Marine-derived n-3 fatty acids (EPA and DHA) carry a qualified health claim in the USA, and the US Food and Drug Administration has approved two highly concentrated n-3 products (Lovaza (GlaxoSmithKline) and Vascepa (Amarin Corporation)) for the treatment of severe hypertriacylglycerolaemia. In the package inserts of both Lovaza and Vascepa, the Drug Interactions section states that ‘Some studies with omega-3-acids demonstrated prolongation of bleeding time. The prolongation of bleeding time reported in these studies has not exceeded normal limits and did not produce clinically significant bleeding episodes.’
In spite of the statement that these agents ‘did not produce clinically significant bleeding episodes’, many physicians and, in particular, surgeons continue to be concerned about bleeding and recommend discontinuing the use of n-3 fatty acids before an invasive or surgical procedure. The basis of a concern for bleeding with the use of these compounds originated from studies in the late 1970s by Dyerberg & Bang( Reference Dyerberg and Bang 1 ) who reported that platelets from Greenland Inuits were markedly enriched with EPA compared with healthy Danes and that bleeding times were significantly prolonged. They went on to show that EPA was five times less active than its n-6 counterpart, arachidonic acid (AA), in stimulating platelet aggregation in vitro ( Reference Dyerberg, Bang and Stoffersen 2 ). Based on these observations, they proposed that the cardioprotective effects of n-3 fatty acids in the Inuit population were mediated by the inhibition of platelet function by EPA. (Interestingly, a study in ninety river- or coastal-dwelling Alaskan Eskimos did not find increased bleeding times despite markedly elevated plasma levels of EPA and DHA( Reference Parkinson, Cruz and Heyward 3 ).) Nevertheless, multiple studies have confirmed that platelet function is affected by n-3 fatty acids( Reference Knapp 4 ). The practical question is not whether n-3 fatty acids can affect haemostatic pathways – they clearly can; it is whether they, alone or in combination with either anti-platelet drugs or anticoagulant therapies, increase the risk of clinically significant bleeding.
In 2007, Harris( Reference Harris 5 ) reviewed nineteen studies investigating the use of high-dose n-3 fatty acids (1–6 g/d) and the associated risk of bleeding in patients undergoing major vascular interventions. None of the studies has revealed evidence of clinically significant bleeding. He concluded that n-3 fatty acids ‘do not increase the risk of adverse bleeding episodes’. Since then, several studies have been published across many medical disciplines that further support the view that n-3 fatty acids do not increase bleeding complications. Some of these studies have directly tested this question, whereas others have reported the results of clinical trials with fish oils testing other hypotheses and, in the Adverse Events section, commented on bleeding. The purpose of the present review is twofold: first, to review many of these clinical studies and, second, to provide an update on the mechanisms by which n-3 fatty acids influence platelet function and coagulation.
n-3 Fatty acids and bleeding: direct evidence
Randomised controlled studies
One of the largest studies to directly test the effects of high-dose fish oil on bleeding in patients with coronary artery disease was performed by Eritsland et al. ( Reference Eritsland, Arnesen and Seljeflot 6 ). Patients undergoing percutaneous coronary intervention (PCI) (n 610) were randomised to receive n-3 acid ethyl esters (Omacor; Pronova Biocare) at the Food and Drug Administration-approved dose of 4 g/d (3·4 g EPA+DHA) or placebo, and either aspirin (300 mg/d) or warfarin. After 9 months of treatment, haemostatic tests were performed. The authors concluded that ‘no significant long-term influence by omega-3 fatty acids on the measured haemostatic variables was seen. Furthermore, no excess of bleeding episodes could be attributed to the use of fish oil concentrate, even when given in addition to aspirin or warfarin.’
In another randomised controlled trial, Gajos et al. ( Reference Gajos, Rostoff and Undas 7 ) studied the effects of n-3 acid ethyl esters v. placebo on platelet function in patients on dual anti-platelet therapy (aspirin 75 mg/d and clopidogrel 600 mg loading dose before PCI, followed by 75 mg/d) undergoing elective PCI. Patients were randomised to receive 1 g Omacor (1 g/d; n 33) or placebo (n 30) for 1 month. Platelet function was measured by light transmission aggregometry and by the P2Y12 reactivity index (the phosphorylation status of vasodilator-stimulated phosphoprotein) before and after 3–5 and 30 d. The treatment with n-3 fatty acids was associated with decreased platelet aggregation; however, there was no evidence of increased bleeding compared with the control group. The authors concluded that the addition of n-3 fatty acids to the standard dual anti-platelet therapy significantly potentiates platelet response to the anti-platelet therapy after PCI and does so without increasing the risk of bleeding. In a follow-up study, the same authors demonstrated that n-3 fatty acids enhanced clot lysis susceptibility and reduced thrombin formation, again, without any evidence of an increased risk of clinically significant bleeding( Reference Gajos, Zalewski and Rostoff 8 ). In a second follow-up study( Reference Gajos, Zalewski and Nessler 9 ), they demonstrated that in patients with loss-of-function mutation in CYP2C19 (which renders patients resistant to the anti-platelet effects of clopidogrel), the co-administration of n-3 fatty acids overcame the resistance, again with no increased the risk of adverse bleeding events.
