d before the experiment, control (standard (STD) diet) mice were fed pelleted A04 standard diet (UAR, Villemoisson-sur-Orge, France) whereas RAF-, TEQ- and DAS-diet mice received a diet prepared by mixing 90 g A04 standard diet with 10 g corresponding fructan (RAF P95, TEQ and DAS respectively). The A04 standard diet contained the following (g/100 g dry diet): protein 19·3 (consisting of equivalent mix of soyabean and fish proteins); total carbohydrates obtained from maize, wheat, barley and bran 70·4 (including starch 38, sucrose 3·0, cellulose 5·0, non-digestible carbohydrate 8·0); lipid 3·0; mineral mixture 6·0; vitamin mixture 1·3. Food intake, taking into account spillage, was assessed three times per week. The mean daily energy intake (kJ/d) was calculated as follows: food intake (g) × energy value of diet (kJ/g). The energy value for the STD diet was 13·86 kJ/g; for RAF, DAS and TEQ diets it was 13·08 kJ/g.
Chemicals
RAF P95 (Orafti, Tienen, Belgium) is a mixture of glucosyl-(fructosyl)n-fructose and (fructosyl)m-fructose with an average DP of 4·8. Fructans from TEQ were analysed by MALDI-TOF-MS (data not shown) and present a range of DP of 3-22 with a predominance of 7 and fructans from DAS show a range of DP of 3-20.
Body weight, intake and faeces
Body weight and food intake were monitored twice per week and faeces collection was performed three times during the experimental period to evaluate the 24 h production.
Blood samples
Blood samples were taken once per week from the mice tails in order to measure serum glucose, TAG, cholesterol and NEFA, using kits coupling enzymatic reactions and spectrophotometric detection of reaction end-products (Elitech, Brussels, Belgium).
On day 37, mice were anaesthetized by intra-peritoneal injection of sodium pentobarbital solution (60 mg/kg body weight; Nembutal®; Sanofi Santé Animale, Brussels, Belgium). Portal vein blood samples were collected in EDTA tubes (Sarstedt, Nümbrecht, Germany) with or without dipeptidyl peptidase IV inhibitor (Linco Research, St Charles, MO, USA); after centrifugation, serum was stored at − 80°C. The concentration of GLP-1 (7-36) amide was measured using an ELISA kit specific for GLP-1 (7-36) amide without cross-reactivity towards GLP-1 (9-36) amide, GLP-2 or glucagon (GLP-1 active ELISA kit; Linco Research).
Tissue samples
Segments of the caecum and proximal, medial and distal colon (corresponding to segments taken just above the caecal junction, in the middle of the colon and just below the rectum, respectively) were immediately excised, flushed with ice-cold saline solution (9 g NaCl/l), immersed in liquid N2 and stored at − 80°C for further mRNA and peptides analysis. Full and empty caecum, liver and epididymal fat tissue were weighed. Liver was removed; one sample was clamped immediately in liquid N2 and kept at − 80°C for lipid analysis and another section was frozen in isopentane and kept at − 80°C for histological analysis.
Liver analysis
Liver samples were homogenized and TAG, cholesterol and NEFA were measured as previously described for blood samples after an extraction with chloroform-methanol according to Folch et al. Reference Folch, Lees and Sloane-Stanley13. Protein concentration was measured by the method of Bradford using bovin serum albumin as standardReference Bradford14. Haematoxylin/eosin and oil red staining were performed on liver tissue cryostat sections.
Intestinal glucagon-like peptide-1 (7-36) amide extraction
Extraction of GLP-1 (7-36) amide from intestinal segments (caecum and colon) was carried out with an ethanol-acid solution (10 ml/g tissue). Samples were homogenized at maximum speed and placed at 4°C for 24 h. The homogenate was centrifuged (2000 g) and the supernatant fraction was decanted and diluted 100- and 250-fold in saline solution (9 g NaCl/l) for caecum and colon, respectively. Concentrations of intestinal GLP-1 (7-36) amide were measured as previously described for blood samples.
