Berry fruits are of great importance due to the high content of polyphenols such as phenolic acids and flavonoids, particularly anthocyanins( Reference Maatta-Riihinen, Kamal-Eldin and Mattila 1 , Reference Paredes-Lopez, Cervantes-Ceja and Vigna-Perez 2 ). Even in populations who have low consumption of berries, the contribution of these fruits to the overall ingestion of polyphenols can be high, in comparison to other food sources( Reference Pinto, Cardoso and Pimpao 3 ). Epidemiological and experimental studies have suggested that intake of polyphenols reduces the risk of developing several pathological conditions such as cancer and CVD( Reference Arts and Hollman 4 – Reference Manach, Scalbert and Morand 6 ). In this regard, in vitro studies are important to elucidate the mechanism of action of polyphenols. However, for the credibility of these studies, it is essential to use those compounds that can actually be found in the human body and to use them in physiologically relevant concentrations. The role of intervention studies in calculating bioavailability and in understanding the metabolic fate of ingested compounds is very important( Reference Manach, Williamson and Morand 7 , Reference Crozier, Del Rio and Clifford 8 ).
Recently, it has been revealed that quantification of polyphenols in biological samples collected from human subjects and animals might be underestimated, since many metabolites resulting from the catabolism of polyphenols by the colonic microbiota can still be absorbed into the blood, and are usually not accounted for( Reference Czank, Cassidy and Zhang 9 , Reference Stalmach, Edwards and Wightman 10 ). Many of these compounds can undergo further metabolism and be conjugated by phase II enzymes, to form sulfated, glucuronidated and methylated compounds( Reference Williamson and Clifford 11 ). Previously, we identified several conjugated phenolic metabolites in the urine of healthy human volunteers, after ingestion of a purée composed of five different berry fruits( Reference Pimpao, Dew and Figueira 12 ). Some of these compounds have been identified for the first time in humans, which have been associated with the consumption of polyphenols. It is now important to quantify the plasma appearance of these metabolites and assess the physiological concentration they can reach, in order to account for the bioavailability of polyphenols.
In the present study, previously identified sulfated compounds were chemically synthesised and used as standards for quantification in human plasma, after ingestion of berry fruit purée. A cross-over intervention in which participants ingested either the fruit purée or a standard polyphenol-free meal was also conducted to assess any endogenous or non-polyphenol dietary origin of the metabolites under study.
Materials and methods
Reagents and reference materials
All chemicals used in the present study were purchased from Sigma-Aldrich, unless stated otherwise, and purchased reference standards were all HPLC grade (>95 %). Acetonitrile (ACN, liquid chromatography–MS grade) was purchased from Fisher Scientific Limited. Liquid chromatography–MS grade water was produced by an Elix/Milli-Q purification system (Millipore). 4-O-Methylgallic acid (4-MeGA) and 2-methylpyrogallol (2-MePyr) were obtained from Apin Chemicals. Taxifolin was obtained from Extrasynthese. Hydroxycinnamic acid conjugates were chemically synthesised and characterised as described previously( Reference Fumeaux, Menozzi-Smarrito and Stalmach 13 ). They were kindly provided by Professor Denis Barron, Nestlé Institute of Health Sciences, Lausanne, Switzerland. Protocatechuic acid-3-O-sulfate (PA-3-sulf) and protocatechuic acid-4-O-sulfate (PA-4-sulf) were kindly provided by Dr Paul Needs, Institute of Food Research, Norwich, UK.
Fruit purée
The fruit purée was prepared on the day of the study, whose composition has been described previously( Reference Pimpao, Dew and Figueira 12 ). Briefly, 100 g of each of five fruits frozen at − 20°C were used. Blueberries (Vaccinium spp. variety Georgia Gem), blackberries (Rubus L. subgenus Rubus Watson variety Karaka Black) and raspberries (Rubus idaeus L. variety Himbo Top) were harvested at the Fataca experimental field in Odemira, Portugal; strawberry tree fruits (Arbutus unedo L.) were harvested in the Alentejo region, Portugal; and Portuguese crowberries (Corema album L.) were harvested in the Comporta region, Portugal. Fruits were blended on a domestic food processor for 1 min at room temperature.
Subjects and study design
A total of twenty-two volunteers (four males), aged between 22 and 54 years, with an average BMI of 22·6 (se 2·7) kg/m2, were recruited in the present study. All individuals were considered healthy based on a medical questionnaire and standard blood tests. Volunteers did not have any history of CVD or any other medical illnesses, were non-smoking, and were not receiving any medication (including antibiotics) or taking vitamins that could interfere with the study.
