A great deal of attention has been paid to the ability of dietary fibre to lower cholesterol levels. Recent studies have shown that dietary fibre with ion-exchange capacity is particularly efficient in lowering cholesterol levelsReference Guillon and Champ1. Algae are a natural source of dietary fibre that differs from land-plant fibre chemically and physico-chemicallyReference Jiménez-Escrig and Sánchez-Muniz2–Reference Fleury and Lahayen5. Although a decoction of algae has traditionally been used to treat hyperlipidaemia in oriental culturesReference Tseng and Chang6, consumption of algal fibre to treat hypercholesterolaemia has scarcely been testedReference Jiménez-Escrig and Sánchez-Muniz2, Reference Dvir, Chayoth, Sod-Moriah, Shany, Nyska, Stark, Madar and Arad7.
In addition to their high fibre content, algae are also very rich in mineralsReference Mabeau and Fleurence8–Reference Bocanegra, Nieto, Blas and Sánchez-Muniz10. Some time ago, KlevayReference Klevay11 hypothesized that CHD is predominantly a disease of Zn and Cu metabolic imbalance. Moreover, our group found that lipoprotein levels in newborn infants were related to serum Zn:Cu ratios and Fe valuesReference Bastida, Vaquero, Veldhuizen and Sánchez-Muniz12. Thus, the evidence for the beneficial effects of algae consumption, with regard to both algal minerals and fibre, must be taken into consideration. In the scientific community there are concerns that a high intake of fibre may impair mineral absorption, due to the ability of dietary fibre to chelate ions in vitro Reference Frolich13, but few studies have investigated the bioavailability of algal minerals in young ratsReference Bocanegra, Nieto, Blas and Sánchez-Muniz10. Moreover, as far as we know, no study to date has tested the hypothesis that algal minerals can act as hypocholesterolaemic agents in growing hypercholesterolaemic rats.
Certain adverse effects, including liver failure, toxic hepatitis and death, have been associated with herbal medications containing algaeReference Ernst14. The relatively high amount of some trace elements could be related to those adverse effects. Nonetheless, considering normal rations, the heavy metal contributions of Nori and Konbu are generally below the toxic limits allowed in several countries as indicated by Indegaard and MinsaasReference Indegaard, Minsaas, Guiry and Blunden15.
In normocholesterolaemic rats, our group observed no effect of algae consumption on organ weight, except in the spleen, but did detect some histological changes in the liver and colonReference Bocanegra, Nieto, Blas and Sánchez-Muniz10. Thus, a large dietary alga supplement could exert a double-edged effect, beneficial, due to its hypocholesterolaemic effect, and harmful, for its negative effects on organ weight and structure.
Recognizing the increasing consumption of diets rich in cholesterol and the high prevalence of hypercholesterolaemia among childrenReference Rodríguez-Artalejo, Garces and Gorgojo16–Reference Bastida, Sánchez-Muniz, Cuena, Aragonés and Bravo18, the present study in growing male Wistar rats aimed to determine: (i) the acceptability of cholesterol-enriched diets supplemented with alga (Konbu or Nori); (ii) the effects of these cholesterol-alga diets on growth and dietary efficiency ratio; (iii) the effect of the alga diets on size and microstructure of several organs; (iv) the bioavailability of several minerals contained in those diets; (v) the possible relationship between the intake of certain minerals and their effect on cholesterolaemia.
Materials and methods
Materials
Nori (Porphyra tenera) and Konbu (Laminaria digitata) were obtained from a local supplier (Algamar C.B., Redondela, Pontevedra, Spain). These commercial edible marine seaweeds were freeze-dried and milled by using a cyclotic mill (Tecator 1093; Foss Tecator, Hoeganaes, Sweden) to a particle size of < 1·0 mm. The composition of the seaweeds employed in the current study consisted of a matrix of soluble, insoluble and total dietary fibre (% dry weight): 9·15 g/100 g, 26·98 g/100 g and 36·12 g/100 g, respectively in Konbu and 14·56 g/100 g, 19·22 g/100 g and 33·78 g/100 g, respectively in NoriReference Rupérez and Saura-Calixto19. The protein content (dry weight) of Konbu was 10·7 g/100 g and that of Nori, 28·29 g/100 g, while fat content (dry weight) was 1·83 g/100 g in Konbu and 1·64 g/100 g in Nori.