Iacoviello et al. ( Reference Iacoviello, Amore and De 10 ) investigated the effects of n-3 fatty acids and aspirin, separately and in combination, on fibrinolytic response (process of dissolution of fibrin in blood clots) to venous occlusion in six healthy subjects. Co-administration, compared with either monotherapy, resulted in a decrease in fibrinolytic activity. This small study suggested, if anything, the possibility of a decreased risk of bleeding (i.e. inhibited fibrinolysis) in those given fish oil and aspirin.
The effects of fish oil alone and in combination with low-molecular-weight heparin on the incidence of restenosis after PCI were examined by Cairns et al. ( Reference Cairns, Gill and Morton 11 ). There were 814 patients who were initially randomised to receive fish oils (5·4 g EPA+DHA/d) or placebo for a median of 6 d before PCI and continued for 18 weeks. Of these patients, 653 continued to receive fish oils (n 325) or placebo (n 328) and were then sub-randomised to receive low-molecular-weight heparin (n 325) or control (n 328). Although the authors found no evidence for a clinically important reduction of restenosis by either n-3 fatty acids or low-molecular-weight heparin, for present purposes, they reported significantly less bleeding complications in the fish oil group compared with the two low-molecular-weight heparin groups, and no difference from the untreated (with either agent) controls.
The Japan EPA Lipid Intervention Study (JELIS) was a randomised controlled trial that tested the effects of EPA (1·8 g/d) in addition to statin therapy in high-risk patients for CVD( Reference Yokoyama, Origasa and Matsuzaki 12 ). Patients (n 18 645) with multiple cardiac risk factors (36 % hypertensive, 15 % diabetic and 20 % coronary artery disease) were included. They were randomised to receive statin therapy (pravastatin 10 mg/d or simvastatin 5 mg/d; n 9319, control group) or statin therapy and EPA (1·8 g/d; n 9326, treatment group) and followed for a mean of 5 years. In a subgroup analysis of the JELIS, the effects of EPA on stroke incidence were examined( Reference Tanaka, Ishikawa and Yokoyama 13 ). In the primary prevention subgroup (i.e. those with no history of stroke), there was no difference in stroke incidence (control v. EPA: 1·3 v. 1·5 %). However, in the secondary prevention subgroup (control v. EPA: n 457 v. 485), stroke incidence was reduced from 10·5 to 6·8 %. This constituted a 20 % relative risk reduction for recurrent stroke (P< 0·05). Importantly, there was no difference in cerebral or subarachnoid haemorrhage rates (0·4 v. 0·5 % of the whole cohort, respectively), despite the concomitant use of anti-platelet agents in both cohorts (40 % of the secondary prevention group and 13 % of the primary prevention group). This finding on both the reduced risk for recurrent stroke and the non-effect on haemorrhage rates in the EPA group argues against the risk of clinically significant bleeding.
A dose–response study covering a range of 0·84–6·72 g EPA+DHA given daily for 6 weeks to three groups of ten subjects otherwise randomised to receive placebo, aspirin (325 mg) or aspirin plus clopidogrel (75 mg) was reported by Cohen et al. ( Reference Cohen, Rossi and Garbarino 14 ). Their primary interest was in the effects on platelet surface charge (discussed in the Mechanisms section below), but for present purposes, no bleeding episodes were observed.
Nilsen et al. ( Reference Nilsen, Dalaker and Nordoy 15 ) investigated the influence of n-3 fatty acids on thrombosis and haemostasis in twenty patients with stable angina undergoing coronary artery bypass graft. Patients were randomised to receive either Omacor (4·85 g EPA+DHA/d; n 10) or placebo (6 g maize oil/d; n 10) for 6 months. Clinical recordings and blood sampling were obtained at different times (pre-intervention, pre-operatively and post-operatively) to measure bleeding time, total platelet count, mean platelet volume, mean platelet aggregation, release of thromboxane B2 and coagulation parameters. Post-operative bleeding, blood transfusion rates, bleeding time, platelet number, mean platelet volume and platelet aggregation did not differ significantly between the two groups. It should be noted that higher rates tended to be in the n-3 fatty acid group for several of these end-points, and the failure of the differences to be ‘significant’ were at least partly due to the very small sample size.
Epidemiological studies
Watson et al. ( Reference Watson, Joy and Nkonde 16 ) retrospectively reviewed bleeding complications in patients with coronary artery disease on dual anti-platelet therapy (aspirin and clopidogrel; n 182) and n-3 fatty acids compared with sex-matched controls only on anti-platelet therapy (n 182). After a mean follow-up of 33 months, one patient in the n-3 group had a major bleeding episode (rectal bleeding from rectal carcinoma, requiring transfusion) compared with none in the control group. In addition, four patients in the n-3 group experienced minor bleeding compared with seven patients in the control group. The authors concluded that high-dose n-3 fatty acids (approximately 3 g EPA+DHA/d) in combination with anti-platelet agents do not increase the risk of clinically significant bleeding.
