Obesity and asthma are considered global public health problems. If no intervention is done, the prevalence worldwide is estimated to be 775 million of overweight individuals(Reference Dobbs, Sawers and Thompson1) and more than 400 million of asthmatics(2) in 2025. There seems to be an association between the diseases(Reference Rasmussen and Hancox3), and it remains a question whether asthma itself can occur first and increase the risk of obesity due to the use of corticosteroids. However, to date, there is no evidence to support this hypothesis, while there is ample evidence that obesity is a strong risk factor for asthma(Reference Lang4).
Observational studies have found higher prevalence of asthma in the obese population(Reference Ford5, Reference Beuther and Sutherland6), and in prospective studies, the excess of visceral adiposity has been shown to be a risk factor for asthma(Reference Brumpton, Langhammer and Romundstad7, Reference Papoutsakis, Chondronikola and Antonogeorgos8). Recently, the results of a meta-analysis have shown a significant relationship between overweight and asthma in children and adolescents(Reference Azizpour, Delpishe and Montazeri9). In addition, obese non-asthmatic individuals may present impaired pulmonary function and exercise-induced bronchospasm(Reference Hayden, Stoloff and Colice10, Reference Tassoudis, Ieropoulos and Karanikolas11). These findings highlight the impact of obesity on the pathophysiology of asthma.
The present research suggests that the link between obesity and asthma occurs through two major phenotypes – (1) atopic asthma, primary, early-onset, aggravated by overweight; (2) late-onset non-atopic asthma, mainly neutrophilic inflammation – and by three routes – (1) mechanical effects of obesity, such as deeper inspirations that increase airway hyperresponsiveness; (2) inflammatory, stimulated by adipokines; (3) lifestyle and environment, such as uterine exposure, physical activity and diet(Reference Rasmussen and Hancox3, Reference Mohanan, Tapp and McWilliams12). So, asthma-obesity is a multifactorial disease with specific asthma phenotypes that aggravate due to overweight and the modern lifestyle, such as an unbalanced diet(Reference Mohanan, Tapp and McWilliams12, Reference Dixon and Poynter13).
Fast-food ingestion, twice or three times a week, is associated with a higher risk of severe asthma in adolescents, whereas fruit intake, with the same frequency, is related to a reduction of asthma prevalence in this population. This is possible as a fat-rich and fibre-poor diet may influence systemic inflammation of airways(Reference Ellwood, Asher and Garcia-Marcos14).
There is evidence that saturated fats contribute to higher lipopolysaccharide levels in circulation(Reference Harte, Varma and Tripathi15), which lead to systemic endotoxaemia and toll-like 4 receptor activation, which signals the NF-κB(Reference Halns, Baines and Berthon16) pathway. This pathway increases the secretion of IL-17, IL-6, leptin and TNFα(Reference Shore and Cho17, Reference Sideleva, Black and Dixon18), which induces hyperresponsiveness of the airways(Reference Han, Forno and Holguin19). On the contrary, fibre consumption can inhibit NF-κB activity through the metabolite butyrate, which signals the PPAR(Reference Berthon, Macdonald-Wicks and Gibson20). Therefore, a diet with low levels of lipids and rich in fruit and vegetables seems to reduce inflammation and reactivity of airways(Reference Maslowski, Vieira and Ng21).
Similarly, the visceral adipose tissue, through hyperplasia and hypertrophy of the adipocytes, also secretes proinflammatory substances such as C-reactive protein and leptin(Reference Boulet22, Reference Gruchala-Niedoszytko, Malgorzewicz and Niedoszytko23), which can reach target organs such as the lung and contribute to neutrophilia(Reference Scott, Jensen and Wood24) and airway hyperresponsiveness(Reference Lang4). However, adiponectin, an anti-inflammatory protein, seems to inhibit NF-κB pathway activation(Reference Berthon, Macdonald-Wicks and Gibson20, Reference Lee, Lee and Choue25).
In this sense, a prior study with a normo-energetic and nutrient-balanced dietary intervention improved the nutritional status and reduced asthma-related symptoms in obese asthmatic pubescent adolescents(Reference Luna-Pech, Torres-Mendoza and Luna-Pech26). Moreover, our group(Reference Da Silva, De Mello and Cheik27) previously showed that an interdisciplinary intervention with the participation of an endocrinologist, psychologists, physical education professionals, physiotherapists and registered dietitians was effective in asthma-obesity treatment, improving body composition, lung function and inflammatory biomarkers.
Asthma and asthma-obesity treatments are similar. Both aim to control the symptoms, maintain and recover lung function. However, the latest publication on asthma handling mentions that weight reduction in obese patients and a balanced diet may be effective in improving the lung function(2, 28).
Consequently, understanding the role of nutrients in asthma-obesity is important as these patients present a limited response to corticotherapy(Reference Dias and Farzan29, Reference Liu, Lin and Zhao30). However, there are not enough studies investigating the effects of an interdisciplinary treatment in asthma-obesity, particularly the role of food consumption and pro- and anti-inflammatory adipokines on lung function and symptoms related to asthma. Therefore, we designed a study to evaluate the effect of an interdisciplinary intervention in food consumption, pulmonary function and adipokines of obese asthmatic patients, as well as to investigate the influence of nutrients on lung function.
