Nutritional epidemiologists encounter a multitude of challenges when conceptualising research studies that are designed to explore the beneficial effects of dietary intake on health outcomes. From a reductionist perspective, the focus is on a single nutrient, followed by foods and/or food groups. However, a healthy diet is not based on the consumption of a single nutrient or a specific food that is consumed in an isolative manner. Additionally, the synergy that exists from nutrients within and between foods can be beneficial or deleterious to specific health outcomes, and substitution effects generally occur with dietary manipulations (e.g. high intake of fibre is accompanied by a reduction in fat intake). Thus, the optimal method to evaluate diet and metabolic disorders and to promote chronic disease prevention is through a more holistic approach using dietary patterns, which has real-world applications and is becoming the gold standard in nutritional epidemiological research.
Studies of dietary patterns generally address the amounts, proportions, frequency and variety of foods that are habitually consumed in a population and their subsequent relationship with metabolic disorders and chronic diseases. A caveat in the study of dietary patterns is that universal definitions of dietary patterns vary from study to study, which makes comparisons between studies challenging. Although many dietary patterns (e.g. Western(Reference Heidemann, Schulze and Franco1, Reference Paradis, Perusse and Vohl2), healthy or prudent(Reference Pryer, Cook and Shetty3), Mediterranean(Reference Rumawas, Meigs and Dwyer4)) are associated with an increased or decreased risk for developing metabolic disorders and chronic diseases, the primary focus of the present study will be limited to presenting the existing evidence on vegetarian dietary patterns and the risk of developing the metabolic syndrome (MetS). The present paper was written based on the articles used for developing a scientific presentation titled ‘Vegetarian Diets and Risk of Metabolic Syndrome – Mediterranean Diets as a Paradigm’ for the 1st World Forum for Nutrition Research Conference, which was held in Reus, Spain on 21 May 2013. Also, a MEDLINE search was performed in PubMed using the MeSH terms ‘metabolic syndrome’ and ‘vegetarian diet’ in the abstract and title of the article, which yielded six additional relevant articles that were incorporated into the paper.
Metabolic syndrome
The MetS is a cluster of disorders that is associated with an increased risk for developing type 2 diabetes and CVD(Reference Ambrosini, Huang and Mori5, Reference McNeill, Rosamond and Girman6). The MetS is present in 78 % of persons with prediabetes and in 86 % of individuals with type 2 diabetes(Reference Grundy7). The prevalence of the MetS in the USA is approximately 20 %(Reference Beltran-Sanchez, Harhay and Harhay8) and is currently increasing in developing countries in line with the obesity epidemic(Reference Misra and Khurana9). The health care costs associated with the MetS are on a magnitude of 1·6 overall compared with healthy individuals(Reference Boudreau, Malone and Raebel10), which makes it an important public health problem.
Two main classification systems are in place for the clinical identification of the MetS, the 2001 National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III criteria, which was updated in 2005 by the American Heart Association and the National Heart Lung and Blood Institute, and the 2005 International Diabetes Foundation (IDF) criteria. The NCEP ATP III defines the MetS as the presence of three or more of the following five criteria: waist circumference (WC)>40 inches (male) or >35 inches (female); blood pressure (BP)>130/85 mmHg; fasting TAG>1·69 mmol/l; fasting HDL < 1·03 mmol/l (male) or 1·29 mmol/l (female); and fasting blood glucose (FBG)>5·56 mmol/l(Reference Grundy, Cleeman and Daniels11). The IDF criteria includes the same criteria as the NCEP ATP III; however, it requires that obesity, but not insulin resistance (IR), be present(Reference Zimmet, Magliano and Matsuzawa12). Also, population-specific cutpoints for obesity exist to account for the variable distributions of norms for body weight and WC across different populations, ethnicities and nationalities. Hence, in light of the increasing prevalence of the MetS worldwide and the associated health consequences, there is a pressing need to identify dietary patterns and other lifestyle factors that may be effective in the prevention and management of the MetS.