Diet preparation and experimental design
Freeze-dried algae (Porphyra or Laminaria) were homogeneously mixed with a rodent diet by passing both components several times through a sieve. The control diet consisted of 93 % rodent diet (AIN-93M Purified Rodent Diet; DYETS, Inc. Bethlehem, PA, USA) mixed with 7 % cellulose:wheat-starch mix (35:65, w/w). The Konbu diet was obtained by mixing the rodent diet (93 %) with freeze-dried Laminaria (7 %), while the Nori diet included 93 % rodent diet and 7 % freeze-dried Porphyra.
Diets contained roughly 14 % protein, 4 % fat and 7 % total dietary fibre; 4·65 g/100 g total dietary fibre came from the AIN-93 diet and the rest from the fibre supplement. Cellulose content of the control diet was 2·45 g/100 g, while that of the Laminaria diet was 2·52 g/100 g and that of the Nori diet was 2·36 g/100 g. Diets contained 2 % cholesterol and 0·4 % sodium cholate. The detailed composition of all the experimental diets is shown in Table 1.
Table 1 Composition of the control, Nori (Porphyra tenera) and Konbu (Laminaria digitata) experimental hypercholesterolaemic diets§
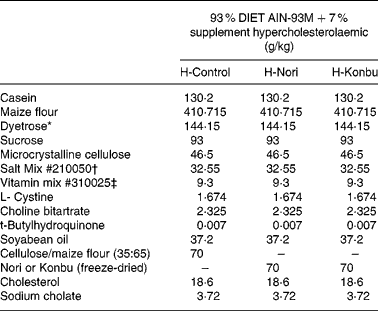
* Dyetrose (carbohydrate composition) (% by weight): monosaccharides 1; disaccharides 4; trisaccharides 5; tetrasaccharides and higher 90.
† Mineral mix contained AIN-93M Mineral Mix (g/kg): calcium carbonate 357·00; potassium phosphate monobasic 250·00; potassium citrate H2O 28·00; NaCl 74·00; potassium sulfate 46·60; magnesium oxide 24·00; ferric citrate u.s.p. 6·06; zinc carbonate 1·65; manganous carbonate 0·63; cupric carbonate 0·30; potassium iodate 0·01; sodium selenate 0·01 025; ammonium paramolybdate 4 H2O 0·00 795; sodium metasilicate 9H2O 1·45; chromium potassium sulfate 12H2O 0·275; lithium chloride 0·0174; boric acid 0·0815; sodium fluoride 0·0635; nickel carbonate 0·0318; ammonium vanadate 0·0066; sucrose finely powdered 209·806.
‡ AIN-93VX Vitamin Mixture (g/kg): niacin 3·00; calcium pantothenate 1·60; pyridoxine HCl 0·70; thiamine HCl 0·60; riboflavin 0·60; folic acid 0·20; biotin 0·02; vitamin E acetate (500 IU/g) 15·00; vitamin B12 (0·1 %) 2·50; vitamin A palmitate (500 000 IU/g) 0·80; vitamin D3 (400 000 IU/g) 0·25; vitamin K1/dextrose mix (10 mg/g) 7·50; sucrose 967·23.
§ For details of diets and procedures, see Materials and methods.
Animals and maintenance
Thirty male Wistar rats weighing approximately 127 g at the outset, obtained from the breeding centre at the Facultad de Farmacia (Universidad Complutense de Madrid, Spain), were randomly divided into three groups of ten animals each, according to average body weight and housed individually in metabolic cages kept in a temperature-controlled room (22·3 ± 18°C) with a 12 h light–dark cycle. The present study was approved by the Spanish Science and Technology Advisory Committee (Comisión Asesora de Ciencia y Tecnología) and by an ethics committee from the Universidad Complutense de Madrid (Spain). Rats were handled according to the Guide for the Care and Use of Laboratory Animals of the National Research Council20.
Dietary treatments
After weaning, rats were given commercial rat pellets (Panlab, Barcelona, Spain) during a 1 week period of adaptation to environmental conditions, then subsequently switched to the experimental diets. Water and food were provided ad libitum over a 3-week experimental period.