In a case–control study from Korea, Park et al. ( Reference Park, Park and Yi 17 ) investigated the relationship between erythrocyte EPA+DHA (the omega-3 index) and either haemorrhagic or ischaemic stroke. The authors recruited cases which consisted of patients with previously diagnosed ischaemic stroke (n 34) or haemorrhagic stroke (n 34), and compared them with controls (n 40). Both the omega-3 index (P= 0·003) and erythrocyte DHA levels (P= 0·011) were significantly lower in both haemorrhagic and ischaemic stroke patients compared with the controls. EPA levels were significantly lower in patients with ischaemic stroke compared with the controls. Thus, lower n-3 fatty acid levels were observed in patients with a history of stroke.
Finally, in another cross-sectional study, Salisbury et al. ( Reference Salisbury, Harris and Amin 18 ) examined the relationship between the omega-3 index( Reference Harris and von Schacky 19 ) and the risk of bleeding in patients admitted with an acute myocardial infarction (n 1523). The authors found no relationships between the omega-3 index and the risk of in-hospital bleeding. In addition, bleeding complications were not different between patients who reported taking fish oil supplements and those who did not. The authors concluded that concerns about bleeding should not preclude the use of n-3 supplements or increased fish consumption when clinically indicated.
Indirect evidence for the safety of n-3 fatty acids by medical discipline
Neurology
Yoneda et al. ( Reference Yoneda, Shirao and Kurokawa 20 ) investigated whether EPA inhibited cerebral vasospasm in patients after subarachnoid haemorrhage. After craniotomy and clipping, patients were allocated to receive 1·8 g EPA/d (n 78) or no EPA (n 28) and followed from day 4 (study initiation) throughout their hospital stay. The authors found that EPA significantly reduced the occurrence of both symptomatic cerebral vasospasm (14 % EPA group v. 36 % control group, P= 0·019) and cerebral infarction (4 % EPA group v. 29 % control group, P= 0·001). Patients in the EPA group were more likely to have a ‘good’ clinical course rating than those in the control group (85 v. 64 %, respectively, P= 0·022), with no deaths reported in the EPA group compared with three (11 %) deaths in the control group (P= 0·020). The authors concluded that not only were there no adverse bleeding effects associated with EPA administration, but it appeared to inhibit symptomatic cerebral vasospasm and cerebral infarction and improve clinical prognosis after subarachnoid haemorrhage.
Nephrology
The effects of fish oil on synthetic haemodialysis graft patency in stage 5 chronic kidney disease patients (n 201) were examined by Lok et al. ( Reference Lok, Moist and Hemmelgarn 21 ). Patients were randomised to receive four 1 g capsules of fish oil (2·6 g EPA+DHA/d; treatment group, n 101) or placebo (maize oil; control group, n 100) on day 7 after graft creation and were followed for 12 months. The prevalence of anticoagulant use was 55 % aspirin, 24 % warfarin and 12 % clopidogrel. The authors found better graft patency and decreased rate of thrombosis in the treatment group compared with the control group. Importantly, there was no difference in bleeding complications (nine in the treatment group and eight in the control group) between the two groups despite the concomitant use of both anticoagulation and anti-platelet therapies.
IgA nephropathy is the most common type of glomerulonephritis in adults. Ferraro et al. ( Reference Ferraro, Ferraccioli and Gambaro 22 ) compared 6 months of standard treatment for IgA nephropathy (renin–angiotensin system blockers) without (n 15, control) and with (n 15) 3 g n-3 fatty acids/d. The primary end-point was the percentage reduction of proteinuria from baseline. For the purpose of the present review, secondary end-points included the change in erythrocyturia from baseline. After 6 months of treatment, the percentage reduction of proteinuria was 73 % in the treatment group and 11 % in the control group (P< 0·001). Erythrocyturia was significantly lower in the treatment group compared with the control group (P= 0·031). Thus, there was less renal bleeding with n-3 fatty acid treatment.
Critical care
Depending on the underlying disease and specific haemostatic abnormality, critically ill patients are at a high risk of developing bleeding complications. The utility and safety of n-3 fatty acids in the intensive care unit continues to be an area of active research. In their recent review, Martin & Stapleton( Reference Martin and Stapleton 23 ) summarised three enteral (providing between 7 and 10 g EPA+DHA/d) and five parenteral feeding studies (exact doses impossible to determine) using fish oils in patients with sepsis, acute lung injury, acute respiratory distress syndrome and acute pancreatitis. Mortality was significantly reduced (P< 0·05) in patients receiving >0·1 g fish oil/kg per d. Favourable outcomes observed were reduced intensive care unit length of stay, ventilator time, new organ failure, 28 d mortality and inflammatory markers, as well as improved oxygenation. Although not directly commenting on bleeding, the authors concluded that ‘clinical use of fish oil in critically ill patients appears to be safe’.
In a similar study, Friesecke et al. ( Reference Friesecke, Lotze and Kohler 24 ) evaluated parenteral nutrition with fish oil in critically ill patients. The study was a single-centre, placebo-controlled, double-blind, randomised clinical trial. Intensive care unit patients (n 166) requiring parenteral nutrition for >6 d were randomised to receive either a 1:1 mixture of medium-chain TAG and long-chain TAG with an n-3:n-6 ratio of 1:7, or the same medium-chain TAG/long-chain TAG emulsion supplemented with fish oil (resulting in an n-3:n-6 ratio of 1:2). There were no significant differences detected between the groups with regard to the primary end-point or bleeding complications. Bleeding frequency (thirteen in the treatment group and eighteen in the control group) and the amount of erythrocytes transfused did not differ between the groups. Thus, parenteral nutrition with fish oil was safe.