Vegetarian dietary patterns
A vegetarian dietary pattern is operationally defined in epidemiological studies based on what is excluded from the diet (e.g. degree of animal food intake) v. what is consumed on a regular basis, which becomes problematic when comparing health outcomes across cohort studies. Additionally, the questions that frequently arise are ‘What do vegetarians eat?’ and ‘Do vegetarians eat healthy?’ The vegetarian dietary pattern is traditionally a plant-based diet that includes fruits, vegetables, cereals, legumes, nuts, vegetable oils, soya, and possibly dairy products and/or eggs. Vegetarians(Reference Fraser13, Reference Key, Fraser and Thorogood14) and other populations that follow a plant-based diet(Reference Bamia, Trichopoulos and Ferrari15), plant-based low-carbohydrate diet(Reference Fung, van Dam and Hankinson16), Mediterranean diet(Reference Knoops, Groot de and Fidanza17–Reference Trichopoulou, Costacou and Bamia20) and healthy or prudent diet(Reference Knoops, Groot de and Fidanza17, Reference Bazelmans, De Henauw and Matthys21, Reference Waijers, Ocke and van Rossum22) enjoy longevity. Specifically, vegetarian dietary patterns have been associated with a lower risk for developing IHD(Reference Fraser13, Reference Fraser and Fraser23), type 2 diabetes(Reference Tonstad, Butler and Yan24, Reference Tonstad, Stewart and Oda25), hypertension(Reference Appleby, Davey and Key26, Reference Pettersen, Anousheh and Fan27), specific cancers(Reference Tantamango-Bartley, Jaceldo-Siegl and Fan28), lower all-cause mortality(Reference Orlich, Singh and Sabaté29) and reduction in cause-specific mortality(Reference Orlich, Singh and Sabaté29).
Obesity and insulin resistance
Obesity and IR are both significant contributing factors in the development of the MetS. Consistent findings across six epidemiological studies of adult vegetarians (Adventist Mortality Study, Adventist Health Study-1 (AHS-1), AHS-2, Heidelberg Study, Oxford Vegetarian Study, EPIC-Oxford) have shown that a progressive increase in BMI is observed as meat and animal products are increasingly included in the diet(Reference Sabaté, Blix and Sabaté30). Further, strict vegetarian and lacto-ovo-vegetarian diets afford greater protection against overweight than omnivores. Irrespective of age, sex, race and geography, vegetarians are leaner than non-vegetarians and have a lower risk of obesity(Reference Tonstad, Butler and Yan24).
In a case–control study involving 391 female Buddhist vegetarians (approximately 80 % lacto-ovo-vegetarian) and 315 female Buddhist non-vegetarians attending a health clinic in Taiwan(Reference Chiang, Lin and Chen31), a subgroup of non-diabetic participants who were vegetarian were found to have a 29 % lower risk of homoeostasis model of assessment-IR (HOMA-IR) compared with non-vegetarians (OR 0·71, 95 % CI 0·48, 1·06; P= 0·094). Similarly, a case–control study of healthy normal-weight adults (age 19–64 years) residing in Bratslava(Reference Valachovicová, Krajcovicova-Kudlackova and Blazicek32) found lower FBG (P< 0·01) and HOMA-IR (P< 0·001) values among ninety-five long-term lacto-ovo-vegetarians compared with 107 non-vegetarians (e.g. Western diet). With respect to age, HOMA-IR was significantly higher in the 31- to 40-year age range and in the later age decades among the non-vegetarians compared with the lacto-ovo-vegetarians. These findings are supportive of dairy products as a component of a healthy vegetarian diet(Reference Weaver33) and are consistent with the evidence from the Coronary Artery Risk Development in Young Adults (CARDIA) study(Reference Pereira, Jacobs and Van Horn34) that found that each daily serving of dairy products reduced the risk of developing IR by 21 %.