Food intake, growth rate, faeces and organ weights
Food intake was checked daily and body-weight variations were measured on alternate days. At the end of the experiment, one animal at a time was taken at random from each of the three groups in turn, anaesthetized with an intraperitoneal injection of sodium pentobarbital (45 mg/kg body weight) and killed, in non-fasting conditions, by extracting blood from the descending aorta with a syringe.
Faeces were collected every day during the last experimental week, weighed and frozen at − 20°C until analysis.
The heart, lateral liver lobe, spleen, left kidney and ascendant colon were removed, weighed and stored in liquid N2 until analysis.
Minerals
Aliquots of diets and faeces were dissolved in 50 % HNO3 (Suprapure; Merck, Darmstadt, Germany) at 500°C to total calcination. Ash was gravimetrically quantified and dissolved in 50 % HNO3 (50 ml), with 5 g/l LaCl3 (Merck) added to samples and standards to avoid interferences. Ca, Mg, Fe, Cu, Mn and Zn were determined in samples by flame atomic absorption spectrophotometry, while Na and K were determined by emission with a Perkin-Elmer Model 5100 PC atomic absorption spectrophotometer with an air-acetylene flameReference Vanhoof and De Schrijver21. Certified reference materials (CRM 63, CRM 185, Community Bureau of Reference, Brussels) were used to assess accuracy. All laboratory equipment employed in the trace elements analysis was washed with 10 N nitric acid to avoid contamination. Dietary concentration of some heavy metals was measured by inductively coupled plasma MS (Perkin Elmer Elan model 6000, ICP-MS), following wet ashing of the organic matter. Each sample (2·5 ml) was digested with 2 ml 65 % HNO3 (Suprapure; Merck) in Teflon bombs for 8 h at room temperature and subsequently heated at 100°C for 12 h. After cooling, solutions were filtered and made up to 25 ml with deionized water. The accuracy of the instrumental methods was validated by replicating all samples as well as by taking measurements of reference material (lobster hepatopancreas, NRC Canada TORT 2) every ten samples. Quantification was based on the most abundant isotope of each element free of analytical interferences. The mean recovery rates were between 90 and 95 %.
The mineral apparent absorption was calculated according to the formula: 100 % × (intake – faecal loss) / (intake).
Plasma cholesterol
Plasma cholesterol was determined by the enzymic colorimetric method of Boehringer Mannheim (Mannheim, Germany).
Relationship between plasma cholesterol and mineral absorption
Plasma cholesterol concentrations were related with mineral intakes and apparent absorption. The differential cholesterolaemic effect of diets was also tested after standardizing data for different mineral intakes.
Histological procedure
For the histopathological study, specimens from the lateral liver lobe, large intestine, spleen, heart and kidney were fixed in formalin, embedded in paraffin, cut in 4 μm sections and stained with haematoxylin and eosin.
Slide-mounted liver sections were investigated by microscopy for hepatocellular degeneration and inflammatory-cell infiltration. Segments of large intestine were also examined for epithelial ulceration, presence of inflammatory cells in lamina propria, submucosal oedema and presence of algae in the lumen. Each slide was scored as follows: 0 for null; 1 for weak; 2 for moderate; 3 for severe changes in liver morphology, infiltration of inflammatory cells in the liver and in the lamina propria of the colon and submucosal oedema. For ulceration, the score was 0 for null, 1 for 0–33 % loss of intestinal epithelium between, 2 for a 34–66 % loss and 3 for intestinal epithelial loss of 67–100 %. Presence or absence of algae in the colon was tested.
Statistical analyses
Results are expressed as mean values and standard deviations. ANOVA one-way and the Welch robust tests for media similarity were used to compare responses with the dietary intervention. Pairwise comparisons of diet responses between groups were conducted employing the Bonferroni test. Results from the histological study were compared using the MonteCarlo exact bilateral signification method. Subsequently, the groups were pairwise compared using the Fisher statistic exact test. Differences were accepted as significant when P < 0·05. Pearson product-moment correlations were applied to study the possible relationship between mineral intake, excretion and bioavailability and plasma cholesterol levels. Statistical analyses were conducted by using SPSS version 13 statistical analysis packages (SPSS Inc., Chicago, IL, USA).
Results
Food intake and body weight gain
Table 2 shows that food intake and body weight gain did not significantly differ among groups.