Surgery
Bleeding complications are always major concerns with any surgical procedure. The potential risk of bleeding commonly associated with the use of fish oils explains why many surgeons recommend discontinuing these agents in patients undergoing elective procedures. Although we are aware of no studies that have been specifically designed to test the effects of pre-surgical administration of n-3 fatty acids on the risk of bleeding, several studies have tested the effects of fish oils in the surgical setting on other end-points and then commented on bleeding as a safety outcome.
Kepler et al. ( Reference Kepler, Huang and Meredith 25 ) and Meredith et al. ( Reference Meredith, Kepler and Hirsch 26 ) performed studies examining the relationships between n-3 fatty acid pre-treatment and bleeding in the setting of lumbar decompression surgery. The objective of both retrospective case–control studies was to assess the pre-operative use of fish supplements and whether these compounds increase intra-operative blood loss or post-operative bleeding complications. In the first study, bleeding outcomes in nineteen patients who had taken fish oil supplements for at least 14 d before surgery were compared with those who took n-3 fatty acid supplements, stopping an average of 2·3 d before the scheduled surgery( Reference Kepler, Huang and Meredith 25 ). The estimated blood loss was not different between the control (n 79) and fish oil (n 16) groups (154 v. 138 ml, P= 0·53). There were two bleeding complications in the former group and none in the latter group. In the second study, bleeding in twenty-eight patients who had taken n-3 fatty acid supplements (and who stopped on average 5·2 d before surgery) were compared with bleeding complications in fifty-six matched controls who had not taken n-3 fatty acid supplements( Reference Meredith, Kepler and Hirsch 26 ). The mean estimated blood loss tended to be higher in the control group (n 56) than in the treatment group (771 v. 697 ml, P= 0·36) as did the transfused volume of Cell Saver (321 v. 282 ml, P= 0·30). In both studies, there was no increase in intra-operative or post-operative bleeding complications. Other related outcomes such as the decline in post-operative Hb levels, transfusion requirements and surgical drain output were not different between the treatment and control groups. The authors concluded that pre-operative use of n-3 fatty acids did not increase bleeding complications during spinal decompression surgery. As noted, in both studies, patients were asked to discontinue n-3 fatty acid supplements 2–5 d before surgery. Because of the very long half-life of n-3 fatty acids in tissues (e.g. 28 d for EPA in erythrocyte membranes( Reference Katan, Deslypere and van Birgelen 27 )), discontinuations of these supplements for this short duration would have had virtually no effect on tissue n-3 fatty acid levels. In other words, the same outcomes would have been expected had they not stopped fish oil pre-operatively.
Another surgical study to further support the safety of n-3 fatty acid supplementation in nutrition was published by Heller et al. ( Reference Heller, Fischer and Rossel 28 ). This was a prospective, randomised, double-blind clinical trial in which forty-four patients undergoing elective major abdominal surgery were randomised to receive total parenteral nutrition containing either soyabean oil (n 20) or a combination of n-3 fatty acids and soyabean oil (n 24) post-operatively. (Again, actual doses of EPA+DHA are not calculable from the information presented.) Blood samples were taken at different times (pre-operatively, intra-operatively and 5 d post-operatively) to measure both platelet function and coagulation parameters (prothrombin time, activated partial thromboplastin time, factor VIIa, factor XIIa, fibrinogen and antithrombin III). After 6 d of treatment, measures of platelet function and coagulation parameters were similar in both groups and bleeding complications were comparable. The authors concluded that total parenteral nutrition infusion with fish oils was safe.
Cardiology
The Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico-Heart Failure (GISSI-HF) trial provides another example of the safety of n-3 fatty acids in patients at increased risk of bleeding( Reference GISSI-HF, Tavazzi and Maggioni 29 ). This randomised trial included over 6900 patients with heart failure who were treated with either placebo or 1 g n-3 acid ethyl esters for a median of 3·9 years. Most of the patients took either anti-platelet (58 %) and/or anticoagulant drugs (29 %). Despite this, bleeding events were not different between the groups.
In the aforementioned JELIS study( Reference Yokoyama, Origasa and Matsuzaki 12 ), the adverse event ‘haemorrhage’ was tracked and compared between groups. It was defined as cerebral, fundal, epistaxis or subcutaneous bleeding. There was a significantly higher rate of haemorrhage in the EPA group (0·6 v. 1·1 %, P= 0·0006), but no indication was given as to how frequent each component was observed.