Methods
Outline and target population
This longitudinal study was approved by the Research Ethics Committee of the Federal University of Goiás through substantiated report no. 126140/2016, as required by the Declaration of Helsinki. The inclusion criteria were postpubescent adolescents aged 15–19 years(Reference Marshall and Tanner31, Reference Marshall and Tanner32), of both sexes, with BMI above the 95th percentile of the curve referred to by the Centers for Diseases Control(Reference Kuczmarski, Ogden and Guo33) and not presenting any change in resting and stress electrocardiogram. The study excluded teens who participated in other nutritional intervention programmes, smoke, have musculoskeletal deformities, heart disease, or cold and flu in the last 6 weeks of the spirometric evaluation, and those who did not attend ≥75 % of the interdisciplinary treatment programme. Then, the adolescents were divided into two groups: obese non-asthmatic (control group; n 42) and obese asthmatic (n 21). All participants and legal guardians signed the free and informed consent to give the volunteers of the study the most comprehensive information about the objectives of the study, as well as the evaluations and therapies that would be submitted. Thus, the participation of adolescents in the study was voluntary and conscious.
The volunteers underwent serum biochemical evaluations, body composition and anthropometry, assessment of food consumption and lung function. The assessments were performed at the beginning and end of the interdisciplinary programme in the short and long term, 6 and 12 months, respectively, except for the biochemical tests that were evaluated after 12 months.
Anthropometry and body composition
Body mass measurements, fat and lean mass percentages were determined by Bod POD® air displacement plethysmography (version 1.69; Life Measurement Instruments; with 0·001 g precision). Body volume was measured with participants wearing minimal clothing (underpants or a tightfitting bathing suit and no jewellery) and a bathing cap while sitting quietly and breathing normally in the test chamber. Body weight was assessed using a scale attached to Bod POD with adolescents standing, bare feet, wearing as little clothing as possible. Height was measured with a stadiometer (Sanny; model ES 2030®) with a 0·1 cm precision scale. BMI was calculated as body weight divided by height squared(Reference Lohman, Roche, Martorrel and Martorrel34).
Subcutaneous and visceral fat measurements were performed with ATL/HDI 3000® ultrasound with a 3·5 MHz multifrequency transducer (wideband). The stopping points to define visceral and subcutaneous fats were based according to specific standardisation. Thus, subcutaneous fat was determined by the distance between the skin and the outer face of the abdominal muscle, while the distance between the inner face of the same muscle and the anterior wall of the aortic artery was defined as visceral fat(Reference Ribeiro-Filho, Faria and Azjen35).
Evaluation of lung function
For the evaluation of lung function, we used a portable spirometer (EasyOne®/model 2001). Three reproducible manoeuvres were accomplished and the best manoeuvre was subsequently chosen according the criteria of the American Thoracic Society(36). These evaluated the forced vital capacity (FVC), which represents the largest volume of air mobilized in the lung during expiration; forced expiratory volume in 1 s (FEV1), which is the volume of air exhaled in 1s during FVC manoeuvre; FEV1:FVC ratio, which is the ratio of the volumes mentioned above to evaluate airway obstruction; and peak expiratory flow (PEF), which demonstrates the maximum air flow during FVC manoeuvre(37). The predicted values were obtained by reference equation(Reference Knudson, Lebowitz and Holberg38).
Asthma diagnosis was performed by a pulmonologist, who evaluated the clinical and functional conditions of the volunteers, as recommended by the American Thoracic Society(36). Specifically, patients with asthma should have a medical history of ≥6 months of recurrent respiratory symptoms, such as cough, dyspnoea and wheezing, relieved by bronchodilator treatment, and which demonstrate impairment of functional lung conditions such as reversible airflow limitation, that is, reference values for spirometry below the predicted values for the healthy population(39). The questionnaire from the International Asthma and Allergies in Childhood Study (ISAAC) was used to assess asthma-related symptoms(Reference Bateman, Hurd and Barnes40).
Biochemical tests
Blood samples were collected by an in-clinic nurse through peripheral venipuncture in the forearm after a 12-h overnight fast. After collection, the blood was centrifuged for 10 min at 5000 rpm and stored at –70°C. The materials used for blood collection were disposable and labelled appropriately. The serum concentrations of adiponectin (Phoenix Pharmaceuticals, Belmont) and leptin (Chemicon International, Inc.) were measured by the enzyme immunoassay technique using commercial ELISA kits according to manufacturer’s instructions.
Assessment of food intake
Dietary intake was assessed at baseline, after 6 and 12 months of intervention from the non-consecutive 3-d food registry, including 2 d of the week and 1 d of the weekend. Portions were reported as home measurements. The values obtained were analysed with Nutwin software (version 2.5)(Reference Anção, Cuppari and Tudisco41) based on the guidelines established by the Dietary Reference Intakes(42).
Interdisciplinary treatment
Obese adolescents were submitted to the interdisciplinary treatment for 1 year. All obese adolescents received the same treatment, during the same period and were followed throughout the trial period by the same professionals. The interdisciplinary treatment consisted of medical, nutritional, physical and psychological therapy, and physical exercise.
From the first consultation with an endocrinologist, teens received a monthly clinical follow-up, guidance on changes in lifestyle and monitoring of clinical evolution regarding the control of obesity and its various comorbidities.