Table 2 Food intake, body weight gain, dietary efficiency ratio, protein efficiency ratio, faecal weight and apparent dietary digestibility in rats consuming the control, Nori (Porphyra tenera) and Konbu (Laminaria digitata) experimental diets§
(Values are means and standard deviations for ten animals per group)
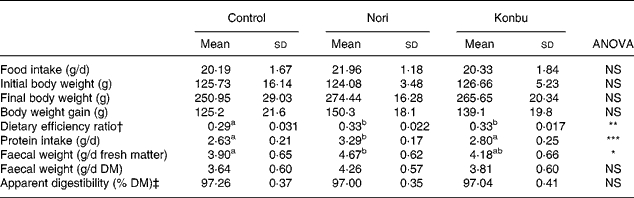
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05, Bonferroni test).
*P < 0·05; **P < 0·01; ***P < 0·001 (ANOVA one-way and Welch robust tests).
† Weight gain/food intake.
‡ 100* (food intake – faecal weight / food intake).
§ For details of diets and procedures, see Materials and methods.
Dietary efficiency ratio, protein intake, faecal weight excretion and apparent diet digestibility
Algae group rats displayed significantly higher dietary efficiency ratio values than control animals (P < 0·01) (Table 2). Nori rats presented significantly higher protein intake (P < 0·001) and significantly higher faecal excretion values for fresh matter (P < 0·05), but not for DM (Table 2). Dietary apparent digestibility did not significantly differ among groups (Table 2).
Mineral intake, excretion and apparent absorption
Mineral intake of rats given an alga supplement was significantly higher (except for Ca in the case of Nori rats and Fe, Mn, Cu and Ca in that of Konbu rats than that of control rats (Table 3). Mineral intake of Nori rats was generally higher than that of Konbu animals, except in the case of K, which was significantly lower in the former animals. Excretion of all minerals, except Ca, was significantly higher in the Nori group than in the control rats, while excretion of all minerals except Na, K, Ca and Mg was significantly higher in Konbu animals than in the control group.
Table 3 Intake, faecal excretion and apparent absorption of several mineral in rats fed the control, Nori and Konbu diets‡
(Values are means and standard deviations for ten animals per group)

a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05, Bonferroni test).
*P < 0·05; **P < 0·01; ***P < 0·001 (ANOVA one-way and Welch robust tests).
† 100 * (food intake – faecal weight / food intake).
‡ For details of diets and procedures, see Materials and methods.
Apparent mineral absorption of Na (P < 0·001) and K (P < 0·001) was significantly higher in both algae groups than in control rats. Apparent absorption of Fe (P < 0·001), Mn (P < 0·001) and Zn (P < 0·001) was significantly lower in the Nori animals than in the other groups. Apparent absorption of Ca, Cu and Mg did not differ significantly between groups.
The ratios for the intake and absorption of Zn and Cu differed significantly (P < 0·01 and 0·05, respectively). Nori animals showed the lowest Zn:Cu ratio intake and apparent absorption values and the highest Zn:Cu faecal excretion levels (Table 3).
Plasma cholesterol
Plasma cholesterol was affected by alga consumption. The level was 5·20 (sd 1·78) mmol/l in the control rats, 4·33 (sd 0·64) mmol/l in the Nori fed rats and 5·67 (sd 1·46) mmol/l in the Konbu rats. Differences were significant between Nori and Konbu diets (P = 0·016) (Fig. 1). After standardizing for mineral intakes the plasma cholesterol also appeared to be significantly affected by diets. Cholesterol/Na (mmol cholesterol/l per mg Na): 0·19 (sd 0·10); 0·05 (sd 0·01); 0·09 (sd 0·02) (P = 0·000); cholesterol/K (mmol cholesterol/l per mg K): 0·07 (sd 0·03); 0·03 (sd 0·01); 0·02 (sd 0·01) (P = 0·000); cholesterol/Zn (mmol cholesterol/l per mg Zn): 8·57 (sd 3·38); 6·29 (sd 1·32); 8·27 (sd 2·19) (P = 0·093); cholesterol/Mg (mmol cholesterol/l per mg Mg): 0·42 (sd 0·20); 0·19 (sd 0·04); 0·28 (sd 0·07) (P = 0·002); cholesterol/Cu (mmol cholesterol/l per mg Cu): 29·03 (sd 11·02); 16·97 (sd 5·34); 31·06 (sd 9·15) (P = 0·003); for control, Nori and Konbu groups, respectively.