Post-operative atrial fibrillation is a common complication of cardiac surgery. There have been inconclusive reports of whether n-3 fatty acids reduce post-operative atrial fibrillation. To further elucidate on this topic, Calo et al. ( Reference Calo, Bianconi and Colivicchi 30 ) investigated this hypothesis of n-3 fatty acids and their relationship to atrial fibrillation in patients undergoing coronary artery bypass graft. This was a randomised controlled study where patients (n 160) were prospectively randomised to receive n-3 fatty acids (n 81, 1·7 g EPA+DHA/d) or placebo (n 79) for at least 5 d before elective coronary artery bypass graft and throughout their hospital course. n-3 Fatty acid treatment was associated with a reduction in post-operative atrial fibrillation (twelve patients in the treatment group v. twenty-seven patients in the control group, P= 0·013). For the purpose of the present review, there was only one clinically significant bleeding episode in each group. Additionally, non-fatal post-operative complications and mortality rates were similar between the two groups (1·3 % in the treatment group v. 2·5 % in the control group).
Mozaffarian et al. ( Reference Mozaffarian, Marchioli and Macchia 31 ) tested the same hypothesis of pre-operative use of n-3 fatty acids and their prevention of post-operative atrial fibrillation. Omega-3 Fatty Acids for Prevention of Post-operative Atrial Fibrillation (OPERA) was an international, multi-centre, double-blind, placebo-controlled, randomised clinical trial. Patients (n 1516) scheduled for cardiac surgery in twenty-eight international centres were randomised to receive 10 g EPA+DHA (n 758) or placebo (n 758), 3–5 d (or 8 g over 2 d) followed post-operatively by 2 g/d until discharge or post-operative day 10. The primary end-point was post-operative atrial fibrillation lasting longer than 30 s, and bleeding episodes were a secondary end-point. The treatment group required less blood transfusion (1·6 v. 1·9 units, P< 0·001). There were non-significant trends favouring the n-3 group for fatal bleeds (0 v. 3 patients, P= 0·08), surgical bleeding episodes as defined by the International Society on Thrombosis and Hemostasis (19 v. 32 % patients, P= 0·06), bleeding as defined by the Thrombolysis in Myocardial Infarction studies (21 v. 26 patients, P= 0·46) and bleeding requiring re-exploration or surgery (20 v. 25 patients, P= 0·45).
Krysiak & Okopien( Reference Krysiak and Okopien 32 ) studied the haemostatic effects of n-3 fatty acids and bezafibrate in patients with hypertriacylglycerolaemia. Patients were randomised to receive either n-3 fatty acids (4 g/d, n 29) or placebo (n 28) for 3 months. The authors measured and compared different haemostatic variables including fibrinogen, von Willebrand factor, plasminogen activator inhibitor-1 plasma levels and factor VII coagulant activity in both groups for 30 and 90 d. They found that not only did the n-3 fatty acids–fibrate combination reduced TAG levels compared with the placebo group, but also diminished procoagulant activity by reducing serum levels of plasminogen activator inhibitor-1 and stimulating fibrinolysis. Additionally, all haemostatic parameters measured were reduced in the treatment group. Although the authors did not address the safety of n-3 fatty acids, they clearly demonstrated the beneficial effects on bleeding parameters in association with these agents.
Haematology
In a study from Sudan, Daak et al. ( Reference Daak, Ghebremeskel and Hassan 33 ) investigated the therapeutic potential of n-3 fatty acids in patients with homozygous sickle-cell disease. In this study, patients (n 140) were randomised to receive either n-3 fatty acids (about 25 mg EPA+DHA/kg body weight or 1·8 g/d for a person weighing 70 kg; n 70) or placebo (n 70) for 12 months. Efficacy and safety outcomes, including blood transfusion rates, were compared between the two groups. The treatment group had no vaso-occlusive events and the control group had one. Severe anaemia (3·2 v. 16·4 %, P= 0·05) and need for blood transfusions (4·5 v. 16·4 %, P= 0·05) were less in the treated group. The authors concluded that n-3 fatty acids are a safe, effective and affordable therapy for sickle-cell anaemia patients and clearly did not cause bleeding.
Obstetrics
There has long been an interest in the effects of fish oil supplementation on pregnancy outcomes, one being the duration of gestation, as observational studies in the Faroe Islands suggested that a high-fish diet may prolong gestation( Reference Olsen and Joensen 34 ). Olsen et al. ( Reference Olsen, Secher and Tabor 35 ) reported the effects of EPA+DHA supplementation (between 2·2 and 5 g/d v. placebo) in six trials including 1647 women. Bleeding was carefully monitored. Overall, fish oil supplementation reduced the risk of pre-term birth but did not significantly prolong pregnancy. Importantly for this discussion, there were no significant differences between the groups in relation to the incidence of vaginal bleeding, blood loss at delivery or the lowest maternal Hb levels. Thus, in this setting as well, no increased risk of bleeding was observed.
Dentistry
Bleeding on probing, also known as gingival bleeding, is routinely used to assess gum health. It refers to bleeding induced by gentle probing of the tissue at the depth of the gingival sulcus, or the interface between the gingiva and the tooth. Gingival bleeding is a sign of inflammation and indicates some destruction and erosion to the lining of the sulcus or the ulceration of sulcular epithelium. Gingivitis and periodontitis are the main causes of gingival bleeding. Since n-3 fatty acids have been reported to have anti-inflammatory effects, Elkhouli( Reference Elkhouli 36 ) conducted a randomised, double-blind, placebo-controlled study to test the efficacy of n-3 fatty acids (1·35 g/d) and low-dose aspirin (75 mg/d) in chronic periodontitis. Patients were randomised to receive either decalcified freeze-dried bone allograft plus n-3 and aspirin (n 20) or plus placebo (n 20). Clinical parameters including gingival bleeding index were monitored and compared between the two groups before and after 6 months of treatment. The authors found a reduced gingival bleeding index and inflammation in the treatment group compared with the control group (P< 0·05). This is further evidence that, even when combined with other anti-platelet agents, n-3 fatty acids do not increase the risk of bleeding and, in this case, can even reduce it.