Nutritional therapy was conducted by nutritionists, composed of individual consultations, standard food plan prescription with a replacement list and group guidance sessions. Individual consultations were monthly and a hypoenergetic menu model was provided based on the recommendations proposed by sex, age and level of physical activity(Reference Anção, Cuppari and Tudisco41). Thus, the menu featured a total energy value of 6276–6695 kJ and 7112–7531 kJ for female and male adolescents, respectively, and the following distribution of macronutrients: 50–60 % carbohydrates, 30 % total fats and 10–15 % proteins in relation to total energy content. No dietary supplements were prescribed during therapy. Once in a week, a nutritional education session was delivered to groups with a maximum of twenty adolescents. Each session lasted 60 min and practical, playful and theoretical activities were developed, aimed at promoting healthy habits and nutritional education, with topics about food pyramid, food for weight loss, concepts of fat, sugars, healthy eating and eating disorders(Reference Corgosinho, Elia and Tufik43).
Physical education professionals submitted adolescents to the combined exercise programme of combined exercises, three times a week, consisting of 30 min of aerobic exercise (exercise bike or treadmill) and another 30 min of resistance training per training session. All volunteers were familiar with the training protocol 2 weeks before starting the programme. Aerobic exercises were performed according to the intensity of the effort related to ventilatory threshold 1, determined by a direct analysis of gases. The protocol was developed with a weekly change of the load, divided into heavy-load week (6–8 repetition maximum (RM)), moderate-load week (10–12 RM) and light-load week (15–20 RM). The volunteers performed eighteen sets per session, divided into three sets for each exercise. The rest interval between sets depended on the load adopted during the training session, with 2-min intervals for heavy-load week and 1-min intervals for moderate-load week(Reference Donnelly, Blair and Jakicic44).
Physical and psychological therapies were performed weekly on different days. These consisted of lessons lasting 60 min, in groups of twenty teenagers. Physiotherapists taught classes on various topics that addressed the human body, such as notions of structures, conditions and ergonomics classes at school, at work, at home and during physical exercise. In addition, practical lessons were taught in global postural re-education groups, stretching and postural guidance(Reference Da Silva, De Mello and Cheik27). Psychologists worked with self-esteem, body image, behaviour, prejudice, eating disorders and family issues to assist teens with lifestyle and quality-of-life changes(Reference Corgosinho, Elia and Tufik43). All adolescents were submitted to the same programme of physical exercises and assessed for physical activity level(Reference Craig, Marshall and Sjöström45).
Statistical analysis
The sample size was based on our previous study(Reference Da Silva, De Mello and Cheik27). Additionally, from our data, we calculated the effect size associated with the sample size, with 80 % power and significance of 5 % of the FEV1:FVC ratio between the periods, which was 1·33’ (Cohen’s d), and this calculation estimated that twenty volunteers would be needed, ten in each group.
The removal of outliers was performed when the peripheral value of the variables (x) was: (1) x > 1·5 × upper quartile + (upper quartile−lower quartile), or (2) lower quartile x + 1·5 × (upper quartile−lower quartile)(Reference Rosner46). After removing outliers, we verified the Gaussian distribution of variables through the Shapiro–Wilk test, and the variables with normal distribution were expressed as means and standard deviations. It is noteworthy that for all the analyses, sex was controlled, that is, treated as a covariate.
The intragroup and intergroup comparison of variables with normal distribution, at baseline, 6 months and 1 year after therapy, was performed by repeated-measures of ANOVA, using paired correlation, followed by Tukey’s test. Cochran's Q test was used to analyse the proportion of symptoms related to asthma between the evaluation periods.
The Spearman test(Reference Mukaka47) was performed for correlations, but only as an exploratory method. The backward method (Wald) for multiple regression analysis was used to determine the influence of dietary intake, body composition and adipokines on lung function in all periods. From the correlation study, we selected the variables that most correlated with the markers of lung function (FVC, FEV1, FEV1:FVC and PEF). Then for the multiple regression analysis, four models with five to eight variables were created, using α = 0·05. We found R = 0·4–0·7 and power from 0·72 to 1·0.
The value of P ≤ 0·05 was adopted for the level of statistical significance in the analyses. Regarding the pulmonary function variables, it is emphasised that the predicted values were used in all calculations.
Results
Initially, 135 volunteers were enrolled in the interdisciplinary intervention programme. However, sixty-three patients completed 12 months of intervention with 75 % participation in therapies, including in physical activity, and both groups showed a significant activity level. The reasons for dropout in our study included family and financial problems, followed by internship opportunity and employment (Fig. 1).