Fig. 1 Plasma cholesterol levels of rats consuming the control, Nori and Konbu diets. Bars bearing different letters were significantly different (P < 0·05; ANOVA one-way and Welch robust tests followed by Bonferroni test). For details of diets and procedures, see Materials and methods.
Mineral intake and apparent absorption and plasma cholesterol
Plasma cholesterol levels appear negatively and significantly correlated with the intake of Fe (r–0·53; P = 0·003), Mn (r–0·518; P = 0·004), Zn (r–0·375; P = 0·045), Ca (r − 0·38; P = 0·042). None of the apparent mineral absorptions was significantly correlated (P>0·05) with the plasma cholesterol levels.
Liver, spleen, kidney and heart weights
Organ weights did not differ significantly between groups (Table 4). However, spleens of Konbu-fed rats tended to weigh less, kidneys of Nori-group animals tended to weigh more and livers of both algae groups tended to weigh more than the same organs of the other groups (Table 4).
Table 4 Organ weights and somatic index of rats consuming the control, Nori and Konbu diets‡
(Values are means and standard deviations for ten animals per group)
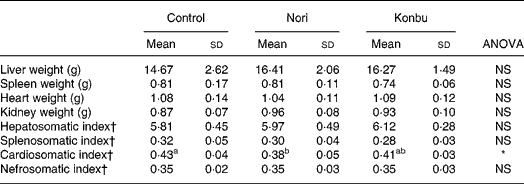
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05, Bonferroni test).
*P < 0·05 (ANOVA one-way and Welch robust tests).
† Organ weight * 100/body weight.
‡ For details of diets and procedures, see Materials and methods.
Considering that organ weights would appear to be highly dependent on body size and/or body weight, the somatic index (100 × organ weight/body weight) was also tested. Of all the somatic indexes, only the cardiosomatic index, which was lower in Nori rats, differed significantly between groups (Table 4).
Histological changes
A microscopic study of the organs revealed no relevant histological changes in heart, spleen and kidney tissues between groups. The presence of fat-like vacuoles in hepatocytes was significantly less frequent in Nori rats (P < 0·05). Control and Konbu groups displayed a large amount of mixed-size vesicles. Light focal infiltration of lympho-plasmocytic cells was found in some livers, mostly in the Nori rats (Fig. 2; Table 5).

Fig. 2 Effect of algae supplementation on hepatic histological changes induced by cholesterol enriched diet. (A) Control diet rat; (B) Nori rats; (C) Konbu rats. Note that Konbu and control rats have large numbers of hepatocytes with mixed-size lipid-like vesicles that were much less evident in Nori rats. Haematoxylin and eosin ( × 100). For details of diets and procedures, see Materials and methods.
Table 5 Histological changes in liver and ascending colon of rats consuming the control, Nori and Konbu diets§
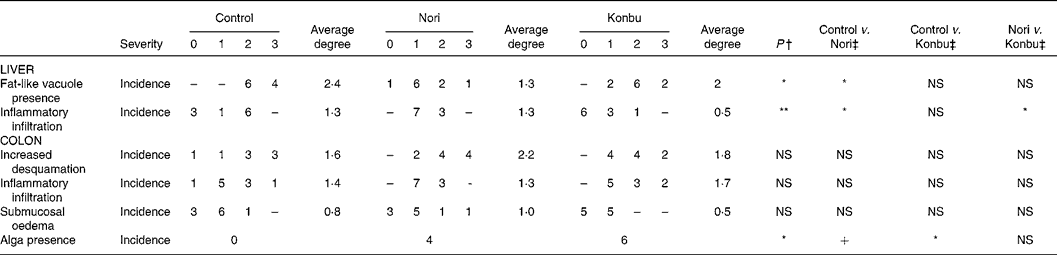
+ P < 0·1; *P < 0·05; **P < 0·01; ***P < 0·001.
† Monte Carlo exact bilateral signification method.
‡ Pairwise comparison using the Fisher statistic exact test.
§ For details of diets and procedures, see Materials and methods.
Regarding the colon wall, losses of epithelial surface, inflammatory infiltration and oedema were not significantly different in the three groups studied (Table 5). However, desquamation and submucosal oedema tended to be greater (although not significantly) in Nori rats, while inflammatory infiltration was more pronounced (although not significantly) in Konbu animals. The presence of algae in the intestinal lumen of Nori and Konbu rats was a relatively frequent finding.