Participants were allocated to two groups (Fig. 1). In the first group, nine volunteers (eight females and one male) followed a polyphenol-free diet (excluding fruit and fruit-containing products, vegetables, whole flour-based cereal products, chocolate, and beverages rich in polyphenols (tea, coffee, cocoa drinks, cider, wine and beer)) for 2 d before the study and throughout the study day. After an overnight fast, volunteers ingested 500 ml of the aforementioned fruit purée along with a standard polyphenol-free breakfast consisting of bread, with ham or cheese, yogurt and biscuits. Blood samples were collected into EDTA-containing tubes before ingestion of the purée and at 0·5, 1, 2, 4 and 6 h after ingestion. The second group, comprising thirteen volunteers (ten females and three males), participated in a cross-over controlled trial. The procedure was similar to that described for the first group; however, the two stages, which occurred 2 weeks apart, varied with respect to the ingestion of the purée or a standard polyphenol-free breakfast, where the purée was substituted by plain yogurt. Blood samples were collected into EDTA-containing tubes before ingestion of the fruit purée and at 2 and 4 h after ingestion.
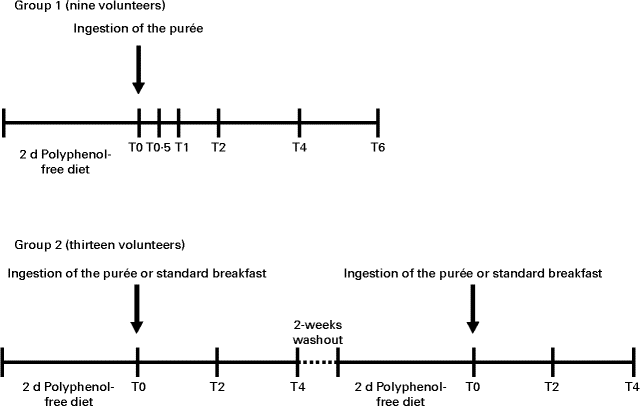
Fig. 1 Representation of the timeline of the intervention study. In the time scale, the number of volunteers in each study, the time of ingestion of the fruit purée or the standard breakfast and the time of blood collection (T0, baseline; T0·5, 0·5 h; T1, 1 h; T2, 2 h; T4, 4 h; T6, 6 h) are indicated.
Compliance with food restriction was confirmed through a questionnaire.
The study protocol was conducted in accordance with the Declaration of Helsinki of 1975, as revised in 1983, and all procedures involving human subjects were approved by the ethical committee of the Faculty of Pharmacy, University of Lisbon, Portugal (02/CEECFFUL/2012). The protocol was explained to each volunteer, who gave written informed consent before participation.
Sample treatment
Immediately after collection, blood samples were centrifuged at 2200 g for 15 min, and plasma samples (1 ml) were stored in cryotubes containing 30 μl formic acid (50 %, v/v). The samples were then stored at − 80°C until analysis.
Protein precipitation was based on a modification of a previously described method( Reference Farrell, Gomez-Juaristi and Poquet 14 ). Briefly, 20 μl ascorbic acid (4 mg/ml) were added to 380 μl of plasma sample (final concentration of 1 mm), and taxifolin, as the internal standard, was added to a final concentration of 250 nm. The sample was combined with 1 ml hexane, homogenised and centrifuged for 10 min at 17 000 g . The aqueous phase was recovered and added dropwise to 1200 μl ACN. The mixture was vortexed for 2 min and centrifuged for 10 min at 17 000 g . The supernatant was recovered and the pellet was reconstituted with 400 μl ACN. After centrifugation, ACN supernatants were combined and dried on a centrifugal evaporator. The samples were reconstituted in 100 μl of water containing 1 μm-sinapic acid as the second internal standard. The samples were centrifuged at 17 000 g for 10 min, and the supernatants were filtered before HPLC–MS/MS analysis.
HPLC–MS/MS analysis
Analysis of plasma sample was carried out on an Agilent HPLC 1200 series comprising a micro degasser, an SL binary pump, an SL autosampler with a chilled sample compartment (8°C), a column oven (30°C) and a diode array detector (Agilent Technologies) connected with a 6410a triple quadrupole liquid chromatography–MS/MS system. Separation was achieved on an Atlantis T3 Column (100 Å, 3 μm, 2·1 mm internal diameter × 100 mm HPLC column; Waters) at a flow rate of 0·26 ml/min over a gradient of 100 % solvent A (95 % H2O, 5 % ACN with 0·5 % (v/v) formic acid) for 10 min, reaching 15 % B (95 % ACN, 5 % H2O with 0·5 % (v/v) formic acid) from 10 to 20 min. Solvent B increased to 25 % at 40 min and to 100 % at 43 min where it was maintained for 5 min, returning to 0 % in 2 min. The injection volume was 10 μl.
