Non-alcoholic fatty liver disease is becoming increasingly prevalent in developed and developing countries. Although non-alcoholic fatty liver is considered benign, it can progress to non-alcoholic steatohepatitis (NASH), which can then lead to cirrhosis, hepatocellular carcinoma and liver failure(Reference Bacon, Farahvash and Janney1). Clinically, the occurrence of non-alcoholic fatty liver disease/NASH in men is more prevalent than in women(Reference Fan, Zhu and Li2, Reference Browning, Szczepaniak and Dobbins3). However, to date, there is no effective therapy for NASH(Reference Matteoni, Younossi and Gramlich4, Reference Bedogni and Bellentani5), and people are recommended to avoid high-fat foods. An alternative, and more attractive, approach would be the development of protocols to prevent non-alcoholic steatosis.
Isoflavonoids including genistein, the most abundant phyto-oestrogen present in soya, have been reported to have beneficial effects in numerous diseases(Reference Adlercreutz and Mazur6, Reference McCue and Shetty7). Genistein also plays an important role in the regulation of lipid metabolism and has been shown to inhibit high-fat diet (HFD)-induced obesity(Reference Lee, Choi and Kim8–Reference Yang, Lee and Park10). Likewise, genistein has been shown to prevent progression to NASH in a rat model(Reference Yalniz, Bahcecioglu and Kuzu11). By enhancing gene expression for enzymes of fatty acid oxidation and inhibiting the expression of those involved in lipogenesis, genistein may lower serum lipid concentrations and fat storage in the body(Reference Lee, Choi and Kim8, Reference Yang, Lee and Park10, Reference Choi, Jung and Yeo12, Reference Ae Park, Choi and Cho13). Thus, it is possible that genistein supplementation may be beneficial in preventing or treating hepatic steatosis. However, prolonged genistein treatment of adult male rats has been reported to cause structural changes in the urethroprostatic complex(Reference Strauss, Mäkelä and Joshi14) and changes in prostate weight(Reference Weber, Setchell and Stocco15). In contrast, neonatal exposure to moderate doses of genistein during the first few days after birth does not result in later reproductive changes, in terms of sperm count, sperm motility, testis weight and morphology, in adult rats(Reference Nagao, Yoshimura and Saito16–Reference Maff18). We are not aware of any studies that investigated the prolonged effects of neonatal treatment with genistein on the adult response to lipid metabolism. In the present study, we tested the hypothesis that subcutaneous exposure of male rats to genistein during the neonatal period would protect the liver from HFD-induced NASH after weaning. The results indicate that genistein had beneficial effects in preventing HFD-induced NASH through 6 weeks post-weaning.
Experimental methods
Animals
Female Sprague–Dawley rats were purchased from the Beijing Experimental Animal Center, China, at 8 weeks of age. The rats were acclimatised to the laboratory for 2 d before the start of the experiments. Rats were housed individually in a room maintained at 22°C with a constant light–dark cycle (14 h light–10 h dark), and were provided isoflavonoid-free rat chow and water ad libitum. The studies were in accordance with the Guidelines for Experimental Animals and approved by the Animal Care and Use Committee of China Agricultural University. Oestrous female rats were cohabited overnight with a male rat. After copulation was confirmed by vaginal smear and sperm detection, the females were weighed and randomly assigned to four experimental groups. Dams were allowed to deliver naturally, and on the day of birth, pups were weighed, sexed and litters culled to six males. Pups were nursed until weaning at day 21. Genistein was administered by injection (subcutaneously), which bypasses the gastrointestinal tract and thus any modifications that may occur that are related to absorption and/or microbial action. However, injected genistein is reported to produce similar plasma aglycone levels(Reference Doerge, Twaddle and Banks17) as oral administration, and injection avoids the difficulties in delivery by gavage in neonatal rats. We selected three doses of genistein (30, 300 and 1200 μg/rat per d, equivalent to 4, 40 and 160 mg/kg body weight), with the lower two doses covering doses equivalent to the nutritional exposure of infants to total isoflavones in soya milk(Reference Maff18). The highest dose (1200 μg/rat) was selected since this dose has been reported to down-regulate hormone receptors in the testes of male mice and thus represents potentially toxic amounts of genistein(Reference Adachi, Ono and Koh19). On days 1–5 after birth, the pups received subcutaneous injections of genistein (Sigma Chemical Company, St Louis, MO, USA) in 20 μl of maize oil. Control pups received daily subcutaneous injections of the vehicle (20 μl maize oil). After weaning, pups were allocated to receive a HFD (Table 1) for 6 weeks (9 weeks of age). Pups were weighed weekly, and food intake was monitored twice a week. At 9 weeks of age, food was withdrawn for 12 h overnight. All pups in one litter received the same treatment, and one pup per litter was used for analysis. On the day of the experiment, rats were anaesthetised with sodium pentobarbital (30 mg/kg body weight, intraperitoneally). Blood was drawn by cardiac puncture, and the rats were killed by exsanguination. All rats in the treatment groups were killed for analysis. Samples of the liver were immediately frozen in liquid N2 for mRNA and protein analysis, and samples from different areas of the liver were fixed in buffered formalin for histological examination.