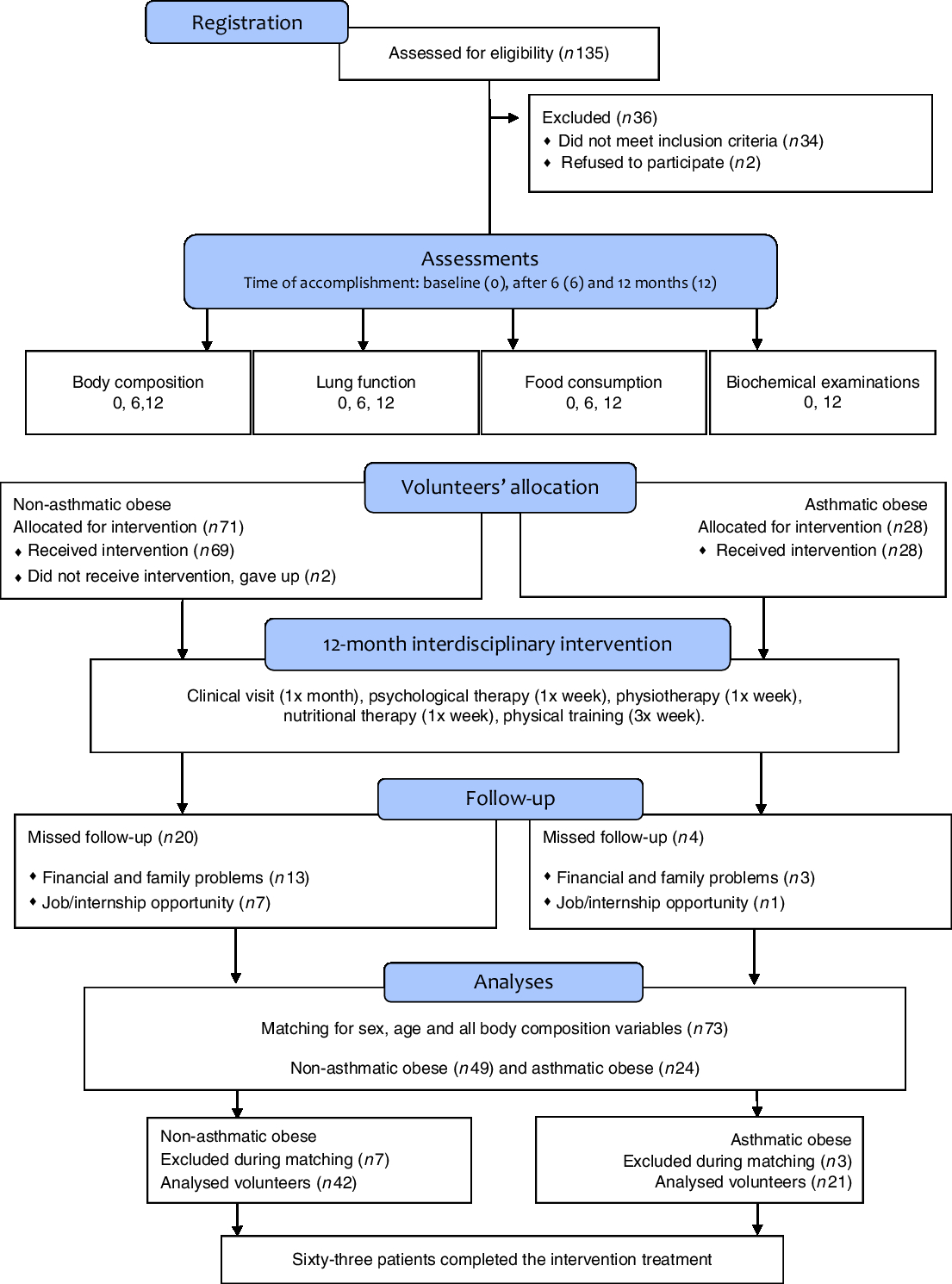
Fig. 1. Study design.
The groups were matched according to age, sex and anthropometric variables and body composition for homogeneity (P>0·05) and in order to observe only the impact of asthma. In the group of non-asthmatics obese, 76 % of adolescents were female with a mean age of 16 (sd 1·5) years and height 167 (sd 0·06) cm, whereas the obese asthmatic group was composed of 67 % female volunteers with a mean age of 16 (sd 1·7) years and height 167 (sd 0·09) cm.
Effects of interdisciplinary treatment
Both groups showed significant reduction (P < 0·01) of fat, BMI, visceral and subcutaneous fat percentage. On the contrary, lean mass percentage increased after 1 year of therapy (Table 1). After a year of intervention, lung function variables increased (P < 0·01) in both groups, except for FEV1:FVC ratio in the obese non-asthmatic group (Table 1). In addition, there was a reduction (P < 0·05) in respiratory symptoms in both groups after 12 months, with the exception of wheezing (P = 0·183) in the obese non-asthmatic group. There was no improvement in adipokine concentrations in both groups.
Table 1. General characteristics before and after interdisciplinary therapy in obese adolescents according to asthma diagnosis
(Mean values and standard deviations)
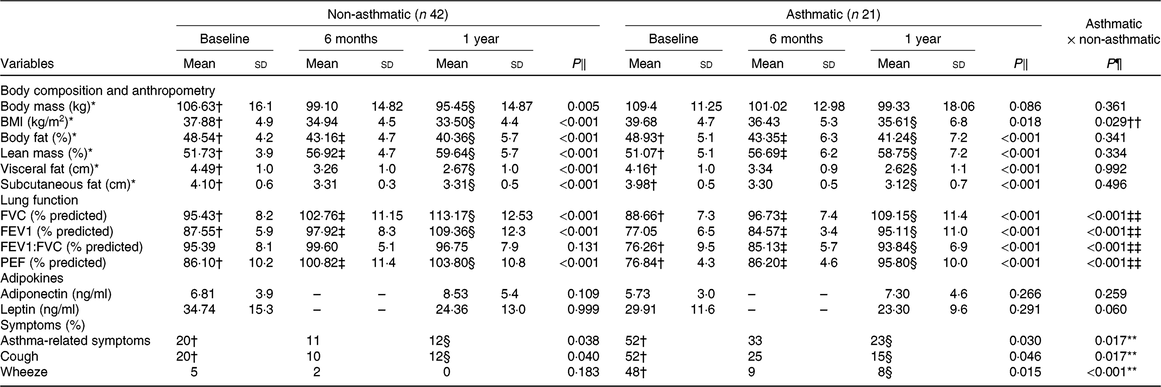
FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; FEV1:FVC, ratio between respiratory volume in 1 s and FVC; PEF, peak expiratory flow.