Cross-sectional studies
A cross-sectional study of 773 subjects (age 30–94 years) that participated in a Calibration Sub-study of the AHS-2 was conducted to evaluate the risk of the MetS according to dietary pattern(Reference Rizzo, Sabaté and Jaceldo-Siegl35). Dietary patterns were categorised as vegetarian (meat, poultry or fish < 1 time/month), semi-vegetarian (fish at any frequency and consuming other meat products < 1 time/month or total meat ≥ 1 time/month and < 1 time/week) and non-vegetarian (red meat or poultry ≥ 1 time/month and total of all meat products ≥ 1 time/week) using a validated FFQ. A vegetarian dietary pattern was associated with a 56 % lower risk of having the MetS compared with a non-vegetarian dietary pattern (OR 0·44, 95 % CI 0·30, 0·64; P< 0·001) and yielded significantly lower means for TAG, FBG, BP and WC (Table 1). The more favourable metabolic risk factor profile persisted after adjustments were made for other lifestyle (e.g. educational status, smoking, alcohol intake, physical activity and dietary energy intake) and demographic (e.g. age, sex and ethnicity) factors. Further, the prevalence of the MetS was 25·2 % among vegetarians, 37·6 % among semi-vegetarians and 39·7 % among non-vegetarians (P for trend < 0·001).
Table 1 Overview of select studies on vegetarian dietary patterns and metabolic syndrome (MetS) risk factors
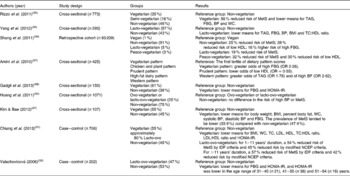
FBG, fasting blood glucose; BP, blood pressure; WC, waist circumference; TC, total cholesterol; HOMA-IR, homoeostasis model of assessment-insulin resistance; IDF, International Diabetes Foundation; NCEP, National Cholesterol Education Program.
Consistent findings were observed in another cross-sectional study(Reference Yang, Li and Zhang36) that was conducted among 196 healthy Chinese lacto-vegetarians and 126 healthy Chinese omnivores (age 21–76 years), which found to have lower BMI, BP and serum levels of TAG, total cholesterol, LDL, FBG and the total cholesterol:HDL ratio in the lacto-vegetarian group (all P< 0·05) (Table 1). Additional analysis showed that the lacto-vegetarians (age 24–55 years) had a lower probability of developing CVD in 5–10 years compared with the omnivores using the same age range criteria.
In a cross-sectional study among 425 adults (age 35–55 years) with impaired glucose tolerance participating in the Isfahan Diabetes Prevention Study(Reference Amini, Esmaillzadeh and Shafaeizadeh37), a Western diet was positively associated with high TAG (OR 1·76, 95 % CI 1·01, 3·07) and high BP (OR 2·62, 95 % CI 1·32, 5·23) whereas a prudent diet was associated with reduced odds of low HDL (OR 0·55, 95 % CI 0·31, 0·96). Additionally, a vegetarian diet was inversely associated with elevated FBG (OR 2·26, 95 % CI 1·25, 4·06). Consistent with these observations, Gadgil et al. (Reference Gadgil, Anderson and Kandula38) have recently reported that among 150 Asian Indians (age 45–84 years) participating in the Metabolic Syndrome and Atherosclerosis in South Asians Living in America (MASALA) study, a vegetarian diet was associated with lower HOMA-IR (P= 0·05) compared with a Western diet in the context of lower HDL concentrations (P= 0·09). Lower HDL concentrations have similarly been reported in the aforementioned case–control study involving the female Buddhist vegetarians in Taiwan(Reference Chiang, Lin and Chen31). Compared with the non-vegetarians, a reduced risk for the MetS was found for lacto-ovo-vegetarians of 1–11 years' duration and >11 years' duration by 45 % (OR 0·55, 95 % CI 0·32, 0·92) and 42 % (OR 0·58, 95 % CI 0·33, 0·997), respectively.