Table 1 Composition of the experimental diet
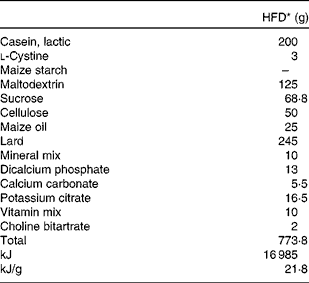
HFD, high-fat diet.
* The HFD of rats is an American Institute of Nutrition (AIN)-93-modified diet with 35 % fat (60 % fat energy) content.
Experimental diet
Dams were provided an American Institute of Nutrition (AIN)-93 rodent diet, containing 20 % casein, 0·3 % dl-methionine, 15 % maize starch, 50 % sucrose, 5 % fibre-celfil, 3·5 % mineral mixture, 1 % vitamin mixture and 0·2 % choline bitartrate, ad libitum throughout gestation, parturition and lactation. After weaning (21 d), offspring were provided free access to a modified AIN-93 diet (Table 1) of the HFD formula containing 60 % energy as fat.
Biochemical analysis
Plasma glucose was determined with a Chemistry Analyzer (RA1000; Bayer Corporation, Leverkusen, Germany) and a Glucose Reagent Kit (Biosino Bio-technology and Science, Inc., Beijing, China). Serum insulin and glucagon were measured using a Rat Insulin ELISA Kit and a Rat Glucagon ELISA Kit (Rapidbio, West Hills, CA, USA). Plasma alanine aminotransferase was measured using a kit from Roche Diagnostics (Mannheim, Germany). Plasma and hepatic (Folch(Reference Folch, Lees and Sloane Stanley20)-extracted) total cholesterol and TAG concentrations were determined using enzymatic methods (Sigma Chemical Company).
Histological examination
Formalin-fixed and paraffin-embedded liver samples were processed for routine haematoxylin and eosin staining. Liver histology was examined and graded according to the magnitude of steatosis, inflammation and ballooning degeneration of hepatocytes as described previously(Reference Matteoni, Younossi and Gramlich4, Reference Brunt, Janney and Di Bisceglie21). The degree of steatosis was graded 0–4 based on the average percentage of fat-accumulated hepatocytes per field at 200 × magnification under haematoxylin and eosin staining (grade: 0 = < 5%, 1 = 5–25%, 2 = 26–50%, 3 = 51–75%, 4 = >75%). Inflammation was evaluated by the number of inflammatory cells counted in ten random fields at 200 × magnification. Mean values were calculated and are reported as inflammatory cells/mm2. Hepatocellular ballooning was evaluated as either negative (absent) or positive (present).
Hepatocyte apoptosis
Hepatocyte apoptosis was determined in the rat liver by the terminal dUTP nick end labelling assay using a In Situ Cell Death Detection Kit (Roche Diagnostics), according to the manufacturer's instructions.
Gene expression by real-time PCR
Total RNA was isolated from the liver using an RNAqueous Kit according to the manufacturer's instructions (Amibion, Foster City, CA, USA) and reverse transcribed using M-MLV RT (Invitrogen, Carlsbad, CA, USA). The primers for complementary DNA amplification of fatty acid synthase (FAS), sterol regulatory element-binding protein-1c (SREBP-1c), PPARα, TNFα, phosphoenolpyruvate carboxykinase and glucose-6-phosphatase genes and the corresponding annealing temperatures are listed in Table 2. The β-actin sequence was used as the internal control. Quantitative real-time PCR was performed using DNA Engine Opticon-2 (MJ Research, Waltham, MA, USA) and DyNAmo SYBR Green qPCR commercial kits (Finnzymes, Finland). The PCR system consisted of 5·0 μl of SYBR Green qPCR mix, 1·0 μl of complementary DNA, 3·6 μl of double-distilled water and 0·4 μl of primer pairs (25 mmol/l for each primer) in a total volume of 10 μl. All samples were assayed in triplicate. The relative mRNA levels of target genes were determined using the relative standard curve method(Reference Zhou, Li and Yin22).