Conclusion for clinical studies
Most of the above-reviewed studies found no increase in the risk of clinically significant bleeding, the only possible exceptions being the very small study of Nilsen et al. ( Reference Nilsen, Dalaker and Nordoy 15 ) and the JELIS study( Reference Yokoyama, Origasa and Matsuzaki 12 ). Indeed, Kepler et al. ( Reference Kepler, Huang and Meredith 25 ) stated: ‘Although (past) conservative recommendations based on likely clinical effect are prudent when no clinical information is available, our developing understanding of the risk associated with n-3 fatty acids suggests no reason these supplements should be stopped before surgery nor why surgery should be cancelled should a patient fail to stop taking them.’
n-3 Fatty acids and platelet biology
The mechanisms by which nutritional levels of n-3 fatty acids are currently believed to reduce the risk of CVD involve anti-arrhythmic effects and blood pressure reduction, with TAG-lowering and anti-platelet effects only playing a possible role in supraphysiological intakes( Reference Mozaffarian and Wu 37 ). This derives in part from the fact that benefits from n-3 fatty acids have been observed in epidemiological (non-supplementation) studies( Reference He, Song and Daviglus 38 ) and in relatively low-dose randomised controlled trial( Reference GISSI-HF, Tavazzi and Maggioni 29 , Reference Marchioli, Barzi and Bomba 39 ), but the effects on platelet function were believed to only occur with high doses. Fish oils do clearly affect platelet aggregation( Reference Gao, Cao and Mao 40 ), but even at high doses, the effects on bleeding times were only inconsistently seen( Reference Nilsen, Dalaker and Nordoy 15 , Reference Thorngren and Gustafson 41 – Reference Harris, Silveira and Dujovne 45 ). This heterogeneity in response again suggested that the effects of fish oil on platelet function were minor and probably irrelevant at nutritional doses. There was, however, a report in 1984 that only 150 mg supplemental EPA significantly reduced ex vivo platelet aggregation( Reference Driss, Vericel and Lagarde 46 ). This study went largely unnoticed because the use of multi-gram, ‘Eskimo’ doses of EPA+DHA was in vogue at the time. Together with the more recent discovery of novel metabolites of EPA and DHA (resolvins, epoxides, poxytrins, etc.), some of which are able to inhibit platelet aggregation at low micromolar concentrations( Reference Dona, Fredman and Schwab 47 – Reference Chen, Vericel and Lagarde 49 ), the 1984 report that even ‘nutritional’ (non-supplemental) levels of n-3 fatty acids could potentially affect platelet function becomes more credible. Precisely how they do so is only beginning to be understood.
The extent to which their relatively subtle effects on platelet function plays a role in their reported health benefits is a matter of debate; however, the fact that they alter important platelet signalling pathways and may thereby mute haemostatic processes is clear. In preparing this manuscript, we were unable to find a recent review on the effects of EPA and DHA on platelet biochemistry and haemostasis (with the notable exception of that from Lagarde et al. ( Reference Lagarde, Calzada and Guichardant 50 )). What follows is intended to help fill that gap.
Platelet aggregation assays
It is important to appreciate from the outset that much of our understanding of the effects of fish oils on platelet function has been based on ex vivo platelet aggregation studies. This early work was reviewed by Kinsella et al. ( Reference Kinsella, Lokesh and Stone 51 ) and was confirmed and extended in numerous small-scale clinical studies in healthy and diseased subjects given fish oil that showed reductions in platelet aggregation in response to agonists such as collagen, ADP and adrenalin( Reference Thorngren and Gustafson 41 , Reference Lorenz, Spengler and Fischer 42 , Reference Driss, Vericel and Lagarde 46 , Reference Siess, Roth and Scherer 52 , Reference Bradlow, Chetty and van der Westhuyzen 53 ). These types of studies provide a limited and imperfect picture of the in vivo effects of fish oils on platelet biology for several reasons. First, in aggregation studies, platelet-rich plasma is typically used. The removal of erythrocytes constitutes the first step away from physiological relevance since, as normal components of whole blood, erythrocytes themselves can modulate platelet function( Reference Santos, Valles and Aznar 54 ). Second, care must be taken to keep the platelet quiescent during the preparation of the plasma, otherwise ex vivo activation can occur. Platelet aggregation is then measured in response to specific agonists typically added one at a time, which again is unlike the in vivo situation where multiple changes in the levels of many agonists/antagonists occur essentially simultaneously. After agonist addition, the rate and extent of the formation of platelet aggregates is monitored by changes in the turbidity of the plasma( Reference Born 55 ). Accordingly, ex vivo aggregation assays, even when done in whole blood by impedance aggregometry( Reference Dyszkiewicz-Korpanty, Frenkel and Sarode 56 ) (which eliminates the erythrocyte concern) have always been imperfect predictors of in vivo haemostasis. Glanzmann's thrombasthenia offers a clinical example. These patients (regardless of genotypic variety) show a near-total lack of the platelet fibrinogen receptor GPIIb/IIIa (integrin αIIbβ3)( Reference George, Caen and Nurden 57 ), and consequently display an almost total abrogation of ex vivo aggregation in response to low-dose agonists( Reference Burgess-Wilson, Cockbill and Johnston 58 ). Similarly, monoclonal antibody-mediated blockage of GPIIb/IIIa also produces a near-total loss of platelet aggregation( Reference Bennett, Hoxie and Leitman 59 ). However clinically, the bleeding phenotype of Glanzmann's patients is quite variable, and can change over time within the same person( Reference George, Caen and Nurden 57 ). As such, it is difficult, if not impossible, to draw clinical conclusions based on the effects/non-effects of an intervention (here, n-3 fatty acids) on platelet aggregation tests. In other words, even if in some studies n-3 fatty acids had no effect on platelet aggregation tests, this would not necessarily mean that they had no impact on haemostasis. With this caveat in mind, we will briefly consider the commonly held view that n-3 fatty acids affect platelet function only via the cyclo-oxygenase (COX) pathway, and then we will discuss other possible mechanisms (beyond platelet–platelet aggregation) by which n-3 fatty acids could affect thrombosis.