Discussion
Food intake and body weight gain
Diets containing seaweeds were consumed at a rate similar to that of the control diet. Data are in agreement with those of Ren et al. Reference Ren, Noda, Amano, Nishino and Nishizana22 and Wong et al. Reference Wong, Sam, Cheung and Ang23 and also similar to those of rats consuming diets containing the same alga supplements but without added cholesterolReference Bocanegra, Nieto, Blas and Sánchez-Muniz10. Naim et al. Reference Naim, Morley, Kare and Ingle24 have pointed out that factors such as taste, smell and texture are decisive in the selection of dietary intake, apart from the fact that, in the long run, post-intake factors may affect the acceptability of a diet. Thus, data suggest that alga diets, cholesterol-enriched or not, appear to be well accepted by rats.
Body weight gains were similar to those found by Wong et al. Reference Wong, Sam, Cheung and Ang23 in rats consuming different seaweed-based diets, but slightly lower than those observed in a previous study in rats fed alga diets with no added cholesterolReference Bocanegra, Nieto, Blas and Sánchez-Muniz10. Wang & YangReference Wang and Yang25 showed that an 8-week diet including 1 % cholesterol, 0·2 % cholate, 5 % sodium alginate and 5 % or 10 % carrageenan did not affect food intake and growth in rats. Kikunaga et al. Reference Kikunaga, Miyata, Ishibashi, Koyama and Tano26 showed that body weight gain decreased when sodium alginate was added to the diets.
Dietary efficiency ratio, faecal excretion and apparent diet digestibility
Although all test diets theoretically contained the same energy content (Table 1), energy utilization of the Nori and Konbu diets was higher than that of the control diet, as soluble fibre fermented by caecal flora produces SCFA that are later metabolically utilizedReference Inagaki, Sakata, McCleary and Prosky27, Reference Livesey, Elia, Cummings, Rombeau and Sakata28. This would explain the fact that dietary efficiency ratio values were higher in the alga groups than in control rats (Table 2). The tendency for Nori rats to present higher body weights could be at least partially explained by their higher protein intake. Nonetheless, algae contain resistant protein, which may pass through the intestine without being absorbed, and can retain some dietary mineral components together with fibreReference Holland, Unwin and Buss29.
The present results are in line with those reported by Gudiel-Urbano & GoñiReference Gudiel-Urbano and Goñi30 and Bocanegra et al. Reference Bocanegra, Nieto, Blas and Sánchez-Muniz10, who found that seaweed supplements significantly increased faecal weight (as fresh matter) and that dietary apparent digestibility did not significantly differ among groups.
Mineral intake and apparent absorption
Algae are rich in a wide variety of minerals, due to their capacity to retain inorganic sea substancesReference Jiménez-Escrig and Goñi31. Seaweeds from the genus Porphyra contain, among other minerals, high amounts of Fe (0·2–0·7 g/100 g)Reference Shaw and Liu32 and Zn (2–20 g/100 g)Reference Nisizana, Noda, Kikuchi and Watanabe33. These data explain the fact that the algae groups, and in particular the Nori rats, presented the highest intake of most minerals. Our group previously reported similar results in rats fed algae-enriched normocholesterolaemic dietsReference Bocanegra, Nieto, Blas and Sánchez-Muniz10. Fibre has been shown to retain divalent cations such as Ca and MgReference Kashimura, Kimura and Itokama34 and, thus, to decrease their bioavailability. Therefore, the increased faecal excretion of several minerals should be, at least partially, ascribed to the alga fibre in these diets. Similar effects were also seen in a previous studyReference Bocanegra, Nieto, Blas and Sánchez-Muniz10. However, caecal microflora releases the chelated cations from the fermentable fibre in the caecum and proximal colon, increasing their bioavailabilityReference Ohta, Ohtsuki, Baba, Adachi, Sakata and Sakaguchi35, Reference Greger36.