Isolation of total RNA
Total RNA was isolated from each intestinal segment using the TriPure Isolation Reagent (Roche, Indianapolis, IN, USA). Approximately 50–100 mg intestinal tissue was used to extract total RNA. The quantity and the purity of RNA were determined by UV spectrophotometry at 260 nm and 280 nm.
Proglucagon and β-actin mRNA by RT-PCR
RT-PCR was performed with an input of 1 μg RNA using the kit for RT-PCR (Access RT-PCR system; Promega Corporation, Madison, WI, USA). Primers of interest for the amplification of cDNA were for the sequences of the sense and antisense primers respectively: 5′-GTAATGCTGGTACAAGGCAG-3′ and 5′-TTGATGAAGTCTCTGGTGGCA-3′ for proglucagon gene, and 5′-CTGACCGAGCGTGGCT ACAG-3′ and 5′-GGTGCTAGGAGCCAGGGCAG-3′ for β-actin gene. Twenty-three cycles were performed for the detection of the proglucagon and β-actin transcripts. Control tubes without RNA templates were used to check contamination. RT-PCR products (3 μl from each) were resolved in an 18 g/l agarose gel in Tris–acetic acid–EDTA (TAE) buffer and visualized by ethidium bromide UV light-staining. Quantification of the PCR products was performed using the fluorimetric method Picogreen® dsDNA Quantitation Reagent and Kit (Molecular Probes, Leiden, The Netherlands). β-Actin was amplified and used for normalization.
Statistical analysis
Results are expressed as mean values with their standard errors of the mean. Statistical differences between groups were evaluated using one-way ANOVA followed by a Bon-ferroni or least squares difference or Tukey post hoc test using SPSS 11.0 for Windows (SPSS, Chicago, IL, USA). For portal vein GLP-1, the analysis was done with logarithmic values. Differences were considered significant at P ≤ 0·05. Correlations between parameters where assessed by Pearson's correlation test, using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA, USA; www.graphpad.com). P < 0·05 was regarded as statistically significant.
Results
Food intake, body weight and faeces
In general, fructan supplementation decreased daily food and/or energy intake (Table 1) and body weight gain (Fig. 1) and increased faeces excretion (Table 1) compared with the STD diet. Concerning food intake (Table 1), the mice with diet supplemented with RAF and TEQ ate 11 % and 10 % less food than STD, respectively. Total energy intake (Fig. 2) was significantly lower in all fructan-fed groups than in the STD group. Mice receiving TEQ, DAS and RAF diets had a significantly lower body weight gain throughout the treatment (Fig. 1). Only the TEQ diet significantly increased total faeces excretion compared with the STD group (17 % more on dry basis), the increase being non-significant in the other groups, namely, RAF and DAS (Table 1).
Table 1 Food intake, faeces, weights of liver and epididymal tissue, liver TAG, cholesterol and NEFA of mice fed a standard (STD) diet or diet supplemented with Raftilose (RAF) P95, Agave tequilana Gto. (TEQ) or Dasylirion spp. (DAS)*
(Mean values with their standard errors of the mean)
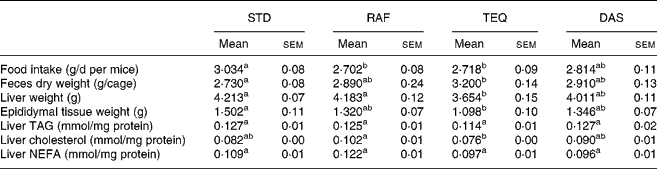
a,b Mean values with unlike superscript letters were significantly different (P ≤ 0·05). n 11 for food intake; n 4 for faeces. Liver weight: n 7 for RAF and TEQ; n 8 for STD and DAS. Epididymal tissue: n 7 for STD and TEQ; n 8 for RAF and DAS; n 8 for liver TAG, cholesterol and NEFA.
* For details of diets and procedures, see Materials and methods.