* Homogeneous groups (P > 0·05) at baseline; therefore, an intergroup analysis of these variables was done considering only the periods of 6 months and 1 year.
† Significant intragroup change baseline v. 6 months (P < 0·05).
‡ Significant intragroup change 6 v. 12 months (P < 0·05).
§ Significant intragroup change baseline v. 12 months (P < 0·05).
‖ Interdisciplinary therapy effect (ANOVA and Tukey post hoc).
¶ Difference between groups (ANOVA and Tukey post hoc).
** Difference only in baseline.
†† Difference only after 1 year of intervention.
‡‡ Difference in all periods (P ≤ 0·05).
There was a reduction in total energy intake and consumption of carbohydrates (P < 0·01) after 6 months of intervention in both groups. However, between 6 months and 1 year of treatment, there was no change in the total energy intake of both groups. Carbohydrate intake was similar to the baseline in both groups after intervention. Furthermore, in the short term, there was a reduction (P < 0·01) in lipids, saturated fats and cholesterol intake in both groups (Table 2).
Table 2. Macronutrient consumption before and after interdisciplinary therapy in obese adolescents according to asthma diagnosis
(Mean values and standard deviations)
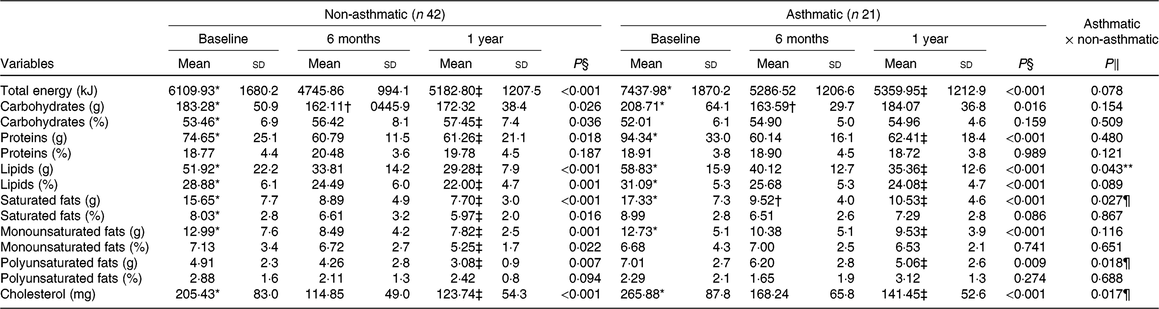
* Significant intragroup change baseline v. 6 months (P < 0·05).
† Significant intragroup change 6 v. 12 months (P < 0·05).
‡ Significant intragroup change baseline v. 12 months (P < 0·05).
§ Interdisciplinary therapy effect (ANOVA and Tukey post hoc).
‖ Difference between groups (ANOVA and Tukey post hoc).
¶ Difference only after 1 year of intervention.
** Difference in all periods (P ≤ 0·05; ANOVA and Tukey post hoc).
The obese asthmatic group decreased Mg and vitamin E consumption (P = 0·01) in the short term, but after 1 year of intervention, the intake was similar to baseline. After a year of intervention, Na was reduced (P < 0·05) in both groups. Contrarily, in both groups, the consumption of fibre, Ca, K, vitamins A and C did not change significantly (Table 3).
Table 3. Micronutrient consumption before and after interdisciplinary therapy in obese adolescents according to asthma diagnosis
(Mean values and standard deviations)
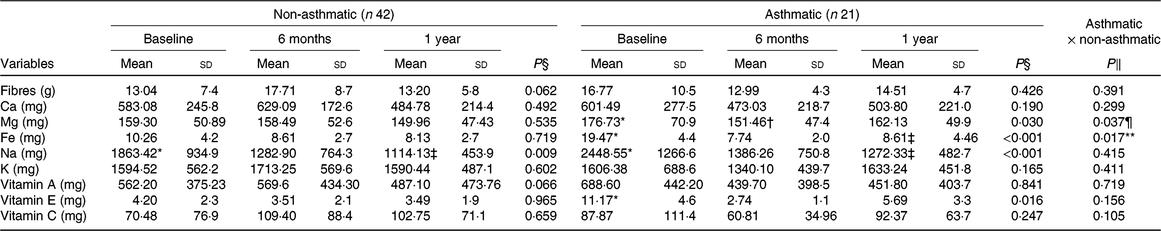
* Significant intragroup change baseline v. 6 months (P < 0·05).