The operating parameters were as follows: MS interface temperature, 350°C; source voltage, 4 kV; N2 flow rate, 11 litres/min; pressure, 30 psi (NM30LA; Peak Scientific). The analysis was performed in a negative multiple reaction monitoring mode optimised using the reference standards (see Table 1). The system was controlled and data were processed by Agilent MassHunter software (version B.01.03; Agilent Technologies). The m/z transitions optimised for each standard were optimised previously( Reference Pimpao, Dew and Figueira 12 ). Quantification of plasma metabolites was obtained using calibration curves of the available standards. At least eight concentration curves, ranging from 0·05 to 20 μm, were constructed from analytical standards, and each concentration point was injected in triplicate. Standard curves were all linear within the concentration range and linearity was ensured as R 2 0·997–1·000 (Table 1). Limit of quantification was determined by analysis in triplicate of standards at low concentrations, and was defined as signal:noise ratios of 1:10.
Table 1 HPLC–MS/MS parameters for the quantification of phenolic metabolites in human plasma after ingestion of 500 ml fruit purée

Statistical analysis
Pharmacokinetic profile of plasma metabolites was constructed using GraphPad Prism v 5.00 for Windows (GraphicalPad software). This package was also used for statistical analysis. Box-and-whisker plots for minimum and maximum values were generated. Comparisons in relation to the baseline were performed by a two-tailed Wilcoxon matched-pair test with a confidence level of 99 %. For the cross-over intervention study, comparisons were made between consumption of fruit purée and the control breakfast for each time point using the same test.
Synthesis of sulfated compounds
Products were synthesised by the treatment of initial aglycones (500 mg) with sulfur trioxide–pyridine. The aglycones (500 mg) and sulfur trioxide–pyridine (one equivalent for all compounds, with the exception of vanillic acid (VA) for which two equivalents were used) were dissolved in 10 ml of anhydrous pyridine and kept at 65°C with constant stirring for 24 h. The reaction was stopped by the addition of water. Solvents were dried in vacuo and the residue was dissolved in water. The unreacted aglycones were separated with ethyl acetate, and the product in the water phase was purified on a Dowex 50W-X8 ion-exchange column (Sigma-Aldrich) loaded with Na+. The purified compounds were then dried in vacuo and characterised by 1H and 13C NMR. NMR chemical shifts are reported using the residual solvent peak as reference. Peak assignments were based on COSY (homonuclear correlation spectroscopy) and HMQC (heteronuclear multiple-quantum correlation spectroscopy) experiments.
Catechol-O-sulfate ((2-hydroxyphenyl)oxidanesulfonic acid): 674 mg, 66 % yield; 1H NMR (400 MHz, fully deuterated dimethyl sulfoxide (DMSO)-d6): δ 8·85 (s, 1H, OH), 7·12 (dd, 1H, J= 1·6, 7·96 Hz), 6·96 (ddd, 1H, J =1·64, 7·96, 9·26 Hz), 6·83 (dd, 1H, J= 1·6, 8 Hz), 6·75 (ddd, 1H, J= 1·68, 7·92, 9·24 Hz); 13C NMR (100 MHz, DMSO-d6): δ 149·21, 140·92, 124·88, 123·13, 119·25, 117·24.
Pyrogallol-O-sulfate: 677 mg, 75 % yield; mixture of two compounds in an approximately similar proportion; pyrogallol-2-O-sulfate ((2,6-dihydroxyphenyl)oxidanesulfonic acid): 1H NMR (400 MHz, D2O): δ 6·97 (t, 1H, J= 8·24 Hz, H-4), 6·51 (d, 2H, J= 8·28 Hz, H-3+H-5) and pyrogallol-O-sulfate ((2,3-dihydroxyphenyl)oxidanesulfonic acid): 1H NMR (400 MHz, D2O): δ 6·90–6·86 (m, 1H, H-5), 6·79–6·74 (m, 2H, H-4+H-6); 13C NMR (100 MHz, D2O): δ 149·84, 145·42, 139·76, 127·19, 120·34, 114·31, 113·80, 108·93.