Fig. 1 Body weight gain of mice fed a standard diet (STD) or a diet supplemented with Raftilose P95 (RAF), Agave tequilana Gto. (TEQ) or Dasylirion spp. (DAS). Mean values (n 8) with their standard errors of the mean. Mean values with unlike letters were significantly different (P ≤ 0·05). For details of diets and procedures, see Materials and methods.

Fig. 2 Food intake of mice fed a standard diet (STD) or a diet supplemented with Raftilose P95 (RAF), Agave tequilana Gto. (TEQ) or Dasylirion spp. (DAS). Mean values (n 11) with their standard errors of the mean. Mean values with different letters were significantly different (P ≤ 0·05). For details of diets and procedures, see Materials and methods.
Liver and epididymal tissue weight and lipid contents
Only the TEQ diet significantly decreased both liver and adipose tissue weights as compared with STD. The sole biochemical modification observed in this group was a decrease in hepatic cholesterol level.
Serum glucose, TAG, cholesterol and NEFA
In the postprandial state, serum glucose concentrations were significantly lowered by 19, 15 and 14 % – as compared with STD – in mice fed RAF, TEQ and DAS diets, respectively (Table 2). TAG concentrations v. STD were reduced by 31 %, 11 % and 7 % in mice fed RAF, TEQ and DAS diets, respectively. Reduction of cholesterol concentrations reached about 20 % in animals receiving DAS, TEQ and RAF diets v. STD diet. NEFA were not significantly modified by any treatment. Plasma glucose and plasma cholesterol levels positively correlated with body weight gain (Fig. 3)
Table 2 Effect of a standard (STD) diet or diet supplemented with Raftilose (RAF) P95, Agave tequilana Gto. (TEQ) or Dasylirion spp. (DAS) on serum glucose, TAG, cholesterol and NEFA of mice*
(Mean values (n 8) with their standard errors of the mean for each parameter measured)

a,b Mean values with unlike superscript letters were significantly different (P ≤ 0·05).
* For details of diets and procedures, see Materials and methods.

Fig. 3 Relationship between plasma glucose and body weight gain, plasma TAG and body weight gain, and plasma cholesterol and body weight gain taking into account the animals from all groups. Values of r and P have been calculated by using Pearson's correlation statistical test. For details of animals and procedures, see Materials and methods.
Histological analysis
The histological analysis of the liver did not reveal any differences between groups. A normal structure of the hepatocytes arranged in typical centriportal trabeculi characterized all groups. Fat stained with oil red led to a negative reaction.
Caecum weight
Fructans had a pronounced effect on total caecum weight (Fig. 4): significant enlargement of the caecum was observed in mice fed the TEQ diet (almost doubled as compared with the STD diet); the DAS and RAF diets increased total caecum weight by about 65 %. A coordinate and significant increase in the caecum wall weight occurred, this parameter being increased by 77 %, 64 % and 43 % for TEQ, RAF and DAS diets, respectively, compared with the STD diet.

Fig. 4 Weight of full and empty caecum of mice fed a standard diet (STD; ■) or diet supplemented with Raftilose P95 (RAF; □), Agave tequilana Gto. (TEQ; ![]() ) or Dasylirion spp. (DAS;
) or Dasylirion spp. (DAS; ![]() ). Mean values (n 8) with their standard errors of the mean for each parameter measured. Mean values with different letters were significantly different (P ≤ 0·05). For details of diets and procedures, see Materials and methods.
). Mean values (n 8) with their standard errors of the mean for each parameter measured. Mean values with different letters were significantly different (P ≤ 0·05). For details of diets and procedures, see Materials and methods.
Intestinal proglucagon mRNA (precursor) and intestinal and portal glucagon-like peptide-1 levels
Caecum proglucagon mRNA level (Table 3) was increased by more than 30 % in RAF and TEQ diets v. STD diet, but no significant effect was shown in the DAS group. The GLP-1 concentration in the caecum was higher in the diets supplemented with fructans. TEQ, RAF and DAS diets showed concentrations (expressed as pmol per caecum) equivalent to 12·92 (sem 1·20), 11·65 (sem 1·19) and 9·34 (sem 0·62), respectively, whereas in the case of the STD diet, it reached 6·79 (sem 0·70).