Table 2 Primers used in real-time PCR
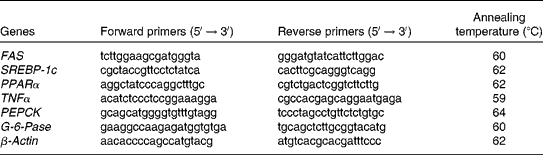
FAS, fatty acid synthase; SREBP-1c, sterol regulatory element-binding protein-1c; PEPCK, phosphoenolpyruvate carboxykinase; G-6-Pase, glucose-6-phosphatase.
Western blotting
Tissues were weighed and homogenised in ice-cold buffer (20 mm-Tris; 0·1 mm-EDTA; 0·1 % Triton X-100; 250 mm-sucrose; protease inhibitor mixture, 50 μl/5 ml; pH 7·6). After centrifugation at 10 000 g for 30 min, supernatants were sampled, and protein (50 μg) was diluted in Laemmli sample buffer(Reference Laemmli23) and resolved on 10 % (w/v) SDS-polyacrylamide gels. Proteins were electrophoretically transferred to a polyvinylidene fluoride membrane (Amersham International, Amersham, Bucks, UK), and membranes were blocked overnight at 4°C in Tris-buffered saline (pH 7·6) containing 5 % (w/v) non-fat dried milk. Membranes were then incubated for 1 h at room temperature in Tris-buffered saline, 0·5 % non-fat dry milk, containing primary antibodies (0·2 μg/ml) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), washed twice at room temperature for 15 min in Tris-buffered saline, and incubated for 1 h at room temperature in Tris-buffered saline, 0·5 % non-fat dried milk, with a 1:2000 dilution of secondary antibody conjugated with horseradish peroxidase (Amersham).
Statistical analysis
All pups in a litter received the same treatments, and one pup per litter was taken for analysis. Results are presented as means with their standard errors, where n indicates the number of litters, and analysed using one-way ANOVA followed by Tukey's multiple comparison test (SAS, version 6; SAS Institute, Cary, NC, USA). Differences were considered significant at P < 0·05.
Results
Body and liver weight
From 5 to 9 weeks (termination of the experiment) after birth, the pups that had received genistein were 15 % lighter compared with the control pups (Fig. 1(A)). There were no differences in body weight among the groups that had received genistein. Similarly, food intake did not differ among any of the groups (results not shown). However, the relative liver weight at 9 weeks was significantly lower in the genistein-treated pups than in control pups. The mid-dose genistein (Gen-M) pups had the smallest livers (Fig. 1(B)).

Fig. 1 Offspring body weights and relative liver weight in high-fat diet-treated rats. (A) Offspring body weight from week 1 to week 9 after birth. (B) Relative liver weights at week 9. Relative liver weight is the liver weight divided by the body weight (%). Values are means, with standard errors represented by vertical bars (n 6). Mean values were significantly different from those of the control: * P < 0·05, ** P < 0·01. a,b,c Mean values with unlike letters were significantly different (P < 0·05). Ctrl (![]() ), maize oil-treated group; Gen-L (
), maize oil-treated group; Gen-L (![]() ), low-dose genistein-treated group; Gen-M (
), low-dose genistein-treated group; Gen-M (![]() ), mid-dose genistein-treated group; Gen-H (
), mid-dose genistein-treated group; Gen-H (![]() ), high-dose genistein-treated group.
), high-dose genistein-treated group.
Liver histology and steatosis
In control pups, the HFD induced an accumulation of fat droplets and infiltration of inflammatory cells into the liver (Fig. 2(A)). This steatosis was characterised by mixed macrovesicular and microvesicular steatosis accompanied by inflammatory cells and ballooning degeneration of hepatocytes. Neonatal exposure to genistein prevented some of the hepatic fat accumulation and inflammation at all three doses (Fig. 2(B)–(D)). The Gen-M treatment displayed the lowest steatosis and inflammatory cell infiltration, together with no evidence of ballooning degeneration of hepatocytes. Again, the Gen-M group presented with the lowest steatosis, hepatic inflammation and ballooning degeneration of hepatocytes (Table 3).