2-Methylpyrogallol-O-sulfate ((3-hydroxy-2-methoxyphenyl)oxidanesulfonic acid): 450 mg, 44 % yield, having 11 % contamination with the disulfated by-product. 1H NMR (400 MHz, DMSO-d6): δ 8·97 (s, 1H, OH), 6·97 (dd, J= 1·52, 8·32 Hz, H-6), 6·74 (t, 1H, J= 8·2 Hz, H-5), 6·51 (dd, 1H, J= 1·52, 8·08 Hz, H-4), 3·34 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ 150·57, 147·20, 139·10, 122·33, 112·30, 110·89, 60·09.
1-Methylpyrogallol-O-sulfate: 500 mg, 58 % yield; (2-hydroxy-6-methoxyphenyl)oxidanesulfonic acid and (2-hydroxy-3-methoxyphenyl)oxidanesulfonic acid, mixture of both compounds in approximately equal amounts (56 %:44 %). 1H NMR (400 MHz, DMSO-d6): δ 9·20 (s, 1H, OH), 8·52 (s, 1H, OH), 6·92 (t, 1H, J= 8·60 Hz), 6·77–6·68 (m, 3H), 6·52–6·44 (dd, 2H); 13C NMR (100 MHz, DMSO-d6): δ 153·39, 150·93, 149·17, 141·35, 139·07, 124·86, 118·07, 115·65, 110·08, 108·64, 104·11, 55·87, 55·72.
4-Methylcatechol-O-sulfate: 604 mg, 66 % yield. Mixture of two compounds, (2-hydroxy-4-methylphenyl)oxidanesulfonic acid and (2-hydroxy-5-methylphenyl)oxidanesulfonic acid, that were not chromatographically separable from each other. One is present at 64 %, 1H NMR (400 MHz, DMSO-d6): δ 8·76 (s, 1H, OH), 6·96 (d, 1H, J= 8·08 Hz), 6·64 (d, 1H, J= 1·76 Hz), 6·55 (dd, 1H, J= 2·04, 8·6 Hz), 2·20 (s, 3H, CH3). The other present at 36 %, 1H NMR (400 MHz, DMSO-d6): δ 8·62 (s, 1H, OH), 6·93 (d, 1H, J= 1·84 Hz), 6·77 (dd, 1H, J= 1·56, 8·04 Hz), 6·71 (d, 1H, J= 8·12 Hz), 2·19 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ 148·87, 146·80, 140·70, 138·80, 134·20, 128·14, 125·27, 123·58, 122·94, 119·87, 117·77, 116·96, 20·06, 20·47.
4-Methylgallic-3-O-sulfate (3-hydroxy-4-methoxy-5-(sulfooxy)benzoic acid): 485 mg, 57 % yield, having a contamination of 13 % of the disulfate by-product and 6 % of the starting material; 1H NMR (400 MHz, D2O): δ 7·46 (d, 1H, J= 2·04 Hz, H-2), 7·32 (d, 1H, J= 2 Hz, H-6), 3·88 (s, 3H, CH3); 13C NMR (100 MHz, D2O): δ 172·07, 149·19, 143·99, 142·85, 129·61, 115·42, 114·84, 61·29.
Vanillic acid-4-O-sulfate (3-methoxy-4-(sulfooxy)benzoic acid): 924 mg, quantitative yield. 1H NMR (400 MHz, DMSO-d6): δ 7·57 (d, 1H, J= 8·16 Hz, H-2), 7·50–7·47 (m, 2H, H-5+H-6), 3·79 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ 167·39, 149·51, 148·4, 146·41, 121·96, 119·33, 112·71, 55·48.
Results
Composition of the fruit purée
Detailed identification and quantification of polyphenols in the fruit purée have been described previously( Reference Pimpao, Dew and Figueira 12 ). The purée (500 ml) containing blueberries, raspberries, blackberries, Portuguese crowberry and strawberry tree fruits was characterised by HPLC diode array detector for major compounds (caffeoylquinic acids and anthocyanins), and the aglycones were also quantified after multi-enzyme hydrolysis of the glycosides. The most abundant compounds were anthocyanins (636 (se 19) mg) and caffeoylquinic acids (5-caffeoylquinic and 3-caffeoylquinic acids, 135·9 (sd 2·1) mg). The aglycones were quantified after hydrolysis using glycosidases from Aspergillus niger as published previously( Reference Pimpao, Dew and Oliveira 15 ). Gallic acid (GA) was the most abundant aglycone (425·9 (sd 14·0) mg/500 ml fruit purée). Caffeic acid (CA) was also abundant (140·4 (se 2·2) mg/500 ml fruit purée), resulting from the hydrolysis of caffeoylquinic acids and conjugated glycosides. After hydrolysis, several other phenolic acids were detected and quantified including the flavanols ( − )-epicatechin and (+)-catechin, and the flavonols quercetin, myricetin and kaempferol. The presence of VA, ferulic acid (FA) and protocatechuic acid (PA) were also observed in the hydrolysed extract.