Table 3 Effects of a standard (STD) diet or diet supplemented with Raftilose (RAF) P95, Agave tequilana Gto. (TEQ) or Dasylirion spp. (DAS) on intestinal proglucagon mRNA concentration†
(Mean values (n 7) for each parameter measured with their standard errors of the mean)

a,b Mean values with unlike superscript letters were significantly different (P ≤ 0·05).
* Values in relative fluorescence units, proglucagon mRNA/β-actine mRNA.
†For details of diets and procedures, see Materials and methods.
Proglucagon mRNA levels measured in the colon were not significantly different between groups (Table 3) except a moderate but significant increase in the TEQ group v. controls (STD) in the medial colon. The measurement of the GLP-1 peptide content in the different segments of the colon revealed (Fig. 5) that mice fed diets supplemented with the different fructans exhibited a higher GLP-1 concentration than in STD diet. This increase was only significant in the proximal colon for the TEQ diet, in the medial colon for the DAS diet and in the distal colon for the RAF diet. When measured in the portal vein (Fig. 6), GLP-1 concentrations in mice fed the fructan diet were significantly higher in all fructans groups than in the STD group; it was almost doubled in the RAF group v. control.

Fig. 5 Intestinal glucagon-like peptide-1 (GLP-1) (7-36) amide concentration of mice fed a standard diet (STD; ■) or diet supplemented with Raftilose P95 (RAF; □), Agave tequilana Gto. (TEQ; ![]() ) or Dasylirion spp. (DAS;
) or Dasylirion spp. (DAS; ![]() ). Mean values with their standard errors of the mean. Mean values with different letters were significantly different (P ≤ 0·05). Proximal colon: n 7 for STD, TEQ and DAS, n 6 for RAF. Medial colon: n 7 for overall group. Distal colon: n 7 for overall group. For details of diets and procedures, see Materials and methods.
). Mean values with their standard errors of the mean. Mean values with different letters were significantly different (P ≤ 0·05). Proximal colon: n 7 for STD, TEQ and DAS, n 6 for RAF. Medial colon: n 7 for overall group. Distal colon: n 7 for overall group. For details of diets and procedures, see Materials and methods.

Fig. 6 Portal vein glucagon-like peptide-1 (GLP-1) (7-36) amide concentration of mice fed a standard diet (STD) or diet supplemented with Raftilose P95 (RAF), Agave tequilana Gto. (TEQ) or Dasylirion spp. (DAS). Mean values with their standard errors of the mean. Mean values with different letters were significantly different (P ≤ 0·05). n 5 for STD; n 6 for RAF and DAS; n 8 for TEQ. For details of diets and procedures, see Materials and methods.
Discussion
The supplementation of diet with soluble fibres has been reported to have beneficial effects in patients with type 2 diabetes mellitus: it helps to improve glycaemic control, decreases hyperinsulinaemia and lowers plasma lipid concentrationsReference Chen, Sheu, Tai, Liaw and Chen15–Reference Groop, Aro, Stenman and Groop19. However, the mechanisms by which fibre may exert some of those effects are not completely understood. The viscosity of the fibre has been proposed as an important propertyReference Vuksan, Sievenpiper and Owen17. However, some fibres, such as non-digestible oligosaccharides, may have effects despite the fact that they have no gelling properties and do not modify viscosity. Fibre fermentation, leading to the production of SCFA, might also be implicated in the modulation of expression of the gut-derived proglucagon gene and, subsequently, secretion of proglucagon-derived peptides such as GLP-1Reference Tappenden, Albin, Bartholome and Mangian20–Reference Reimer and McBurney22. As previously mentioned, this peptide acts as incretin hormone and is known as an antidiabetic agent that combines insulinotropic and anorectic effectsReference Meier, Gallwitz, Schmidt and Nauck23. GLP-1 plays an important role in lowering blood glucose levels, primarily through its ability to potentiate the stimulatory effect of glucose on insulin secretion from pancreatic β-cellsReference Holz, Kuhtreiber and Habener24. It also affects blood glucose levels through its inhibitory effects on gastric emptyingReference Nauck, Niedereichholz, Ettler, Holst, Orskov, Ritzel and Schmiegel25, suppression of appetiteReference Turton, O'Shea, Gunn, Beak, Edward and Meeran26 and inhibition of glucagon secretion from α-cellsReference Komatsu, Matsuyama, Namba, Watanabe, Itoh, Kono and Tarui27.
