Food consumption studies investigating childhood eating habits are useful for the prevention of nutrient deficiencies and nutritional status disorders in that life stage( Reference De Castro, Verly and Fisberg 1 ). With this information, public policies can be developed to mitigate and/or eliminate nutritional problems( Reference Livingstone, Robson and Wallace 2 , Reference Salles-Costa, Barroso Gdos and Mello 3 ).
The methods applied in these studies to evaluate food consumption include FFQ, 24-h dietary recalls (24HDR), food records and diet histories. The 24HDR is based on the foods and amounts that are actually consumed by an individual on a specific day or days( Reference Salles-Costa, Barroso Gdos and Mello 3 , Reference Willett 4 ).
The challenge of assessing the food consumption of infants is to estimate their ‘true’ food and nutrient intake because the types and amounts of food consumed change considerably with growth and development, thus affecting the overall variability of food intake( Reference De Castro, Verly and Fisberg 1 ).
Ideally, diet observation lasts for several days, weeks or months to estimate the individual’s usual intake, but due to the costs and challenges of developing epidemiological studies, short-term methods are often used to estimate usual dietary intake( Reference Huybrechts, De Bacquer and Cox 5 ).
However, the assessment of food consumption includes between-person variation in usual intake and within-person random error, which arise because each individual differs in the types and amounts of food consumed from 1 d to another (intrapersonal variability) and because individuals differ from each other in their food intake (interpersonal variability)( Reference De Castro, Verly and Fisberg 1 , Reference Tarasuk and Beaton 6 ).
Estimated intra and interpersonal variability are also useful for determining the sample size needed to study diet–disease relationships, habitual intake distributions and days of data required to estimate the usual intake of the individual( Reference Willett 4 , Reference Huybrechts, De Bacquer and Cox 5 ).
The number of days required is based on the relationship between intra and interpersonal variability( Reference Black, Cole and Wiles 7 ). Increasing the number of days of dietary data collection increases the accuracy of these consumption estimates for each individual in the population. However, the number of days of sampling differs for each nutrient and population( Reference Huybrechts, De Bacquer and Cox 5 , Reference Nelson, Black and Morris 8 ).
Although several studies have characterised intra and interpersonal variability of nutrient intake in adults( Reference Ouellette, Yang and Wang 9 – Reference Morimoto, Marchioni and Cesar 15 ), the information available for children is still very limited( Reference Huybrechts, De Bacquer and Cox 5 , Reference Nelson, Black and Morris 8 , Reference Jahns, Carriquiry and Arab 16 – Reference Kylberg 25 ). Such data are especially scarce for Brazil, where only two studies have conducted such assessments( Reference De Castro, Verly and Fisberg 1 , Reference Salles-Costa, Barroso Gdos and Mello 3 ). Furthermore, assessments of the adequacy of food consumption of Brazilian children are usually conducted in public health institutions and day care centres( Reference Cavalcante, Tinôco and Cotta 26 – Reference Inoue, Osório and Taconeli 31 ), and few studies have adjusted for within-person variability( Reference Gomes, da Costa and Schmitz 28 , Reference Tavares, Veiga and Yuyama 30 – Reference Bueno, Fisberg and Maximino 34 ).
Black et al.( Reference Black, Cole and Wiles 7 ) report that 3 d are needed to assess the energy consumption of American children aged 2–18 months. Nelson et al. ( Reference Nelson, Black and Morris 8 ) recommend 7 d to estimate energy and 5–7 d to estimate macronutrient intake among Peruvian children aged 1–4 years. In a study of seventy-two children in London ranging in age from 6 months to 2 years, Lanigan et al.( Reference Lanigan, Wells and Lawson 19 ) find that the required number of days for energy, protein, fat and carbohydrate is 5, 4, 4 and 3, respectively, and for micronutrients, Ca, P, Mg, Fe, Zn, ascorbic acid and vitamin A, 2 d are required. Erkkola et al. ( Reference Erkkola, Kyttala and Takkinen 22 ) find that for Finnish children up to 1-year old, 2–4 d of food records were needed to estimate energy and most macronutrients and micronutrients required 3–7 d of data.