Chemical synthesis of sulfated phenolic metabolites
Polyphenol metabolites have been identified previously in urine samples from human volunteers after ingestion of fruit purée( Reference Pimpao, Dew and Figueira 12 ). In the present study, we focused on the quantification of sulfated metabolites in plasma. Some of these compounds were chemically synthesised for this purpose (Table 1). Synthesised compounds were mostly pure as determined by NMR, except for 2-methylpyrogallol-O-sulfate (89 % pure due to a double sulfate-substituted compound) and 4-methylgallic acid-3-O-sulfate (4-MeGA-sulf, 81 % pure due to the presence of some aglycone and a double sulfate-substituted derivative). The liquid chromatography–MS/MS and chromatographic parameters of the synthesised standards are shown in Table 1, with most compounds resulting in single peaks. However, for the compounds 4-methylcatechol (4-MeCat) and 1-methylpyrogallol (1-MePyr), two different sulfated metabolites were obtained after synthesis, as observed by NMR, although they co-eluted as only one chromatographic peak. PA-3-sulf and PA-4-sulf also co-eluted when run together on HPLC–MS/MS.
Plasma appearance of phenolic metabolites
Previously, several metabolites were identified in the urine of volunteers after ingestion of the fruit purée; although only a relative quantification was performed, sulfated phenolic metabolites were more abundant (from 5- to 200-fold) than glucuronidated phenolic metabolites( Reference Pimpao, Dew and Figueira 12 ). In the present study, plasma obtained from the volunteers was analysed after ingestion of the fruit purée for confirmation of the presence of those sulfated phenolic metabolites by liquid chromatography–MS/MS (Fig. 2). GA and its conjugated metabolites 4-MeGA and 4-MeGA-sulf were identified. Sulfated metabolites of VA, FA, dihydroferulic acid and dihydrocaffeic acid (DHCA) were also detected in plasma. Protocatechuic acid-O-sulfate (PA-sulf) was also present; however, its isomers PA-3-sulf and PA-4-sulf could not be distinguished from one another since they co-eluted.

Fig. 2 Structure of phenolic metabolites identified in plasma obtained from the volunteers: 4-MeGA, 4-O-methylgallic acid; PA, protocatechuic acid; VA, vanillic acid; CA, caffeic acid; FA, ferulic acid; IFA, isoferulic acid; DHCA, dihydrocaffeic acid; DHFA, dihydroferulic acid; Cat, catechol; 4-MeCat, 4-methylcatechol; Pyr, pyrogallol; MePyr, methylpyrogallol.
Although one peak of sulfated DHCA was found in plasma at low levels, the presence of two sulfated metabolites was likely since they co-eluted (Table 2). The presence of the sulfated metabolites of catechol, 4-MeCat, 1-MePyr and 2-MePyr was also confirmed. Furthermore, two sulfated metabolites of pyrogallol were identified, corresponding to the synthesised standards.
Table 2 Presence and concentrations of the conjugated phenolic metabolites in the plasma of volunteers after ingestion of 500 ml of fruit purée (Mean values with their standard errors)
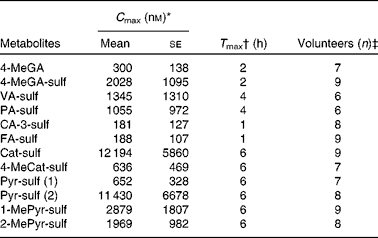
For the description of the compound abbreviations, please refer to Table 1.
* C max= peak plasma concentration calculated from the average concentration in volunteers indicated in the column ‘Volunteers (n)’.
† T max= time to reach C max of the metabolites for the times of collection. It includes higher concentration of some metabolites after 6 h.
‡ Volunteers (n) = volunteers in whom the presence of the indicated metabolites was confirmed.