Fig. 2 Liver histopathology of rats neonatally treated with genistein and fed a high-fat diet (HFD) for 6 weeks. (A) Rat livers treated with maize oil and fed a HFD showed mixed macrovesicular and microvesicular steatosis accompanied by a population of inflammatory cells (arrows shown) and ballooning degeneration of hepatocytes (200 × magnification). (B–D) Rat livers treated with (B) low, (C) mid and (D) high doses of genistein neonatally and fed a HFD showed lower amounts of fat accumulation and inflammation (200 × magnification).
Table 3 Summary of histopathological lesions in the livers of high-fat diet-induced genistein-treated rats
(Mean values with their standard errors, n 6)
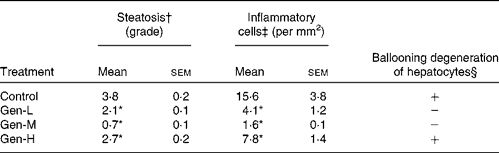
Control, maize oil-treated rats; Gen-L, low-dose (30 μg/d) genistein; Gen-M, mid-dose (300 μg/d) genistein; Gen-H, high-dose (1200 μg/d) genistein.
* Mean values were significantly different for all three treatments from those of the control (P < 0·05).
† These values are the average grade of steatosis. Grades 0–4 are average percentage of fat-accumulated hepatocytes per field at 200 × magnification under haematoxylin and eosin staining (grade: 0 = < 5%, 1 = 5–25%, 2 = 26–50%, 3 = 51–75%, 4 = >75%).
‡ Inflammation was evaluated by the number of inflammatory cells counted in ten random fields at 200 × magnification. The mean of these numbers was calculated and expressed as inflammatory cells/mm2.
§ Hepatocellular ballooning degeneration was evaluated as either negative (absent) or positive (present).
Hepatocyte apoptosis
Control pups fed the HFD showed a higher incidence of hepatocyte apoptosis compared with pups fed a low-fat diet. HFD-induced hepatocyte apoptosis was prevented by neonatal exposure to genistein (Fig. 3). The number of apoptotic cells (per 400 × field) was 4·3 (sem 0·4), 2·6 (sem 0·3), 1·5 (sem 0·2) and 3·8 (sem 0·4) for the control and low-, mid- and high-dose genistein-treated pups, respectively.

Fig. 3 Cell apoptosis in the liver of rats neonatally treated with genistein and fed a high-fat diet for 6 weeks. (A) Terminal dUTP nick end labelling (TUNEL)-positive apoptotic hepatocytes were quantified from twenty randomly selected fields at 400 × magnification. (B) A representative TUNEL staining is shown. Values are means, with standard errors represented by vertical bars (n 20). * Mean values were significantly different from those of the control (P < 0·05). Ctrl, maize oil-treated group; Gen-L, low-dose genistein-treated group; Gen-M, mid-dose genistein-treated group; Gen-H, high-dose genistein-treated group.
Plasma biochemistry
Plasma glucose concentrations did not differ between the groups, but the plasma insulin concentration was lower, and that of glucagon was higher, in the HFD-fed genistein-treated pups than in the HFD-fed control pups (Table 4). Genistein-treated pups also had less plasma alanine aminotransferase activity than the control pups (Table 4), with the Gen-M group showing the lowest (by 25·2 % compared with the control group) levels. Plasma lipid levels were also decreased (Table 4).
Table 4 Plasma glucose, insulin, glucagon, alanine aminotransferase (ALT), plasma lipid levels and hepatic lipid levels in neonatal genistein-treated rats
(Mean values with their standard errors, n 6)
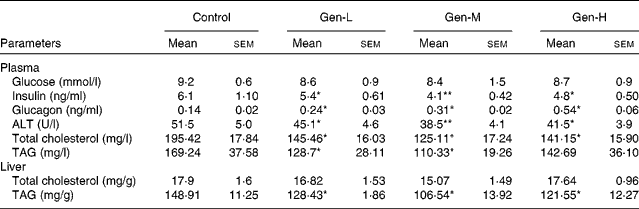
Control, maize oil-treated rats; Gen-L, low-dose (30 μg/d) genistein; Gen-M, mid-dose (300 μg/d) genistein; Gen-H, high-dose (1200 μg/d) genistein.