Retrospective cohort studies
A retrospective cohort study was conducted to assess the risk of the MetS among 93 209 Taiwanese who were categorised as vegan, pesco-vegetarian, lacto-vegetarian and non-vegetarian using data from a longitudinal health check-up database(Reference Shang, Shu and Wang39). After adjusting for demographic and lifestyle factors, the hazard ratios of the MetS for non-vegetarians, pesco-vegetarians and lacto-vegetarians were 0·75 (95 % CI 0·64, 0·88), 0·68 (95 % CI 0·55, 0·83) and 0·81 (95 % CI 0·67, 0·97) compared with vegans. In terms of specific MetS risk factors, non-vegetarians and pesco-vegetarians had a 28 and 30 % reduced risk of developing low HDL than vegans with an adjusted hazard ratio of 0·72 (95 % CI 0·62, 0·84) and 0·70 (95 % CI 0·57, 0·84), respectively (Table 1). The investigators suggested that the low dietary fat intake among the vegans could have induced low HDL levels, which has been described by others comparing low- and high-fat diets on CVD risk factors(Reference Ashen and Blumenthal40, Reference Meksawan, Pendergast and Leddy41). Lastly, compared with vegans, non-vegetarians demonstrated a 1·16 times risk of high FBG (95 % CI 1·02, 1·32), which may be due to the effect of a higher fat intake on diminishing insulin sensitivity(Reference Barnard, Cohen and Jenkins42–Reference Lovejoy, Windhauser and Rood46).
Dietary pattern analysis
To address the complexity of dietary intake, nutritional epidemiologists have embraced dietary pattern analysis to examine the association between dietary patterns and the MetS. A 2006 review of three large robust epidemiological studies(Reference Baxter, Coyne and McClintock47), more specifically the Isle of Ely(Reference Williams, Prevost and Whichelow48), Malmo Diet and Cancer Project(Reference Wirfalt, Hedblad and Gullberg49), and the CARDIA study(Reference Pereira, Jacobs and Van Horn34), has shown that dietary patterns high in fruits, vegetables and dairy products are associated with a lower prevalence of the MetS, and dietary patterns with high meat intake are associated with components of the MetS, particularly impaired glucose tolerance. Further, minimally processed cereals are associated with a decreased risk of the MetS while highly processed cereals with a high glycemic index are associated with a higher risk. These data strengthen the findings from the aforementioned large studies of vegetarians and emphasise the importance of healthy food patterns for preventing metabolic disturbances. The investigators concluded that no single dietary component could be considered wholly responsible for the association of diet with the MetS and that the overall diet quality appears to confer protection against the MetS.
More recently, a prudent dietary pattern (e.g. dairy products, whole grains, fruits, vegetables and coffee) was compared with a Western dietary pattern (e.g. meat, refined grains, fried foods and sweetened beverages) using prospective data from 9514 subjects (age 45–64 years) participating in the Atherosclerosis Risk in Communities (ARIC) Study(Reference Lutsey, Steffen and Stevens50). Over 9 years of follow-up, the Western pattern was adversely associated with incident MetS (P for trend = 0·03). Further analysis of individual food groups showed that meat, fried foods and diet soda were adversely associated with the MetS and dairy intake was protective (P for trend < 0·05 for all). However, no associations were found between incident MetS and a prudent dietary pattern, nor the intake of whole grains, refined grains, fruits, vegetables, nuts, coffee or sweetened beverages.
The use of factor analysis to derive dietary patterns within South Asian(Reference Garduno-Diaz and Khokhar51), Taiwanese(Reference Huang, Fan and Liu52) and South Korean(Reference Kim and Jo53) adult populations has revealed conflicting results in the context of vegetarian diet patterns and the MetS risk factors. First, among South Asians living in the UK who experienced dietary acculturation(Reference Garduno-Diaz and Khokhar51), a reduction in vegetarianism and acceptance of a Western dietary pattern was not associated with a higher prevalence of the MetS. The investigators speculated that the inclusion of full-fat dairy products and low consumption of raw fruits and vegetables may partially explain their surprising findings. Second, using data from 1071 elderly participants (age ≥ 65 years) in the Elderly Nutrition and Health Survey in Taiwan (1999–2000), Huang et al. (Reference Huang, Fan and Liu52) failed to observe an association between the MetS for vegetarians compared with omnivores. Lastly, a dietary pattern that included grains, vegetables and fish was associated with a lower risk of high TAG (P for trend < 0·01) and a 14 % reduced risk of the MetS (P for trend = 0·02) among 9850 South Korean adults who participated in the 2001 and 2005 Korean National Health and Nutrition Examination Surveys(Reference Kim and Jo53).