Quantification of plasma phenolic metabolites
The synthesised standards or pure aglycones were used for the quantification of the phenolic metabolites (Table 1). For the co-eluting compounds in plasma (sulfates of PA, 1-MePyr and 4-MeCat), quantification was done as corresponding to a single compound. Measurement of the plasma metabolites was made between 0 and 6 h after ingestion of 500 ml fruit purée. The pharmacokinetic profile of each metabolite is shown in Fig. 3, and an average maximum concentration of the metabolites when present in six or more volunteers is presented in Table 2. Although GA was present in all volunteers, it was mostly found under the limit of quantification, only being quantifiable in three out of nine volunteers, reaching a maximum concentration of 840 (se 340) nm at 1 h after ingestion of the fruit purée. However, its conjugated metabolites 4-MeGA and 4-MeGA-sulf clearly peaked at 2 h. Dihydroferulic acid-O-sulfate was detected in the plasma of volunteers; however, it was found under the limit of quantification. Dihydrocaffeic acid-O-sulfate was only quantifiable in four volunteers, reaching a maximum concentration of 151 (se 18) nm at 6 h.

Fig. 3 Quantification of plasma phenolic metabolites at baseline and at 0·5, 1, 2, 4 and 6 h after ingestion of 500 ml fruit purée. (a) 4-O-Methylgallic acid, (b) 4-methylgallic acid-3-O-sulfate, (c) protocatechuic acid-O-sulfate, (d) vanillic acid-4-O-sulfate, (e) caffeic acid-O-sulfate, (f) ferulic acid-4-O-sulfate, (g) catechol-O-sulfate, (h) 4-methylcatechol-O-sulfate, (i) pyrogallol-O-sulfate (1), (j) pyrogallol-O-sulfate (2), (k) 1-methylpyrogallol-O-sulfate and (l) 2-methylpyrogallol-1-O-sulfate. Values are medians, with the boxes representing the interquartile range, and the whiskers representing the minimum and maximum values. Median value was significantly different from that at baseline (time zero): * P< 0·05, ** P< 0·01.
The metabolites catechol-O-sulfate (Cat-sulf), 4-methylcatechol-O-sulfate (4-MeCat-sulf), pyrogallol-O-sulfates (Pyr-sulf) and O-methylpyrogallol-O-sulfates were found in several volunteers at baseline. Concentrations of all these metabolites, with the exception of 4-MeCat-sulf, were statistically significantly increased over the collection period time in comparison to the baseline. Interestingly, concentrations of the compounds Cat-sulf and Pyr-sulf reached even up to 20 μm in some volunteers. However, variability in plasma concentrations between the volunteers was high for all the quantified metabolites.
Comparison between ingestion of the fruit purée and the polyphenol-free meal
Confirmation of the provenance of the metabolites analysed was assessed by the ingestion of a polyphenol-free meal (control) as opposed to the ingestion of the fruit purée by the same volunteers. Comparative analysis was carried out in plasma samples collected at baseline and at 2 and 4 h after ingestion, and is summarised in Fig. 4. At baseline, no differences were observed between ingestion of the fruit purée or the polyphenol-free meal. However, for most metabolites, a statistically significant increase was observed after ingestion of the fruit purée at a certain time point. The only exceptions were observed for PA-sulf, whose concentration increased in plasma both after ingestion of the fruit purée and the control meal, and for vanillic acid-4-O-sulfate (VA-sulf), whose concentration was found to be higher in plasma at 4 h after ingestion of the control meal than after ingestion of the fruit purée.

Fig. 4 Quantification of plasma phenolic metabolites at baseline and at 2 and 4 h after ingestion of the fruit purée (■) or the standard breakfast (□). (a) 4-O-Methylgallic acid, (b) 4-methylgallic acid-3-O-sulfate, (c) protocatechuic acid-O-sulfate, (d) vanillic acid-4-O-sulfate, (e) caffeic acid-O-sulfate, (f) ferulic acid-4-O-sulfate, (g) catechol-O-sulfate, (h) 4-methylcatechol-O-sulfate, (i) pyrogallol-O-sulfate (2), (j) 2-methylpyrogallol-1-O-sulfate and (k) 3-methylpyrogallol-1-O-sulfate. Statistical analysis was carried out to compare plasma concentrations of each volunteer after ingestion of the fruit purée or the standard breakfast. Values are medians, with the boxes representing the interquartile range, and the whiskers representing the minimum and maximum values. Median value was significantly different from that at baseline (time zero): * P< 0·05, ** P< 0·01.
For Pyr-sulf, although a small increase in concentration was observed in the plasma of volunteers at 2 h after ingestion of the control meal, there was a marked increase in the plasma concentration of this metabolite in volunteers after ingestion of the fruit purée at 4 h.