Mean values were significantly different for Gen-L, Gen-M and Gen-H from those of the control: * P < 0·05, ** P < 0·01.
Hepatic gene expression
The expression of the gluconeogenic genes, phosphoenolpyruvate carboxykinase and glucose-6-phosphatase, was not significantly different in any of the groups (Fig. 4(A) and (B)). FAS and SREBP-1c are key factors of lipogenesis, and their expression was lower in the genistein-treated groups than in the control high-fat group. Again, the Gen-M treatment showed the largest difference from the control group (Fig. 4(A) and (B)). The expression of PPARα, a key regulator of fatty acid oxidation, was higher in pups that received neonatal genistein than in the control pups. Similarly, the expression of carnitine palmitoyltransferase 1, a target of PPARα, was also higher in genistein-treated pups. Tissue inflammation, as characterised by the expression of TNFα, was lowest in the low and mid doses of genistein treatment, with no effect of the high-dose genistein treatment when compared with the control group. In all cases, protein expression, as determined by Western blotting, paralleled the mRNA abundance results (Fig. 4(B)), indicating functional changes in the expression of these genes.

Fig. 4 (A) Gene quantification and (B) Western blot analysis of rat liver fatty acid synthase (FAS), sterol regulatory element-binding protein-1 (SREBP-1c), PPARα, carnitine palmitoyltransferase 1 (CPT1), TNFα, phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G-6-Pase). (A) Values are means of mRNA abundance, with standard errors represented by vertical bars (n 6). a,b,c Mean values with unlike letters were significanlty different (P < 0·05). (B) Representative Western blots of protein expression in liver homogenates from all groups. Protein expression was normalised to β-actin. Values are means, with standard errors represented by vertical bars (n 6). a,b Mean values with unlike letters were significanlty different (P < 0·05). Ctrl, maize oil-treated group; Gen-L, low-dose genistein-treated group; Gen-M, mid-dose genistein-treated group; Gen-H, high-dose genistein-treated group.
Discussion
Prolonged intake of a HFD is associated with accumulation of liver TAG, inflammatory status and alterations in lipid metabolism-related gene expression that ultimately leads to the development of NASH(Reference Larter and Yeh24, Reference Tessari, Coracina and Cosma25). Soyabean genistein has been proposed as a treatment for NASH(Reference Bhathena and Velasquez26–Reference Tham, Gardner and Haskell28). The present study suggests that neonatal treatment with genistein has the potential to prevent hepatic steatosis in rats fed HFD post-weaning. Neonatal (days 1–5) exposure to genistein resulted in lower indices of NASH in rats fed a HFD for 9 weeks. After 6 weeks of HFD treatment, control rats developed symptoms typical of NASH, while rats that were neonatally exposed to genistein displayed significant amelioration of such indices, including lower inflammation and hepatic lipid accumulation, in all three treatment groups.
In previous studies, three doses of genistein have been selected(Reference Adachi, Ono and Koh19, Reference Shibayama, Fukata and Sakurai29), which span nutritionally relevant and toxicological amounts of genistein exposure in experimental animals, or in human infants consuming soya formulas(Reference Setchell, Zimmer-Nechemias and Cai30, Reference Doerge, Chang and Churchwell31). Similar doses have been used in genistein toxicology tests(Reference Adachi, Ono and Koh19). The effects of neonatal genistein treatment on the amelioration of NASH progression, lower hepatic inflammation and lipid accumulation, and post-weaning were seen in all groups, but the results were not dose-dependent. The liver of pups that received the mid dose of genistein tended to show higher resistance to the effects of high fat feeding, with both the low- and high-dose genistein-treated pups showing milder effects. Although the reason for this pattern is not clear, it is possible that the high dose of genistein could have damaged hepatocytes, as this dose has been reported to damage other cell types(Reference Adachi, Ono and Koh19).