The high levels of Na and K in Nori and KonbuReference Jiménez-Escrig and Goñi31 explain the significantly higher intake (about 2- or 3-fold) of these minerals by seaweed-fed rats. Results for faecal excretion and apparent absorption of Na and K are consistent with those reported by other authorsReference Bocanegra, Nieto, Blas and Sánchez-Muniz10, Reference Gudiel-Urbano and Goñi30. Faecal excretion data from the present study display higher Na (10·81 to 17·03 mg/d) and K (15·60 to 25·84 mg/d) values in all rat groups than those for Na (1·40 to 6·17 mg/d) and for K (3·01 to 15·83 mg/d) of a previous study in rats fed a diet without additional cholesterolReference Bocanegra, Nieto, Blas and Sánchez-Muniz10. Furthermore, hypercholesterolaemic Nori rats presented much higher excretion values for Fe, Mn, Zn, Cu and Mg than their normocholesterolaemic counterparts (data not shown), but no differences were observed for Konbu rats. SCFA, but not lactic or succinic acids, stimulate the absorption of water and Na from the large intestineReference von Engelhardt, Bartels, Kirschberger, Meyer zu Dutting-dorf and Busche37. The higher soluble dietary fibre content of Nori could explain, at least in part, the higher Na absorption in those rats.
However, it is relevant that the apparent mineral absorption of Fe, Mn and Zn in the present study was negative in the case of the Nori diet. This effect had not previously been observed in rats fed a Nori diet without additional cholesterolReference Bocanegra, Nieto, Blas and Sánchez-Muniz10.
Plasma cholesterol levels
Some time ago, KlevayReference Klevay11 suggested that the hypercholesterolaemic effect of diets was related to their high Zn and Cu ratio. The significant inverse relationships between the intake of Fe, Mn and Zn and the plasma cholesterol levels suggest that these minerals play a role in cholesterolaemia values. Bastida et al. Reference Bastida, Vaquero, Veldhuizen and Sánchez-Muniz12 found differences in serum and lipoprotein cholesterol and serum TAG between neonates within the highest and lowest quartile of different mineral concentrations. Present data also indicate that the Zn:Cu dietary intake ratio was lower in the Nori diet than in the other two diets, suggesting a possible role of this mineral ratio on cholesterolaemia. Some trace elements, such as Mn and Zn, are absorbed and quickly re-secreted into the gut through the bileReference Finley, Caton, Zhou and Davison38 and Suzuki et al. Reference Suzuki, Ohsugi, Yoshie, Shiroi and Hirano39 demonstrated in vitro that cholate bound to Nori more than twice as much as with other seaweeds, such as Konbu. Thus, it is possible that the decrease observed in the apparent absorption of these minerals is related to the different hypocholesterolaemic effects of the Nori and Konbu diets.
Animals deficient in scavenger receptor type I (SR-BI) or in angiotensin AII suppresses ATP binding cassette transporter AI gene (ABCAT2) two transmembrane proteins involved in intestinal cholesterol absorption, show a substantial decrease in this activity. Endothelial Zn finger protein 2, a novel transcription factor, is known to regulate the SR-BI expressed by endothelial cells. Thus, intestinal absorption of cholesterol may depend on certain minerals, such as Zn. Chen et al. Reference Chen, Liao, Kuo and Ho40 found that reduced intestinal Zn absorption in rats may be due to a reduction of mucosal Zn efflux into plasma from the basolateral membrane. Seaweeds are known to contain large quantities of soluble dietary fibre. Brown seaweeds, in particular, are rich in alginates, a group of polysaccharides that have many physiological effects on blood pressure and serum lipidsReference Jiménez-Escrig and Sánchez-Muniz2. Differences in cholesterolaemia between Nori and Konbu diets can be related to the alga composition. Nori contains more porphyrans, alginates and furans than Konbu, explaining, at least partially, the present results. The ionic groups of molecules composing fibres are able to sequester minerals and bile acids and consequently increase faecal bile acid and mineral excretions; thus, lowering blood cholesterol levelsReference Lahayen4, Reference Suzuki, Ohsugi, Yoshie, Shiroi and Hirano39.
As previously commented, Nori diet rats displayed the lowest ingestion and apparent absorption of Zn/Cu, suggesting that the Klevay et al. Reference Klevay11 hypothesis can be applied to the present data.