Cyclo-oxygenase inhibition
As noted in the Introduction, reports linking platelet function with these n-3 fatty acids can be traced back to the late 1970s, with the reports of Dyerberg & Bang( Reference Dyerberg and Bang 1 ) that Greenland Eskimos had prolonged bleeding times( Reference Dyerberg, Bang and Stoffersen 2 ) and that exogenous EPA inhibited ex vivo platelet aggregation. The biochemical basis of this effect was proposed to be the ability of EPA to replace AA as a substrate for PG synthesis via the COX pathway( Reference Needleman, Whitaker and Wyche 60 ) (Fig. 1). Similar EPA- and DHA-mediated reductions were also noted in platelet thromboxane production( Reference Lorenz, Spengler and Fischer 42 , Reference Siess, Roth and Scherer 52 , Reference Brox, Killie and Osterud 61 , Reference von Schacky, Fischer and Weber 62 ) and the competition for thromboxane receptor occupancy( Reference Swann, Venton and Le Breton 63 , Reference Swann, Parent and Croset 64 ). A comprehensive series of experiments comparing EPA with AA as a substrate in the COX system was reported by Wada et al. ( Reference Wada, DeLong and Hong 65 ) (Fig. 2). Relative to AA, EPA was found to be equally susceptible to hydrolytic release by phospholipase A2, but it was a much poorer substrate for COX-1 (10 % as good as AA) and COX-2 (about 30 % as good as AA). Once converted to PGH2 (or H3 from EPA), the latter was again a poorer substrate for conversion to the D- and E-series PG, but equally good as AA for the synthesis of prostacyclin and thromboxane. Further down the pathway, the AA-derived metabolites were more effective activators of the respective receptors than were the EPA-derived metabolites. Overall, these experiments suggest that even if EPA and AA were present in platelet membranes at equimolar concentrations (which they are not, even on high-dose fish oil where AA is about 10 × EPA( Reference Larson, Ashmore and Harris 66 )), EPA would have very little impact on the COX pathway, at least in part because of its inability to serve as a competent substrate for COX.
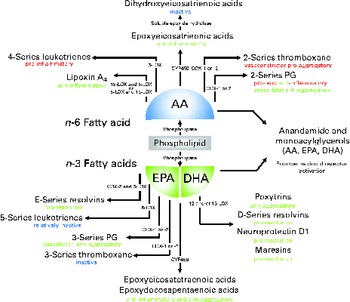
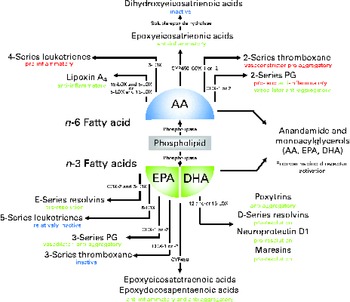
Fig. 1 Overview of lipid mediators derived from the n-6 fatty acid arachidonic acid (AA) and the two marine n-3 fatty acids EPA and DHA. Adapted and updated from de Roos et al. ( Reference de Roos, Mavrommatis and Brouwer 87 ). Reprinted with permission. LOX, lipoxygenase; COX, cyclo-oxygenase; CYP, cytochrome P-450.
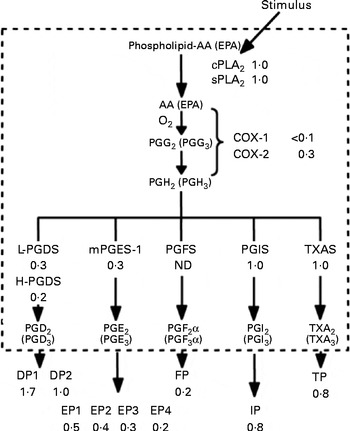
Fig. 2 Effectiveness of arachidonic acid (AA)-derived v. EPA-derived substrates and products with enzymes and receptors of the PG pathway. DP, EP, FP, IP and TP are receptors for PGD, PGE, PGF, PGI and TXA (thromboxane A)/PGH, respectively. cPLA2, cytosolic phospholipid A2; sPLA2, secreted phospholipase A2; COX, cyclo-oxygenase; L-PGDS, lipocalin PGD synthase; H-PGDS, haematopoietic PGD synthase; mPGES1, microsomal PGE synthase-1; ND, not determined; TXAS, thromboxane A synthase. Reprinted from Wada et al. ( Reference Wada, DeLong and Hong 65 ).