In Brazil, studies of food consumption in children are limited to descriptive analyses of foods or nutrients, and only two studies have considered diet variability and the number of days required to estimate nutrients from usual intake. Salles-Costa et al. ( Reference Salles-Costa, Barroso Gdos and Mello 3 ) study children 6–30 months old in the city of Duque de Caxias, Rio de Janeiro, and they find that 1–5 d are needed to estimate energy and macronutrients, while for micronutrients, 1 d is needed for Ca and 6 d for vitamin C. De Castro et al. ( Reference De Castro, Verly and Fisberg 1 ) observed that for most nutrients, 7 d( Reference Black, Cole and Wiles 7 ) is sufficient to correctly classify the intakes of children 1–6 years old enrolled in creches and pre-schools in the cities of Manaus, Natal, Recife, Brasilia, Cuiaba, Rio de Janeiro, Viçosa, São Paulo and Caxias do Sul.
Thus, the few studies of children in low and middle-income countries are limited to descriptive analyses of foods or nutrients and investigate neither the variability of diet nor the number of days required to estimate usual dietary intake. This study aimed to calculate intrapersonal and interpersonal variability and to estimate the number of days needed to assess usual intakes of energy and nutrients in children 13–32 months old in São Luís, Maranhão, Brazil.
Methods
Design
This cross-sectional study was developed in São Luís, Maranhão, Brazil as part of a project entitled ‘Aetiology of Preterm Birth and Consequences of Perinatal Factors in Child Health: Birth Cohorts from Two Brazilian Cities, São Luís (MA) and Ribeirão Preto (SP) – BRISA’ developed by the Federal University of Maranhão (UFMA) in partnership with the Ribeirão Preto Medical School (FMRP), São Paulo.
Population and study sample
The target population consisted of children of both sexes during a follow-up visit in their 2nd year of life as part of the BRISA project. These children were born in São Luís, Maranhão, Brazil, which is located in northeastern Brazil and has one of the lowest human development index values in the Federation. The present study used a non-probabilistic sample of 241 children.
Children 13–32 months old whose mothers or guardians provided signed informed consent and three complete 24HDR were included in the study. Children with atypical food intake on any 24HDR were excluded. Exclusion was based on the responsible party’s answer to the question ‘Was the child fed as usual yesterday?’ If the was answered ‘No,’ the child was excluded from the study. After these checks, ten children (4·15 %) were excluded from the sample. Thus, the final sample consisted of 231 children.
Data collection and study variables
Data were collected on socio-economic and demographic conditions using a pre-prepared BRISA questionnaire. Information on the children’s food consumption was collected via the 24HDR method. All this information was collected from the children’s mothers and/or guardians from April 2011 to January 2013 by a team of researchers and trained interviewers.
The 24HDR was used to assess the food and beverages consumed on the day before the interview. Three 24HDR were collected for 2 non-consecutive weekdays (Monday to Friday) and 1 d of the weekend (Saturday or Sunday) or holiday at the children’s homes. The mother or guardian of the child was questioned in detail about the food and beverages consumed by the child, including the brand, preparation method, portion size or volume consumed, with the aid of a photo album.
To standardise data collection, the team of researchers and interviewers was properly trained in the use of the questionnaire and 24HDR, and we provided a manual to explain their completion.
Before entering the food consumption data into a specific programme, a quality control step was performed. The information on food and beverages was collected and quantified in a standardised way, with the help of the Tables for Food Consumption Assessment using Domesticated Measures( Reference Pinheiro 35 ). Subsequently, the 24HDR data were converted into energy and nutrients using the Virtual Nutri Plus® program (version 2010), University of São Paulo, São Paulo-SP, Brazil, which is based on the Brazilian Table of Food Composition (TACO)( 36 ) and Sonia Tucunduva Food Composition Table( Reference Philippi 37 ). Regional foods or preparations that were not included in these databases were included based on the ingredients and amounts provided in the 24HDR. Later, the data were imported into Stata® (version 12.0), where the daily energy and nutrient intakes were calculated for each child.