In the present work, we have evaluated, for the first time, the effect of fructans from TEQ and DAS on glucose and lipid metabolism in an in vivo assay in rodents. This treatment was well tolerated by mice; TEQ treatment was solely responsible for increased faecal excretion. The observed increase – in the present study – on caecum weight and faeces production agrees with other studies after fructan consumption by rats and hamstersReference Trautwein, Rieckhoff and Erbersdobler28–Reference Delzenne, Aertssens, Verplaetse, Roccaro and Roberfroid30. The effect on the increase in caecum tissue reflects hypertrophy and suggests increased bacterial activity, namely, an increase in SCFA production through fermentation by colonic bacteriaReference Trautwein, Rieckhoff and Erbersdobler28, Reference Campbell, Fahey and Wolf29.
Some positive effects similar to the ones already described for inulin-type fructans were demonstrated, namely, a decrease in energy intake and body weight gain, together with a decrease in glycaemia and triacylglycerolaemia. Fasting triacylglycerolaemia has been considered as a factor involved in dietary obesity in rodentsReference Ji and Friedman31. However, taking into account the data obtained from animals of all groups, there was no significant correlation between body weight gain and triacylglycerolaemia (Pearson's test P>0·05), contrary to what was shown in animals fed with soya isoflavoneReference Demonty, Lamarche, Deshaies and Jacques32. Therefore, it is improbable that the sole decrease in energy intake could be responsible for the improvement of triacylglycerolaemia in fructans-fed animals. However, a positive correlation exists between blood glucose or cholesterolaemia and body weight gain in the present study, thus suggesting that those parameters are more related to energy intake and fat mass development.
A. tequilana was the most efficient to decrease body weight gain, whereas its effect on glycaemia and on triacylglycerolaemia was less pronounced than the one shown for RAF. This suggests that the decrease in body weight is not the sole way by which the dietary fructans tested in this study may modulate lipid and glucose homeostasis. Delzenne & Kok mentioned that the main systemic effect of RAF feeding in rats is a decrease in serum TAGReference Delzenne and Kok33. Kok et al. reported that RAF intake reduces postprandial glycaemia and insulinaemia by 17 and 26 %, respectively, and this could be implicated in lower lipogenesis and thus in lower hepatic TAG productionReference Kok, Roberfroid, Robert and Delzenne34, Reference Hillgartner, Salati and Goodridge35. Here, we confirm the decrease in triacylglycerolaemia due to RAF; whereas agave fructans had no effect on this parameter. However, all types of fructans significantly decreased glycaemia. This would suggest that a decrease in triacylglycerolaemia due to fermentable fibres is not necessarily attributable to a decrease in glucose availability.
Gut fermentation has to be taken into account in the interpretation of the metabolic effects of dietary fructans. The fermentation of fructans in the caeco-colon leads to the production of SCFA, propionate being an inhibitor of hepatic lipid synthesisReference Kok, Roberfroid, Robert and Delzenne34, Reference Delzenne36–Reference Kok, Taper and Delzenne38.
Propionate, which is largely produced through the fermentation of all tested fructans, has been shown to decrease cholesterol synthesis in different modelsReference Demigné, Rémésy, Morand, Gibson and Roberfroid37. Interestingly, we ob-served a significant decrease in serum cholesterol level, with a significant decrease in liver cholesterol for TEQ treatment only.