Discussion
Conjugation reactions, particularly sulfation, glucuronidation and methylation, are known to be involved in the metabolism of phenolic compounds in the human body, generally resulting in stabilisation and increased water solubility, and therefore modifying their distribution and excretion( Reference Wu, Basu and Meng 16 , Reference Kurogi, Alazizi and Liu 17 ). Sulfate conjugation is one of the major metabolic pathways for endogenous and exogenous phenolic compounds( Reference Riches, Stanley and Bloomer 18 ). Sulfation of phenolic compounds is mediated by cytosolic sulfotransferases (SULT), which catalyse the transfer of a sulfonate group from 3′-phosphoadenosine-5′-phosphosulfate to a substrate containing a hydroxyl group. The isoforms SULT1A1, SULT1A3/4, SULT1B1, SULT1E1 and SULT2A1 are considered the most relevant in polyphenol metabolism in human adults( Reference Riches, Stanley and Bloomer 18 ). Besides sulfation, methylation catalysed by catechol-O-methyltransferase is also important for the metabolism of phenolic compounds( Reference Kurogi, Alazizi and Liu 17 ). Previously, we demonstrated that sulfated phenols are detected in the urine after ingestion of a fruit purée containing a mixture of five berry fruits( Reference Pimpao, Dew and Figueira 12 ). In the present study, some of these compounds were chemically synthesised and used for quantification purposes in plasma collected from volunteers. Confirmation of their provenance was also achieved by comparing their amounts in the plasma of volunteers consuming the fruit purée or a polyphenol-free standard meal.
GA, a compound present as aglycone in high amounts in the fruit purée, was previously quantified in human plasma, as well as its methylated metabolite 4-MeGA, after ingestion of GA tablets and black tea( Reference Shahrzad, Aoyagi and Winter 19 ). Its kinetics were similar to those observed in the present study, reaching a maximum concentration at approximately 1·5 h. 4-MeGA-sulf, identified for the first time in the urine of rats by Yasuda et al. ( Reference Yasuda, Inaba and Ohmori 20 ), was quantified in human plasma for the first time in the present study and was undoubtedly derived from GA and its derivatives present in the fruit purée (Fig. 4). Its kinetic profile was similar to those of GA and 4-MeGA, reaching a maximum concentration at 2 h (Fig. 3 and Table 1), suggesting that the absorption and metabolism of GA was fast, with the 4-hydroxyl group being regioselectively preferred for methylation and the 3-hydroxyl group for sulfation.
Previous studies suggested PA as one of the major metabolites derived from the degradation of cyanidin( Reference Tsuda, Horio and Osawa 21 , Reference Kay, Kroon and Cassidy 22 ). Of the compounds studied herein, PA-sulf, VA-sulf and ferulic acid-4-O-sulfate (FA-sulf) have previously been identified in plasma as metabolites resulting from the degradation of 13C-labelled cyanidin-3-glucoside in the digestive tract( Reference Czank, Cassidy and Zhang 9 ). However, in the present study, there was a significant increase in the concentrations of PA-sulf and VA-sulf only at 6 h compared with baseline, and their increase was also observed after ingestion of the control meal. Since the foods in the control meal were selected to contain minimal amounts of polyphenols, the phenolic metabolites present after ingestion of the control meal are likely to be derived from endogenous metabolism. A comparable example is dopamine metabolism, where homovanillic acid is a known metabolite, which would probably exist as homovanillic acid sulfate( Reference Muskiet and Groen 23 ).
Hydroxycinnamic acid metabolism has been widely studied in relation to coffee consumption, and the present results are consistent with these data. Sulfation is dominant over glucuronidation for most of the hydroxycinnamic acids( Reference Wong, Meinl and Glatt 24 ), but methylation also appears to have an important role, resulting in the production of FA and isoferulic acid( Reference Stalmach, Mullen and Barron 25 ). Caffeic acid-3-O-sulfate and FA-sulf clearly resulted from the ingestion of the fruit purée (Fig. 4) and were quantified in plasma, with similar values and absorption kinetics. These results are in accordance with previous studies proposing rapid absorption kinetics for the metabolites of CA and FA in plasma( Reference Stalmach, Mullen and Barron 25 , Reference Stalmach, Edwards and Wightman 26 ). DHCA and dihydroferulic acid conjugates were not quantifiable in plasma, as they were mostly under the limit of quantification. However, these conjugates are derived from the action of colonic bacterial reductases( Reference Stalmach, Mullen and Barron 25 ), and so would only start to appear at 5 h and have a T max between 7 and 11 h( Reference Renouf, Marmet and Giuffrida 27 ).