Because of the difficulty of measuring the daily volume of milk consumed, we used the methodology of Drewett et al. ( Reference Drewett, Woolridge and Jackson 38 ) due to its low cost and ease of application. In this method, the volume of breast milk consumed is estimated based on the amount (kJ (kcal)) of complementary feeding and the child’s age in days. These variables are included in a multiple linear regression model proposesd by the authors, where Y is the estimated breast milk consumption, X' is the age (days), and X" is the consumption of complementary foods (kJ (kcal)):
According to these authors, the number of feedings per day and the consumption of other foods reflect breast milk intake better than the duration of breast-feeding alone. This equation has been used in Brazil in studies by Nejar et al. ( Reference Nejar, Segall-Correa and Rea 39 ) and Garcia et al.( Reference Garcia, Granado and Cardoso 40 ).
The following socio-economic and demographic variables were used in the study: the child’s sex, the child’s age, the child’s skin colour, the mother’s marital status, the family’s income, the family’s social economic class, the mother’s remunerated activity and the mother’s education.
The variables related to food consumption were continuous: energy (kJ (kcal)), carbohydrate (g), protein (g), total fat (g), SFA (g), MUFA (g), PUFA (g), total fibre (g), soluble fibre (g), insoluble fibre (g), cholesterol (mg), vitamin A (retinol equivalents), vitamin C (mg), niacin (mg), Fe (mg), Ca (mg), P (mg), Mg (mg), Na (mg) and Zn (mg).
The foods consumed by the children were classified into nine groups: milk and dairy products, cereals, meat and eggs, vegetables, fruits, breast milk, processed foods, beans and others (sugar, coffee, margarine, butter, olive oil and infant formulas).
Statistical analysis
Initial analyses were performed using Stata® (version 12.0). Descriptions of the categorical variables (socio-economic and demographic) were obtained as sample frequencies and percentages and of the numerical variables, such as age, as mean values and standard deviations.
We investigated the presence of extreme values (outliers) through graphical analysis of the energy consumption data (box plots) and the reviewing the minimum and maximum values for each nutrient. Values below or above the chart limits suggest outliers and maximum. If inconsistencies were found and corrected, the children were not excluded.
Intrapersonal variability (S 2 w ), interpersonal variability (S 2 b ), and variance ratio (VR: S 2 w /S 2 b ) values were estimated, and descriptive statistics for food consumption were obtained as averages, standard deviations, medians and interquartile ranges. We used the Multiple Source Method® (MSM) program (version 1.0.1) developed by the Department of Epidemiology at the German Institute of Human Nutrition Potsdam-Rehbrücke( Reference Harttig, Haubrock and Knuppel 41 ).
The MSM operates in three steps: (a) a logistic regression model is estimated to determine the probability of consuming a nutrient, along with the residual of the corresponding model. The residual is transformed into real numbers, and intra and interpersonal variability are estimated. The intrapersonal variance is then removed, and the reduced residual is back-transformed to the original scale; (b) a linear regression model is estimated, and the model residuals are then normalised through the Box–Cox transformation. As in the first step, the person variance estimated from the processed residual is subsequently removed and the reduced quantities are back-transformed to the original scale; (c) finally, the ‘normal’ dietary intakes (and corresponding distributions) are calculated by multiplying the quantities generated in steps (a) and (b)( Reference Harttig, Haubrock and Knuppel 41 ).
To calculate the number dietary inquiries necessary to estimate children’s intake, we used the formula proposed by Black et al. ( Reference Black, Cole and Wiles 7 ):
where d is the number of days required; r corresponds to the expected correlation coefficient (0·7, 0·8 or 0·9) between the observed intake and the usual intake; S 2 w the intrapersonal variance; and S 2 b the interpersonal variance.
This approach is proposed by Black et al. ( Reference Black, Cole and Wiles 7 ) to determine the number of days of dietary data necessary for estimating the usual intake of a group using the VR, that is, the ratio of the intrapersonal variance:the interpersonal variance, and the hypothetical correlation between the observed intake and the usual intake. This approach is also used to minimise error in the classification of individuals into levels (quartiles) of nutrient intake. The higher the desired correlation and the rate of nutrient variance, the more days of dietary data needed for the group( Reference Nelson, Black and Morris 8 , Reference Gibson 42 ).