Childhood dietary intake studies
The investigative team for the Cardiovascular Risk in Young Finns Study recently explored the associations between childhood dietary factors (e.g. frequency of vegetable, fruit, fish and meat intake, and use of butter on bread) and physical activity and risk of the MetS in adulthood(Reference Jaaskelainen, Magnussen and Pahkala54). After 27 years of follow-up in a cohort of 2218 (age at baseline 3–18 years), a 14 % decrease in the risk of developing the MetS was observed for a higher intake of childhood vegetable consumption (OR 0·86, 95 % CI 0·77, 0·97). Further, a lower intake of vegetable consumption predicted high BP (OR 0·88, 95 % CI 0·80, 0·98) and high TAG (OR 0·88, 95 % CI 0·79, 0·99). These results complement our previous findings from the Child-Adolescent Blood Pressure Study that showed that the frequency of vegetables (OR 0·67, 95 % CI 0·48, 0·94), grains (OR 0·59, 95 % CI 0·41, 0·83), nuts (OR 0·60, 95 % CI 0·43, 0·85) and low-nutrient-dense foods (OR 0·43, 95 % CI 0·29, 0·63) were inversely related to the risk of being overweight in a cohort of 1764 healthy children and adolescents (age 6–19 years)(Reference Matthews, Wien and Sabaté55).
Mediterranean dietary pattern
The Mediterranean dietary pattern has many attributes that resemble a vegetarian dietary pattern (Fig. 1) because it is traditionally characterised by a high consumption of fruits, vegetables, legumes, grains, nuts and olive oil, a moderate intake of wine and dairy products, and a low intake of red and processed meat, cream and pastries. It is beyond the scope of the present paper to discuss the extensive body of published articles that describe the association between the Mediterranean diet and the MetS. However, it is worth discussing the findings from a recent meta-analysis of epidemiological studies and randomised controlled trials in addition to a cross-sectional study and the one-year results of a randomised trial among high CVD risk adults that participated in the Prevención con Dieta Mediterránea (PREDIMED) trial.

Fig. 1 Foods related to the metabolic syndrome (MetS) in two plant-based diets. ↑ , increases the risk of MetS; ↓ , decreases the risk of MetS; ?, uncertain relation to MetS.
First, a meta-analysis of fifty original research studies (thirty-five clinical trials, two prospective studies and thirteen cross-sectional trials) with 534 906 participants was conducted through 30 April 2010 to assess the effect of a Mediterranean diet on the MetS and its individual components(Reference Kastorini, Milonis and Esposito56). Adherence to the Mediterranean diet in the prospective studies and clinical trials was found to be associated with a 31 % reduced risk of the MetS. Further, results from both the epidemiological studies and the clinical trials revealed a protective role of the Mediterranean diet on WC, HDL, TAG, systolic BP, diastolic BP and FBG. Thus, the Mediterranean diet pattern is an option for the primary and secondary prevention of the MetS and its components.
The relationship between adherence to the Mediterranean dietary pattern and risk of the MetS was also evaluated among 808 participants at high CVD risk enrolled in the PREDIMED trial using a cross-sectional approach(Reference Babio, Bullo and Basora57). Compared with the lowest quartile of dietary adherence, participants with the highest dietary adherence had a 56, 47 and 54 % lower odds of having the MetS, low HDL and high TAG, respectively. Further, consumption of olive oil, legumes and red wine was associated with lower odds of having the MetS. Second, in the entire cohort of 1224 participants after 1 year of follow-up(Reference Salas-Salvado, Fernandez-Ballart and Ros58), incident rates of the MetS were similar among the three study arms (Mediterranean diet+olive oil, Mediterranean diet+mixed nuts or a low-fat diet (control)). Compared with a 2 % reduction in the MetS prevalence in the control arm, a 13·7 and 6·7 % reduced prevalence was found in the mixed nut (P= 0·01) and olive oil arm (P= 0·18), respectively. After adjustments for confounders, the OR for the reversion of the MetS were 1·3 (95 % CI 0·8, 2·1) and 1·7 (95 % CI 1·1, 2·6) for the olive oil and mixed nuts arms compared with the control arm, respectively. The investigators concluded that a nut-enriched Mediterranean diet may be a useful tool in the management of the MetS among high CVD risk adults, which is consistent with the views of others(Reference Kendall, Josse and Esfahani59).