The trend of the effects on food/growing behaviour was similar with agave fructans and with RAF. The effect of fructans on energy intake is not due to any ‘direct’ effect of those fructans, but is really attributable to a metabolic effect in the caeco-colon, due to fermentation. Fermentation is a key point, since, in obese Zucker rats, the administration of non-fermentable cellulose in place of oligofructose does not allow the improvement of any parameters linked to fat mass, body weight or lipid metabolismReference Daubioul, Rousseau, Demeure, Gallez, Taper, Declercq and Delzenne39. Moreover, and to support the lack of ‘direct effect’ due to the treatment with fructans, mice lacking the GLP-1 receptor (KO mice or mice treated chronically with GLP-1 receptor antagonist) and treated with inulin-type fructans do not exhibit any effect on satiety, body weight and fat mass as compared with mice receiving the basal corresponding diet, thus showing that the effect of fructans on satiety (and consequences on body weight) are well due to the interaction with GLP-1 production, and might not occur through fructans per se Reference Cani, Knauf, Iglesias, Drucker, Delzenne and Burcelin7.
GLP-1 could play a role in the modulation of food intake and glycaemia, since all types of fructans increased its concentration in the portal vein. An increase in GLP-1 caecal content, and of its mRNA precursor (proglucagon) in different colon sections, are in accordance with the hypothesis that the higher GLP-1 secretion in the portal vein comes from a fermentation-dependent increase in proglucagon expression in L cells, which are present all along the lower part of the gutReference Reimer, Thomson, Rajotte, Basu, Ooraikul and McBurney40. Moreover, recent data suggest that RAF may increase GLP-1 colonic content by promoting L cell differentiationReference Cani, Hoste, Guiot and Delzenne41. SCFA, which are produced in the gut by fructan fermentation, have been reported to stimulate secretion of proglucagon-derived peptides, butyrate being the main acid implicatedReference Tappenden, Drozdowski, Thomson and McBurney42, Reference Tappenden and McBurney43. Recently, Zhou et al., by means of in vitro analysis, found that butyrate increased proglucagon gene expression in a dose-dependent mannerReference Zhou, Hegsted, McCutcheon, Keenan, Xi, Raggio and Martin44. In the present study, we have observed that the increase in the proglucagon mRNA level and GLP-1 in the different intestinal segments was different depending on the fructan source evaluated.
Interestingly, among tested fructans, the one from A. tequilana was the most potent to decrease fat mass, body and liver weight. We propose that this novel source of fructans could be interesting in studies devoted to relate specific modulation of the microbial flora and the risk of diseases associated with obesity.
Are those studies relevant to human health and behaviour? Flint et al. examined the effect of intravenously infused GLP-1 on subjective appetite sensations after an energy-fixed breakfast and on spontaneous energy intake at an ad libitum lunchReference Flint, Raben, Astrup and Holst45. They reported that GLP-1 enhanced satiety and reduced energy intake and thus may play a physiological regulatory role in controlling appetite and energy intake in human subjects. Piche et al. have shown that dietary fructans were able to increase GLP-1 production several hours after ingestionReference Piche, des Varannes, Sacher-Huvelin, Holst, Cuber and Galmiche46; on the other hand, we have recently shown that dietary RAF was able to induce satiety in normal human volunteersReference Cani, Joly, Horsmans and Delzenne47. The door is open to start studies with other types of fructans, from different botanical and geographical origin. Finally, the findings of the present study emphasize the potential of improving glucose and lipid homeostasis as well as the modulation of GLP-1 and proglucagon expression by RAF and fructans from A. tequilana and Dasylirion spp. In addition, the present results show a positive influence of the fructans on body weight control, which might be of interest in the control of obesity.
Acknowledgements
RAF P95 was kindly provided by Orafti (Tienen, Belgium). We thank Mr Luc Geshe and N. Maton for technical assistance. This work was supported by CONACYT Mexico, the Fonds National de la Recherche Scientifique, Belgium (grant F.C. 41 682 2005-2006) and by the Fonds Spéciaux de la Recherche de l'Université catholique de Louvain (UCL/ FSR 2005). Patrice Cani is a post-doctoral researcher from the FNRS.