Ethical considerations
The BRISA project was submitted to the Research Ethics Committee (CEP) of the University Hospital Presidente Dutra Unit (HUUPD) of the UFMA and was approved under Opinion no. 223/09 and record 350/08.
Results
The 231 children studied had a mean age of 19·3 (sd 4·2) months. Children were predominantly males (55·0 %), younger than 24 months (86·5 %) and had mixed /mulatto/cabocla/dark skin (64·8 %). Their mothers were married or in a consensual union (78·8 %), had 9–11 years of education (65·4 %) and were not engaged in remunerated activity (56·3 %). Their families had incomes of 1–3 times the minimum wage, which is equivalent to US$301·65 to US$904·95 (50·5 %), and were ranked as socio-economic class C (59·7 %) (Table 1).
Table 1 Socio-economic and demographic characteristics of children 13–32 months old (Numbers and percentages; mean values and standard deviations)

Source: BRISA, São Luís, Maranhão, Brazil, 2010–2012.
* n 230.
† n 212.
‡ Current minimum wage in 2012: R$622·00 or US$301·65 (based on the dollar exchange rate in 2012).
§ Socio-economic class verified by the Brazilian Association of Research Companies (ABEP) using the Economic Classification Criteria Brazil (class A has the highest purchasing power and E the lowest).
Table 2 provides the descriptive analysis of energy and nutrient intake for these children. Notably, the average intake of Na was 905·0 (sd 352·0) mg/d; Ca was 990·0 (sd 428·0) mg/d; Fe was 13·0 (sd 9·0) mg/d and Zn was 7·9 ( sd 4·9) mg/d. The most consumed food groups were milk and dairy products (20·3 %), cereals (19·7 %) and processed foods (17·8 %) whereas meat and eggs (10·5 %), vegetables (9·0 %) and fruits (7·4 %) were the least consumed (Table 2).
Table 2 Distribution of usual energy consumption, macronutrients and food groups of 2 non-consecutive weekdays and 1 d of the weekend or holiday for children aged 13–32 monthsFootnote * (Mean values and standard deviations; 25th, 50th and 75th percentiles and percentages)

Source: BRISA, São Luís, Maranhão, Brazil, 2010–2012.
* Values estimated using the Multiple Source Method® program.
The intrapersonal variability was <1 for all nutrients, except soluble fibre. For interpersonal variation, only SFA, MUFA, and Zn had interpersonal variability values of >1. The vast majority of nutrients had intrapersonal variability values below those of interpersonal variability, resulting in low VR (<1). A VR between 1 and 2 was observed only for PUFA. Vitamin C, total fibre, soluble fibre and insoluble fibre showed VR >2 (Table 3).
Table 3 Nutrients in 2 non-consecutive weekdays and 1 d of the weekend or holiday for children aged 13–32 monthsFootnote * (Intrapersonal variability, interpersonal variability and variance ratio (VR))

Source: BRISA, São Luís, Maranhão, Brazil, 2010–2012.
* Values estimated using the Multiple Source Method® program.
† Intrapersonal variability (S ² w ): mean square within individuals.
‡ Interpersonal variability (S ² b ): (mean square between individuals – S ² w /number of days of dietary survey of each individual).
§ VR: S ² w /S ² b .
The number of 24HDR replications required to estimate the usual intake of children, considering a correlation coefficient of 0·9, ranged from 2 d for energy, carbohydrate, SFA, Ca, Fe, P and Zn to 12 d for soluble fibre. Considering a correlation coefficient of 0·8, the required number of replications ranged from 1 d for the vast majority of nutrients to 5 d for soluble and insoluble fibre (Table 4). For a correlation coefficient of 0·7, the required number of days ranged from 1 d for the vast majority of nutrients to 3 d for soluble fibre. Thus, as the correlation coefficient decreased, so did the number of days required to estimate the nutrient intake (Table 4).