The novel findings from these two PREDIMED studies prompted a cross-sectional analysis of the data from the aforementioned AHS-2 Calibration Sub-study to explore the relationship between total nut (tree nuts and peanuts) consumption, the MetS and obesity(Reference Jaceldo-Siegl, Haddad and Oda60). In addition to finding the odds of the MetS and obesity to be inversely related to the amount, frequency and the pattern of nuts consumed, WC was 40 % lower among high-tree nut consumers (mean intake of 16 g/d) compared with low-tree nut consumers (mean intake of 5 g/d) (OR 0·60, 95 % CI 0·39, 0·93). Complementary to the AHS-2 Calibration Sub-study and PREDIMED findings, a study that evaluated out-of-hand nut consumers (operationally defined as consuming ≥ 1/4 ounce of nuts/d) among the 13 292 adults (age >19 years) participating in the United States National Health and Nutrition Examination Survey (1999–2004) observed that the nut consumers had a higher HDL (P< 0·01) and a 19 % decreased risk of high BP (OR 0·81, 95 % CI 0·67, 0·98) compared with non-consumers, which are two MetS risk factors(Reference O'Neil, Keast and Nicklas61).
Potential mechanisms
Dietary patterns (e.g. vegetarian, Mediterranean and prudent) that feature a high consumption of fruits and vegetables may reduce the risk of the MetS through mechanisms that rely on a beneficial synergistic combination of antioxidants, fibre, K, Mg and phytochemicals, which may result in a decrease in BMI, BP, TAG, oxidative stress, IR and systemic inflammation. Evidence for the latter hypothesis is supported by a cross-sectional study of 486 Tehrani female teachers (age 40–60 years)(Reference Esmaillzadeh, Kimiagar and Mehrabi62) that found an inverse association with plasma C-reactive protein concentrations and fruit and vegetable intakes using a validated semiquantitative FFQ. Participants in the highest quintile of fruit intake and vegetable intake had a 34 and 30 % reduced chance of having the MetS than those in the lowest quintiles, respectively.
Omnivores consume heme iron from animal products and have significantly higher concentrations of serum ferritin compared with vegetarians(Reference Haddad, Berk and Kettering63–Reference Wilson and Ball65). Evidence has emerged from cross-sectional studies showing a link between increased serum ferritin concentrations and the MetS(Reference Kim and Jo53, Reference Sun, Franco and Hu66). A recent cross-sectional study on fifty-nine South Korean postmenopausal vegetarians and 48 age-matched postmenopausal non-vegetarian controls revealed that the prevalence of the MetS was 33·9 and 47·9 %, respectively(Reference Kim and Bae67). Further, serum ferritin was positively correlated with FBG (r 0·264, P< 0·01), TAG (r 0·232, P< 0·05) and the NCEP criteria score for the MetS (r 0·214, P< 0·05).
Future research
The current evidence that shows that an association between consumption of a vegetarian dietary pattern and a reduced prevalence or risk of the MetS comes from cross-sectional and case–control studies. There is a need for further research to be conducted, particularly prospective cohort studies to evaluate the effect of vegetarian dietary patterns on reducing the incidence of the MetS, and clinical trials should be designed to explore vegetarian dietary patterns for the reversal of the MetS in high-risk populations. This research could contribute to reduce the societal and economic burdens associated with the disorder.
Acknowledgements
The authors' contributions are as follows: J. S. conceived the manuscript; J. S. and M. W. wrote the manuscript; J. S. and M. W. approved the final manuscript.
The authors have no conflicts of interest.