† Significant intragroup change 6 v. 12 months (P < 0·05).
‡ Significant intragroup change baseline v. 12 months (P < 0·05).
§ Interdisciplinary therapy effect (ANOVA and Tukey post hoc).
‖ Difference between groups (ANOVA and Tukey post hoc).
¶ Difference in all periods (P ≤ 0·05).
** Difference only after 1 year of intervention.
Associations between diet and body composition, adipokines and lung function
Energy consumption and nutrients were predictors of lung function parameters. The total energy value was a FVC predictor, and each 1 kcal (4·184 kJ) increase in the diet caused a 0·02 (adjusted β) reduction of FVC (Table 4).
Table 4. Multiple regression analysis for baseline lung function determinants*
(β Coefficients and P values)
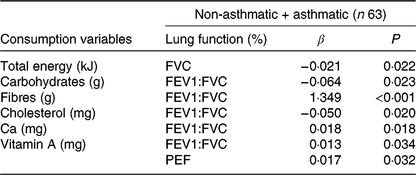
FVC, forced vital capacity; FEV1:FVC, ratio between respiratory volume in 1 s and FVC; PEF, peak expiratory flow.
* P ≤ 0·05; variables controlled by adipokines, BMI, body fat, lipids, saturated fats. Model 1, FVC (%): BMI, total energy, lipids, cholesterol, saturated fats. Model 2, FEV1 (%): leptin, adiponectin, BMI, cholesterol, fibres, vitamin A. Model 3, PEF (%): body fat, carbohydrates, fibres, cholesterol, Ca, vitamin A, saturated fats. Model 4, FEV1:FVC (%): body fat, carbohydrates, fibres, leptin, cholesterol, Ca, vitamin A, leptin.
Regarding macronutrients, carbohydrate consumption was inversely proportional to FEV1:FVC. Each increase of 1 g of carbohydrate reduced FEV1:FVC by 0·06 (adjusted β). Fibre intake and cholesterol were also a predictor of FEV1:FVC, and the addition of each 1 g of fibre in the diet contributed to an increase of 1·34 (adjusted β), while increasing every 1 mg of cholesterol decreased 0·05 (adjusted β) of the ratio (Table 4).
Regarding micronutrients, Ca had a directly proportional association with FEV1:FVC, and every 1 mg increase of Ca and Mg led to an increase of 0·01 (adjusted β) in FEV1:FVC. Vitamin A intake was a predictor of FEV1:FVC ratio and PEF, and each addition of 1 mg of vitamin A in the diet contributed to an increase of 0·01 (adjusted β) of both lung markers (Table 4). After a regression analysis, no influence of adipokines and body composition on lung functions was found.
Differences between obese asthmatic and non-asthmatic groups
Obese asthmatic adolescents showed lower spirometric values (P < 0·01) compared with the non-asthmatic group in all periods (Table 1). Regarding anthropometric parameters and body composition, obese asthmatic had higher BMI (P = 0·02) only after 1 year of therapy compared with the obese non-asthmatic group. In relation to food intake, the obese asthmatic group presented a higher intake of lipids (P = 0·04), polyunsaturated fats (P = 0·01), cholesterol (P = 0·01) (Table 2) and Mg (P = 0·03) (Table 3) in all periods. In addition, they showed higher saturated fat consumption (P = 0·02) (Table 2) after 1 year of treatment and lower Fe intake (P = 0·01) at baseline (Table 3) compared with the obese non-asthmatic group.
Discussion
This is the first study to investigate the effect of an interdisciplinary intervention on food consumption and the relationship of nutrient consumption with adipokines and lung function in obese asthmatic adolescents. The interdisciplinary approach in asthma-obesity treatment increased lean body mass, reduced fat percentage, BMI, visceral and subcutaneous fat, besides reducing lipid, cholesterol and Na consumption, positively influencing the markers of lung function.
Studies indicate worse control of asthma in obese(Reference Egan, Ettinger and Dewan48–Reference Ahmadizar, Uijverberg and Arets50) and improvement of symptoms due to weight loss interventions (Reference Lang4, Reference Liu, Lin and Zhao30). In the present study, there was an improvement of symptoms and pulmonary function in both groups after interdisciplinary intervention. The obese non-asthmatic group had some clinical conditions for the diagnosis of asthma but not functional conditions. Because they underwent spirometry, they had regular airflow, so they were not asthmatics. However, the interdisciplinary intervention further improved lung function in this group.
Clinical and functional status changes of both groups were significant. Breathing is an essential process for survival, and lung function improvements can cause great benefits in the quality of life and in the performance of daily living activities. Mainly because of the increase in the FEV1:FVC ratio, values >80 % are considered normal and accompanied by reduction in asthma symptoms(2, Reference Bateman, Hurd and Barnes40). One of the mechanisms for it is the reduction of adipocytes and lower production of proinflammatory adipokines, such as leptin, which, when found in excess in the blood, are capable of reaching target organs such as the lungs and increasing inflammation of airways(Reference Scott, Jensen and Wood24, Reference Lee, Lee and Choue25).