Table 4 Nutrient intake of children aged 13–32 months (Number of 24-h dietary recalls)
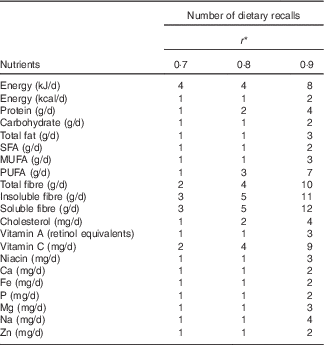
Source: BRISA, São Luís, Maranhão, Brazil, 2010–2012.
* Expected correlation (0·7, 0·8, 0·9) between the observed intake and the usual intake.
Discussion
For the vast majority of nutrients, interpersonal variability was greater than within-person variability, resulting in VR of <1 and fewer 24HDR replications needed, usually 1, 2 or 3 d for correlations of 0·7, 0·8 and 0·9.
In studies evaluating food intake, measurement bias, memory, completion and quantification of the portions reported in 24HDR are limitations that may affect intra and interpersonal variability. In this study, to minimise these potential biases, we used a photo album to train the interviewers and during the collection of the 24HDR from respondents. The data were also standardised using household measures, with interviewers identifying and correcting flaws before the tabulation.
The absence of breast milk volume information could also influence the estimated food consumption. However, this limitation was minimised by estimating the volume using the equation proposed by Drewett et al. ( Reference Drewett, Woolridge and Jackson 38 ), which has been validated and used in other studies( Reference Nejar, Segall-Correa and Rea 39 , Reference Garcia, Granado and Cardoso 40 ). Another limitation of this study was the use of a non-probabilistic sample, which may not be representative of the target population.
A strength of this study is to obtain intrapersonal variability, interpersonal variability and VR from three non-consecutive 24HDR (from 2 weekdays and 1 weekend day or holiday) for all 231 children assessed, using the MSM program. In addition, evaluating the dietary intakes of children who are not enrolled in kindergarten or school can contribute to the development of these other children. As most nutrient intake VR were <1, this study may help find epidemiological associations with the health outcomes of these children, given that these associations are easier to detect when values are <1.
The estimated distributions of habitual intakes of both macronutrients and micronutrients showed small differences between averages, medians (50th percentile) and the first and third quartiles (25th and 75th percentiles), as seen in Table 2. These distributions denote small interpersonal variations, probably due to repetitive consumption of the same energy foods, protein, specific sources of fats, vitamins and minerals throughout the week. The low variability of food intake is also noticed in the analysis of the main food groups consumed by the children, with higher frequencies for milk and dairy products, cereals and processed foods, which were present during the 3 d of food consumption.
In addition, consumption of fruits and vegetables by the children was low, denoting a poor quality diet. Therefore, the low nutrient variation observed over the course of days was not due to a regular consumption of minimally processed or in natura foods, but rather to processed or ultra-processed foods.
The mean intakes of Ca (990·00 (sd 428·00) mg/d), Fe (13·00 (sd 9·00) mg/d) and Zn (7·9 (sd 4·9) mg/d) were within the recommended ranges for children aged 1–3 years, according to the estimated average requirement of dietary reference intakes from the Institute of Medicine, which established values of 500 mg/d for Ca, 3·0 mg/d for Fe and 2·5 mg/d for Zn( 43 ). These micronutrients are of fundamental importance for children growth and development and aid in the immunological and neurological responses of children, preventing against diseases, such as Fe deficiency anaemia( Reference Cavalcante, Tinôco and Cotta 26 ).
The average Na intake (905·00 (sd 352·00) mg/d) was close to the recommended adequate intake of 1000 mg/d( 43 ). An excess of average Na intake was not verified, despite the high consumption of processed foods by the children.
A low VR indicates that interpersonal variability of food consumption is relatively high compared with intrapersonal variability. These findings differ from those in various studies of children( Reference De Castro, Verly and Fisberg 1 , Reference Huybrechts, De Bacquer and Cox 5 , Reference Nelson, Black and Morris 8 , Reference Jahns, Carriquiry and Arab 16 , Reference Piwoz, Creed de Kanashiro and Lopez de Romana 17 ) and are consistent with the studies of Salles-Costa et al. ( Reference Salles-Costa, Barroso Gdos and Mello 3 ) with children 6–30 months of age in Duke Caxias, Rio de Janeiro, Brazil and of Lanigan et al. ( Reference Lanigan, Wells and Lawson 19 ) with children 6–30 months in London. These results may be due to the low variability of infant diets compared with adult diet. Infants consume a smaller number of foods daily, resulting in more monotonous diets( Reference Salles-Costa, Barroso Gdos and Mello 3 , Reference Lanigan, Wells and Lawson 19 ).