Liver, spleen, kidney and heart, weights
Present results suggest that hepatomegaly and steatosis was produced in rats fed cholesterol-enriched diets as a consequence of fatty liver inductionReference Sánchez-Muniz, Cava, Viejo, Bastida, Higón and Marcos41. In agreement with Wong et al. Reference Wong, Sam, Cheung and Ang23, no significant effect on liver weight was found in rats fed different diets containing algae and cholesterol. With the exception of the liver, organ weights were similar to those obtained in a similar study, in which the same algae were added to diets without additional cholesterolReference Bocanegra, Nieto, Blas and Sánchez-Muniz10. Sánchez-Muniz et al. Reference Sánchez-Muniz, Higón, Cava and Viejo42–Reference Sánchez-Muniz, Cava, Viejo, Bastida, Higón and Marcos44 found that the inclusion of fried sardines to cholesterol-enriched diets markedly reduced serum cholesterol but increased liver weights. At present, we have no hypothesis to explain the effect of algae diets on the cardiosomatic index.
Histological changes
The size, form and structure of hepatocyte vacuoles, together with the liver enlargement, suggest a net fat infiltration in rats due to consumption of cholesterol-rich diets. Viejo et al. Reference Viejo, García-Linares, Bastida, García-Arias and Sánchez-Muniz45 indicate that the liver fat increase in cholesterol-fed rats was in the form of TAG and esterified cholesterol. The moderate to severe hepatocellular vacuolization observed in control and Konbu rats was much less pronounced in Nori rats, suggesting that Nori may provide a degree of hepatic protection (Fig. 2). Konbu and control rat livers contained hepatocytes with lipid-like vesicles of different sizes, which were much less observed in Nori rats. Moreover, in the group fed Konbu, the livers were found to contain large lipid droplets. However, the size of nuclei did not differ significantly within the various group hepatocytes. Again, the reduction of fat infiltration in Nori rats can be related to the lower plasma cholesterol level and the suggested mineral/fibre mechanisms. The meaning of light focal infiltration of lympho-plasmocytic cells that was found in some livers, mostly in the Nori groups (Fig. 2) (Table 5) is unclear. Saito et al. Reference Saito, Yoneda, Yokohama, Okada, Haneda and Nakamura46 suggest that fucoidans prevent the concanavalin A-induced liver injury throughout the production of endogenous IL-10 and the inhibition of proinflammatory cytokine in mice. Konbu is rich in foicoidans; thus, it can be suggested that the lower liver inflammatory infiltration observed in Konbu rats is related to these sulphated polysaccharides intakes.
The morphological alterations observed in the colon did not appear to be the result of algae consumption, but were generally more severe than those observed in Wistar rats consuming diets enriched in algae but without added cholesterolReference Bocanegra, Nieto, Blas and Sánchez-Muniz10.
At present we do not have a reliable hypothesis to explain these data, but the alterations found would mainly be related to the irritating mechanical and/or physiological effect of the cholate-cholesterol content of the diets (2·4 %).
Nori rats tended to show higher average degree of desquamation and oedema than the other rats. Non-digested carbohydrates can undergo fermentation by colony microflora producing important amounts of H+, methane, SCFA, lactic and succinic acidsReference Inagaki, Sakata, McCleary and Prosky27. According to HoshiReference Hoshi47, lactic and succinic acids, but not SCFA, are the major determinates of lumen pH in the large bowel. It is interesting that lowering lumen pH inhibits caecal epithelial cell proliferation in vivo Reference Ichikawa and Sakata48. The decrease of colon epithelium and the light to moderate scaling off of the colon epithelium histologically observed in Nori rats seem to be related to the higher fermentability of the Nori fibre. The loss of colonic epithelium in Nori rats would also explain their low apparent absorption values for several minerals, and thus the previously discussed hypocholesterolaemic effect of this alga.
In conclusion, alga supplementation did not significantly change the total food intake or the body weight gain of growing rats but did affect the apparent absorption of several minerals. The relationship found between plasma cholesterol and the intake and bioavailability of some minerals suggests an important role of these micronutrients on cholesterolaemia, particularly in rats consuming Nori. The lower incidence of liver vacuolization in Nori rats seems to be a consequence of the reduction of plasma cholesterol levels. Long-term studies are needed to test the effects of intake and bioavailability of different minerals on cholesterol metabolism in rats fed diets containing different types of algae.
Acknowledgements
We are indebted to Laura Barrios for her help and assistance. This work was granted by the Spanish Ministerio de Investigación y Ciencia, Project AGL 2005-07204-C02-C1/ALI.