Previously, we identified conjugated phenolic metabolites possibly resulting from the catabolism of other polyphenols( Reference Pimpao, Dew and Figueira 12 ). Their colonic origin has been confirmed since some unconjugated phenolic metabolites have been identified in the faeces after ingestion of polyphenols( Reference Stalmach, Edwards and Wightman 10 , Reference Gonzalez-Barrio, Edwards and Crozier 28 ). However, our data suggest that absorption in the colon might also occur, and these compounds can subsequently be found to be conjugated in plasma. The origin of these phenolic conjugates such as sulfated metabolites of catechol, pyrogallol, 2-MePyr and 3-MePyr resulted from the consumption of the fruit purée, and to our knowledge, this is the first study to report that these compounds were quantified in human plasma and associated with polyphenol metabolism. Clearly, this class of metabolites has been neglected in bioavailability studies, and since they reached considerable amounts in plasma, further studies are needed to assess their biological significance. Recently, the importance of colonic catabolism of polyphenols and several metabolites has been appreciated. Due to the ability of the colonic microbiota to catalyse reactions such as O- and C-deglycosylation, ester and amide hydrolysis, and deglucuronidation, flavonoids, once considered to show poor bioavailability, might result in smaller phenolic compounds with a much higher absorption than their parent compounds( Reference Moco, Martin and Rezzi 29 ). Additionally, new classes of metabolites have been specifically assigned to colonic metabolites of flavonoids, such as valerolactones from catabolism of catechins, and urolithins from catabolism of ellagic acid and ellagitannins( Reference Gonzalez-Barrio, Edwards and Crozier 28 , Reference Monagas, Urpi-Sarda and Sanchez-Patan 30 ). These metabolites of colonic origin might be of great importance for the health effects commonly associated with the ingestion of polyphenols.
Glucuronidation has been described as being a major route for the conjugation of phenolic compounds such as acetaminophen( Reference McGill and Jaeschke 31 ), quercetin( Reference Cubitt, Houston and Galetin 32 ), PA and VA( Reference Xu, Zhang and Fu 33 ), and other substances with a 1,2-dihydroxybenzene group( Reference Elovaara, Mikkola and Luukkanen 34 ). However, sulfation is the primary conjugation route of the hydroxycinnamic acids CA, FA and DHCA( Reference Wong, Meinl and Glatt 24 ), and many other sulfated phenolics have been reported, including sulfates of PA and VA( Reference de Ferrars, Cassidy and Curtis 35 ). Methylation may be responsible for the conversion of pyrogallol to 1-MePyr and 2-MePyr, GA to 4-MeGA, CA to FA, DHCA to dihydroferulic acid, and PA to VA. Additionally, all of these compounds could be sulfated, and this dual conjugation of compounds will affect the reactivation of conjugated compounds via deconjugation reactions( Reference Kurogi, Alazizi and Liu 17 ).
In summary, the present results confirm the provenance of several phenolic conjugates resulting from the ingestion of a polyphenol-rich berry fruit purée by human subjects, since their concentrations increase in plasma in comparison to either the baseline or after ingestion of a polyphenol-free control meal. Metabolism of fruit polyphenols results in methylated, sulfated and some dual conjugated compounds. We highlight the importance of catabolism in the colon, generating simple phenols that can then be absorbed from the colon and circulate in conjugated form in the blood. Therefore, they contribute indirectly to the bioavailability of food polyphenols, and can also potentially be used as markers of polyphenol intake.
Acknowledgements
The authors thank Pedro Oliveira (Instituto Nacional de Investigação Agrária, Oeiras, Portugal) for providing Vaccinium spp., Rubus spp. and R. idaeus. The authors also acknowledge the clinical analysis services of the Faculdade de Farmácia da Universidade de Lisboa for collecting the plasma samples, and the volunteers who participated in the study.
The present study was supported by the Fundação para a Ciência e a Tecnologia through grants PEst-OE/EQB/LA0004/2011 and SRFH/BPD/84618/2012, IF/01097/2013 (C. N. S.) and SFRH/BD/63615/2009 (R. C. P.). G. W. acknowledges funding from the European Research Council through an advanced grant (POLYTRUE? 322467), and C. N. S. and R. C. P. thank the EC for the grant EU FP7 EUBerry KBBE-2010–4 265942. G. W. and C. N. S. acknowledge the grant in the ambit of the Treaty of Windsor Programme.
The authors' contributions are as follows: R. C. P., G. W., R. B. F. and C. N. S. designed the research; R. C. P. and C. N. S. recruited and liaised with the volunteers; R. C. P. carried out the experimental work and analysed the data; M. R. V. planned and supervised the chemical synthesis of the standards; R. C. P. wrote the first version of the manuscript. All the authors contributed to the writing of the manuscript and approved the final version.
The authors declare that there are no conflicts of interest.