Higher VR were observed for total, soluble and insoluble fibres and for vitamin C and lower VR were observed for Fe, SFA, Zn and Ca. These results indicate that the usual diets of the evaluated children had larger daily fluctuations from the consumption of food sources of fibre and vitamin C, such as vegetables and fruits, and smaller daily fluctuations resulting from regular consumption of Fe-rich foods (organ and other meats, dark green vegetables), Ca (milk and dairy products), Zn and SFA, which is found in animal foods.
The VR for vitamin C can be explained for its concentration in specific foods, especially fruits. Thus, intake of vitamin C may be too low or too high, depending on the food choices each day( Reference Salles-Costa, Barroso Gdos and Mello 3 , Reference Beaton, Milner and McGuire 11 ). A lack of habitual consumption of fruits was observed among the children despite the wide variety of fruits available in the region.
The VR were lower for Ca and Zn, which will require fewer 24HDR repetitions (1–3 d); this pattern is understandable because the main food sources of these nutrients (meat, milk and dairy products) are characteristic, regular and predominant foods in the diets of children, resulting in low intrapersonal variability( Reference Salles-Costa, Barroso Gdos and Mello 3 , Reference Lanigan, Wells and Lawson 19 ).
Although Na does not exhibit one of the smallest VR (VR=0·83), it is noteworthy that its intrapersonal variability (S 2 w =0·074) is lower than its interpersonal variability (S 2 b =0·089). This is probably because children consume snacks, biscuits, instant noodles, processed juices and soft drinks almost daily, resulting in little variation in Na consumption over the study period. The popularity and availability of processed products in supermarkets in the study area may contribute to this result.
In other Brazilian studies, Salles-Costa et al. ( Reference Salles-Costa, Barroso Gdos and Mello 3 ) obtained a VR for vitamin C >1, while in this study the value was >2. The VR for Ca was <1, which similar to the value found in this study. De Castro et al. ( Reference De Castro, Verly and Fisberg 1 ) found a lower VR for Ca, 1·17, and a higher value for total fat (VR=8·70), while in this study, the corresponding VR were 0·42 and 0·71, respectively. Note that in this study the largest VR was observed for soluble fibre at 2·87, while the lowest was for Fe at 0·37.
The VR for cholesterol was low (VR=0·99), which differs from the results of most studies in other populations, where this VR is usually >1 or 2. In this sample, food sources this nutrient, such as eggs, meat and whole milk, were consumed regularly, explaining the low VR( Reference Huybrechts, De Bacquer and Cox 5 ).
Low variances for energy and macronutrients (carbohydrate, protein and total fat) can be better explained by greater interpersonal variability than intrapersonal variability of intake. Huybrechts et al. ( Reference Huybrechts, De Bacquer and Cox 5 ) found the VR for these nutrients >1, with the exception of carbohydrates. However, as in this study, the VR for micronutrients were generally <1, except for Zn and Na.
The low intrapersonal variability of energy and carbohydrate intake can be attributed to the fact that food sources of carbohydrates and energy are typically repeated each day, mainly as the rice and porridge flour that are frequently fed to children in this age group. Similarly, the presence of meat in main meals (lunch and dinner) and of whole milk and dairy products, especially yogurt, in morning meals and afternoon snacks may have contributed to the low intrapersonal variability observed for protein and total fat.
In general, these lower VR required fewer 24HDR replications. The highest number of days was necessary for total fibre (10), insoluble fibre (11) and soluble fibre (12) and the lowest for energy, carbohydrate, SFA, Ca, Fe, P and Zn (which all had 2 d for r 0·9). Most nutrients required one or two 24HDR replications for r 0·8 and 2–3 d for r 0·9 (Table 4).