However, we did not find differences between the groups, similar to a previous study(Reference Huang, Del-Rio and Torres-Alcantara51) with adolescents in which there was no difference in serum concentrations of adiponectin and leptin in obese non-asthmatic and asthmatic groups. In addition, in the present study, improvement in lung function was independent of systemic inflammation, since there was no difference in adipokine concentrations after the intervention. These findings corroborate a randomised clinical trial with children and adolescents between 8 and 17 years of age who displayed a reduction in BMI after a low-energy diet followed by lung function improvement without alteration in inflammatory markers(Reference Jensen, Gibson and Collins52). In a similar study, leptin of obese adolescents also did not present a significant change after intervention(Reference Campos, Masquio and Corgosinho53).
These results differ from a previous study conducted by our group(Reference Da Silva, De Mello and Cheik27), which showed that an interdisciplinary intervention reduced serum leptin concentrations and increased adiponectin in obese adolescents, regardless of asthma diagnosis. However, obese adolescents had higher concentrations of leptin and adiponectin at the beginning of treatment compared with the present study.
In a separate sex re-analysis, we found that at baseline the mean leptin concentration was similar in both groups and sexes (obese asthmatic boys: 16·0 (sd 3·76) ng/ml; obese asthmatic girls: 16·65 (sd 6·6) ng/ml; obese non-asthmatic boys: 16·45 (sd 1·76) ng/ml; obese non-asthmatic girls: 19·47 (sd 16·38) ng/ml). Obese non-asthmatic girls showed greater dispersion in leptin concentrations. The mean leptin levels were normal compared with the reference values for healthy adolescents, regarding sex and age(Reference Lausten-Thomsen, Christiansen and Louise Hedley54).
Thus, we believe that serum adipokine concentrations in the present study were not problematic, as these were below the values obtained in other studies with obese adolescents(Reference Da Silva, De Mello and Cheik27, Reference Campos, Masquio and Corgosinho53). In addition, the fact that adipokine concentrations remain similar after interdisciplinary intervention suggests that other factors, besides fat percentage and BMI(Reference Campos, Masquio and Corgosinho53, Reference Elloumi, Ben-Ounis and Makni55, Reference Andersen56), may be involved in the expression of adiponectin and leptin, such as sexual maturation(Reference Yarrow, Beggs and Conover57).
In a cohort, an increase in androgen levels during puberty was negatively correlated with blood adiponectin concentrations in both sexes, especially in males(Reference Bottner, Kratzsch and Muller58). In a cross-sectional study with adolescents, leptin was inversely related to testosterone in boys and positively related to estradiol in girls(Reference Xi, Zhang and Guo59). In this sense, an in vitro study verified that testosterone can suppress leptin expression, even at the mRNA level(Reference Wabitsch, Blum and Muche60), confirming the previous finding. Therefore, in the present study, we believe that changes in androgens during puberty may have influenced adipokine concentrations in adolescents.
To the best of our knowledge, there are still no studies on adiponectin and leptin reference values for obese adolescents. Therefore, we suggest the advancement of research in this subject.
In relation to dietary intake and lung function, we observed that obese asthmatics had lower values of lung function and higher consumption of lipids, polyunsaturated fats and cholesterol compared with non-asthmatics in all evaluation periods. However, it is interesting to note that after a 6-month intervention, FVC, FEV1 and PEF in obese asthmatic patients were close to the baseline values of the obese non-asthmatic group. Furthermore, after the interdisciplinary intervention of 1 year, both groups showed a reduction in the consumption of lipids, cholesterol, saturated and polyunsaturated fats, Na and had increased lung function associated with clinical improvement of asthma symptoms, demonstrating the effectiveness of this treatment strategy and suggesting a relationship between nutrient intake and lung function.
In this intervention, cholesterol intake was a predictor of lung function. The new Dietary Guidelines for Americans does not establish a maximum value for cholesterol intake, justifying that there is no direct relationship between dietary cholesterol and its levels in blood(61). However, a recent systematic review(Reference Grundy62) and meta-analysis(Reference Berger, Ramon and Vishwanathan63) showed that only a consumption of high cholesterol can increase serum cholesterol by up to 5 %. Some studies have found it difficult to verify this result because of confounding factors, such as body mass, which has an influence on cholesterol metabolism(Reference Andersen56, Reference Berger, Ramon and Vishwanathan63). Obese people appear to absorb more cholesterol compared with lean individuals(Reference Miettinen and Gylling64). Therefore, studies on the consumption and metabolism of cholesterol in different clinical settings, such as obesity and asthma, are timely.
From this point of view, the literature demonstrates a positive relationship between serum concentrations of cholesterol and asthma severity(Reference Chen, Tung and Tsai65, Reference Ramaraju, Krishnamurthy and Maamidi66). However, studies were conducted mainly with children and adults, and only serum cholesterol was investigated. Obese asthmatics between 10 and 14 years of age showed higher cholesterol levels compared with obese children without asthma. Similarly, in Indian adults, a positive relationship was observed between serum cholesterol and the risk of asthma, regardless of age, sex, BMI and adipokines. Cholesterol is the main lipid fraction of the surfactant, a substance that helps reduce the surface tension of the pulmonary alveolar and respiratory efforts. In this regard, it is believed that excess cholesterol in the blood can change surfactant composition as well as its function, leading to lung collapse(Reference Veldauizen, Nag and Orgeig67, Reference Fessler68). Therefore, the finding of an inverse relationship between cholesterol consumption and FEV1:FVC in the present study suggests the importance of reducing cholesterol consumption to improve lung function and asthma control.