As a phyto-oestrogen, genistein has frequently been studied in relation to the disturbance of reproductive functions(Reference Eustache, Mondon and Canivenc-Lavier32–Reference Akingbemi, Braden and Kemppainen34), and thus neonatal exposure raises some concerns regarding later sexual development(Reference Nagao, Yoshimura and Saito16, Reference Lewis, Brooks and Milburn35–Reference Klein, Wisniewski and Marson38). However, studies of neonatal genistein exposure have yielded inconsistent results(Reference Adachi, Ono and Koh19, Reference Shibayama, Fukata and Sakurai29, Reference Lewis, Brooks and Milburn35–Reference McClain, Wolz and Davidovich41). In some studies, genistein treatment in dams from gestation to lactation resulted in male offspring reproductive deficiency with decreased testosterone levels(Reference Delclos, Bucci and Lomax37, Reference Klein, Wisniewski and Marson38, Reference Wisniewski, Klein and Lakshmanan42), but this morphological deficiency has been reported to be recovered in later life in some studies, and some studies have not found any such differences(Reference Lewis, Brooks and Milburn35, Reference Takagi, Shibutani and Lee39–Reference McClain, Wolz and Davidovich41). These variable results may be due to differences in strains, genistein dosage, treatment period or route of delivery (injection or oral administration)(Reference Adachi, Ono and Koh19, Reference Shibayama, Fukata and Sakurai29, Reference Masutomi, Shibutani and Takagi36). In the present study, we did not observe any differences in testis morphology (data not shown), which suggests that neonatal pretreatment with a safe dose of genistein may be beneficial in the prevention of hepatic steatosis and NASH later in life.
Pharmacokinetic studies of orally administered genistein, in animals(Reference Chang, Churchwell and Delclos43) and human subjects(Reference King and Bursill44) have revealed that plasma genistein concentrations quickly drop to residual levels within 12–24 h. Similarly, subcutaneously injected genistein results in a fall in plasma-conjugated and aglycone genistein from 3 to 1 mmol/l in 20 h in male mice(Reference Doerge, Twaddle and Banks17). Thus, the protective effects of neonatally administered genistein, seen at 9 weeks of age, were not due to residual genistein but were due to a persistent effect in the body. This claim is further supported by the finding that there were no differences in liver morphology, or plasma aspartate aminotransferase and alanine aminotransferase activities, in any of our rats at the end of the experiment (results not shown).
Hepatic expressions of FAS, SREBP-1 and PPARα are all reported to be enhanced on high fat feeding, and this underlies both increased lipogenesis and the development of NASH. In the present study, the expression of FAS and SREBP-1 was lower in the genistein-treated animals. This may reflect a protective effect of genistein to prevent the up-regulation of these genes, thereby preventing the development of NASH. In contrast, the expression of PPARα and the down-stream carnitine palmitoyltransferase genes were higher in the genistein-treated animals, again reflective of a protective effect. The genes encoding key hepatic proteins involved in the regulation of lipid metabolism, such as SREBP-1c and PPARα, are known to be potentially programmed by dietary treatment during the gestation and neonatal period. Recent studies have revealed that feeding dams diets comprising different protein and fat can alter hepatic SREBP-1c, PPARα and downstream gene FAS expression(Reference Erhuma, Salter and Sculley45, Reference Ringseis, Gutgesell and Dathe46) in adult pups. Perinatal exposure to bisphenol A, an endocrine disruptor, has also been shown to change early adipogenesis(Reference Somm, Schwitzgebel and Toulotte47). We found similar results in the present study that neonatal exposure to genistein changed the expression of lipid metabolism genes such as hepatic SREBP-1c, PPARα and FAS when the pups were later fed a HFD as adults. These results allow us to speculate that as an oestrogenic isoflavone, genistein may play a pivotal role, in that, at a given dose, genistein acts as a weak oestrogenic agonist, but at a higher dose, it may compete with endogenous oestrogen as an antagonist.
The present study showed that a short-term exposure to genistein in the neonatal period maintained hepatic PPARα expression for up to 9 weeks of age. These results indicate a prolonged effect of neonatal exposure to genistein on lipid metabolism that persists well beyond weaning and may offer lifelong benefits in protecting against the deleterious effects of high fat consumption. Although, as noted, our use of subcutaneous injection bypasses the gastrointestinal tract, and the use of genistein does not reflect all the phytochemicals in soya, the procedure was designed to span nutritionally relevant amounts of soya consumption by infants. Although there is considerable controversy regarding the use of soya infant formulae, the present study does illustrate that neonatal genistein alone is effective in ameliorating indices of NASH development that are persistent well beyond weaning.
Acknowledgements
C. H. conducted the research. X. Q. provided the essential reagents. They both analysed the data. B. D. designed the research and wrote the manuscript. We are grateful to Dr Malcolm Watford for his help with the preparation of the manuscript. The present study was supported by the National Natural Science Foundation of China (no. 30800789). There are no conflicts of interest in the present study.