De Castro et al. ( Reference De Castro, Verly and Fisberg 1 ) noted the need for 2–15 d of dietary assessment to achieve r 0·8, and 5–37 d to reach r 0·9 in Brazil, while Huybrechts et al. ( Reference Huybrechts, De Bacquer and Cox 5 ) reported a range of 3–38 d for Belgian children to reach r≥0·9. Erkkola et al.( Reference Erkkola, Kyttala and Takkinen 22 ) reported a range from 1 to 9 d for Finnish 1-year-old children to reach r 0·8 and from 2 to 20 d to reach r 0·9. In this study, fewer days were required, ranging from 1 to 3 d for r 0·7, from 1 to 5 d for r 0·8 and from 2 to 12 d for r 0·9 (Table 4).
The number of days required to estimate dietary intakes of macronutrients and micronutrients were similar for the correlation coefficients analysed (Table 4). Likewise, Salles-Costa et al.( Reference Salles-Costa, Barroso Gdos and Mello 3 ) found that 3 d were needed to estimate energy, macronutrients and micronutrients for the majority of children (r≥0·9). However, Lanigan et al.( Reference Lanigan, Wells and Lawson 19 ) found that energy, protein, fat and carbohydrate required 5, 4, 4 and 3 d, respectively, and micronutrients such as Ca, P, Mg, Fe, Zn and vitamins A and C required 2 d for r≥0·9. Huybrechts et al.( Reference Huybrechts, De Bacquer and Cox 5 ) found that the assessment of energy intake and macronutrients (other than fat, fatty acids and cholesterol), required a 7-d diet record to achieve r≥0·9 and 4 d for most micronutrients (except Na and Zn).
This study verified the need for 1–2 d of data to estimate energy, carbohydrate and SFA, 1–3 d for total fat and MUFA and 1–4 d for protein, considering r values from 0·7 to 0·9 (Table 4). Other international studies reported number of days greater than ours. Black et al.( Reference Black, Cole and Wiles 7 ) reported that 3-d diet records were sufficient to estimate the energy consumption of children aged 2–18 months, while Nelson et al.( Reference Nelson, Black and Morris 8 ) recommended 7 d for energy and 5–7 d for macronutrients in children 1–4 years old, both considered r≥0·9.
When comparing our results with previous works, more than 7 d of data are usually needed to estimate nutrient intake in adults( Reference Ouellette, Yang and Wang 9 – Reference Morimoto, Marchioni and Cesar 15 ) and older children. Fewer records are needed for younger age groups( Reference De Castro, Verly and Fisberg 1 , Reference Nelson, Black and Morris 8 , Reference Ollberding, Couch and Woo 21 ), which can be attributed to the lower variability of their diets, which are based on a smaller variety of foods than adult diets( Reference Huybrechts, De Bacquer and Cox 5 ).
Obtaining such data is justified in the fact that the sample includes children from families with monthly incomes ranging from US$301·65 to US$904·95, whose mothers have low levels of education, and who reside in an emerging country with average incomes. Brazil is a member of the so-called emerging BRICS countries (Brazil, Russia, India, China and South Africa), but São Luís, Maranhão is one of its least developed cities, suggesting limited food choices and, consequently, the possibility of applying fewer reminders to obtain the estimate food consumption compared with children in other countries with different realities.
In conclusion, this study contributes to the epidemiological study of food consumption by providing information on the variability and the number of days of dietary data required to estimate the dietary intakes of children aged 13–32 months: 1 d of 24HDR is sufficient for estimating the consumption of most nutrients for r 0·8 and 2–3 d of data are needed for r 0·9.
Acknowledgements
The authors thank the children and mothers or guardians who participated in this study.
This work was funded by the Foundation for Research and Scientific and Technological Development of Maranhão, Brazil (FAPEMA) (Protocol No. 00035/2008). The funders had no role in the design, analysis or preparation of this article.
L. L. P. performed the statistical analysis, interpreted the findings and wrote the manuscript. W. R. C. C., M. A. B., S. I. O. d. C., A. K. T. d. C. F and A. A. M. d. S. assisted in the interpretation of the findings. All authors contributed to the writing of the manuscript, read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.