The pathophysiology of asthma-obesity is complex, and its mechanisms are being elucidated, but our study strengthens the hypothesis that it is impossible for only one pathway to be responsible for the disease. Thus, a lack of healthy lifestyle coupled with an obesogenic diet, characterised by high cholesterol and refined carbohydrate intake as well as low consumption of fibres, vitamins and minerals, has been associated with an increased risk of asthma(Reference Ellwood, Asher and Garcia-Marcos14, Reference Wood69).
The total energy value of the diet was a predictor of reduced FVC, and carbohydrate consumption was a negative predictor of the FEV1:FVC ratio. These findings corroborate previous studies showing that meals with a high carbohydrate content increase the expression of NFκ-B(Reference Patel, Ghanim and Ravishankar70), and energy restriction in the diet contributes to reducing the concentrations of TNF-α and carbonyl proteins, nitrotyrosine and 8-isopropanol, which are markers of oxidative stress, and may influence airway inflammation associated with asthma symptoms(Reference Johnson, Summer and Cutler71).
In our study, adolescents in both groups consumed Ca below reference values(72). Moreover, there was a positive association between Ca intake and lung function in obese adolescents. These findings suggest a possible relationship between Ca deficiency and asthma symptoms and assumes that an increased intake of this mineral may improve lung function. In the literature, there are few studies on micronutrient intake in asthmatic patients. However, a previous study has found low Ca concentrations in asthmatic children(Reference Hijazi, Abalkhail and Seaton73). It is known that Ca influences homeostasis and the contraction of pulmonary smooth muscles(37), but the specific mechanisms of these events are yet to be elucidated.
Regarding fibre consumption, both groups reported levels lower than the reference values(72). In fact, we found, for the first time, a proportional relationship between fibre intake and airway obstruction in obese adolescents. To our knowledge, there are no studies with adolescents; however, our findings corroborate a population study conducted with adults, in which a proportional association was found between fibre intake and lung function(Reference Hanson, Lyden and Rennard74). A reduction in pulmonary inflammation may be one of the mechanisms by which dietary fibres increase airflow. It has already been observed that fibre consumption was inversely associated with airway eosinophilia(Reference Berthon, Macdonald-Wicks and Gibson20) and a reduction of neutrophils, macrophages, lymphocytes and nitric oxide in the respiratory system(Reference Halns, Baines and Berthon16). Further research is needed to investigate the influence of fibre intake on the specific phenotype, non-atopic asthma, obesity and in different audiences. Furthermore, it is known that there are soluble and insoluble fibres. It is possible that both types are important in asthma or one of them may have more benefits than the other; therefore, further studies are necessary.
In foods that are fibre sources, we also found antioxidant nutrients such as vitamin A, which can improve the immune system and reduce asthma symptoms(Reference Barranco, Delgado and Gallego75). Vitamin A is absorbed in the human body in the form of retinoic acid, which increases the expression of IL-4, an anti-inflammatory cytokine(Reference Han, Blatter and Brehm76). However, a meta-analysis has found that vitamin A dietary intake was lower in asthmatic patients compared with non-asthmatic ones and in individuals with severe asthma compared with those with mild asthma. Additionally, a low vitamin A intake was positively associated with wheezing(Reference Allen, Britton and Leonardi-Bee77). Therefore, these findings corroborate our investigation, in which a lower intake of vitamin A was a predictor of lower values of FEV1:FVC and PEF, which are considered inferior airway obstruction markers(37).
One of the limitations of the present study is the absence of eutrophic asthmatic control or untreated obese to check for placebo effect. However, strengths include (1) evaluation of biochemical markers (adiponectin, leptin); (2) analysis of fat and lean mass percentage, visceral and subcutaneous fat in addition to BMI; and (3) investigation of macro- and micronutrients.
We realise the importance of the interdisciplinary treatment in asthma-obesity in order to improve nutrition, body composition and lung function in obese adolescents. However, further studies are needed to more accurately explain the mechanisms linking dietary intake to the pathophysiology of obesity-asthma, so that dietary management aimed at the prevention and treatment of obesity-asthma may be possible in the future. We propose that efforts be made to investigate: (1) reference values of leptin and adiponectin levels in obese adolescents; (2) consumption of micronutrients and their serum concentrations in adolescents, especially obese asthmatics; (3) role of Ca in homeostasis and contraction of the pulmonary muscle, and (4) effects of cholesterol, saturated fats and fibre consumption in the intestinal microbiota of obese asthmatic adolescents and their influence on adipokine production and secretion and pulmonary inflammation.
Acknowledgements
The authors thank the Federal University of Goiás Nutrition and Health Graduation Programme (Programa de Pós-Graduação em Nutrição e Saúde da Universidade Federal de Goiás – PPGNUT) and the Federal University of São Paulo Obesity Studies Group (Grupo de Estudos em Obesidade da Universidade Federal de São Paulo – GEO/UNIFESP). A very special thank you to the adolescents and their parents.
The study did not receive any specific funding from any financing agency, commercial or non-profit sectors.
There were no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519001739