Fish oil can lower platelet AA levels (from about 26 to 24 % of total fatty acids( Reference Larson, Ashmore and Harris 66 )) and possibly thereby slightly diminish the COX-derived signalling cascade; however, there is little reason to believe that n-3 fatty acids affect platelet biochemistry primarily via the effects on the COX pathway. A caveat is appropriate here because, while the conversion of EPA into its metabolites via platelet enzymes was not as efficient as the conversion of AA to its metabolites in Wada et al. ( Reference Wada, DeLong and Hong 65 ), others who examined these interactions over 30 years ago reached somewhat different conclusions( Reference Needleman, Raz and Minkes 67 – Reference Boukhchache and Lagarde 69 ), a detailed discussion of which is beyond the scope of this paper. The occupation of platelet enzyme active sites by n-3 fatty acids and the subsequent prevention of AA binding could contribute to some platelet inhibitory effects. Furthermore, the simultaneous in vivo presence of multiple fatty acids, each of which may alter the metabolism of the other, complicates the attempts to define the pro- or anti-platelet effects of each fatty acid in isolation( Reference Morita, Takahashi and Saito 70 ). As is evident in Fig. 1, these fatty acids serve as substrates for several enzymes besides COX that produce active metabolites that could affect platelet function. The closing words of Wada et al. ( Reference Wada, DeLong and Hong 65 ) are relevant in this regard: ‘All of these values (summarized above) were obtained under optimal in vitro conditions. Obviously, what occurs in vivo cannot yet be predicted with any certainty because the ratios of enzymes and receptors to substrates and agonists involved in PG signaling may well be different in vivo. There may also be other eicosanoid mediators, including those derived from n-3 fatty acids that are importantly involved in PG signaling.’
Inhibition of platelet signalling and function
Platelet signalling and physiology are influenced by the incorporation of EPA and DHA into cell membrane phospholipids, and result in the effects on platelet function beyond platelet–platelet interactions (i.e. aggregation). A summary of these other mechanisms is given in Fig. 3. Increased platelet EPA and DHA inhibit intracellular signalling events following platelet ligation to exposed matrices such as collagen. This has been noted in reductions of collagen receptor-linked phosphotyrosine modification( Reference Larson, Shearer and Ashmore 71 ) and mitogen-activated protein kinase signalling( Reference Guillot, Caillet and Laville 72 ). An n-3 fatty acid-induced increase in platelet antioxidant formation has also been observed( Reference Guillot, Caillet and Laville 72 ). These alterations to intracellular proteins may give rise to other cellular effects such as an impairment of platelet adhesion to collagen and fibrinogen( Reference Li and Steiner 73 , Reference Li and Steiner 74 ), diminished granule secretion and lowered exposure of phosphatidylserine on the outer membrane( Reference Larson, Shearer and Ashmore 71 ). n-3 Fatty acids also increased the negative surface charge on platelet membranes( Reference Cohen, Rossi and Garbarino 14 ).
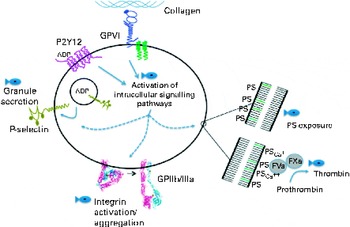
Fig. 3 Platelets respond to vascular damage via collagen ligation to the glycoprotein (GP) VI receptor. Secondary signalling by ADP released from dense granules and binding to the P2Y12 receptor reinforces this initial activation signal. Both receptors activate signalling protein assembly and modulation, and subsequently these signals alter platelet physiology. These alterations include secretion of granules that contain adhesion molecules such as P-selectin (CD62P), activation of the integrin GPIIb/IIIa that facilitates platelet aggregation, and exposure of phosphatidylserine (PS) on the outer leaflet of the platelet membrane. PS allows the assembly of a complex comprising Ca++, factor Xa (FXa), factor Va (FVa) and prothombin. This complex is necessary for the conversion of prothrombin into active thrombin, the enzyme that converts fibrinogen into insoluble fibrin. The fish symbol denotes places where EPA and DHA inhibit signal transduction pathways. The crystal structure of GPIIb/IIIa is derived from Xiong et al. ( Reference Xiong, Stehle and Diefenbach 88 ).
Inhibition of the platelet–coagulation–inflammation interface
Platelets not only interact with each other but with other cell types as well. For example, recent studies have described the EPA- and DHA-induced reduction of platelet-derived microparticles that carry thrombogenic tissue factor( Reference Del Turco, Basta and Lazzerini 75 , Reference Phang, Lincz and Seldon 76 ), and studies in animals showed a reduction in injury-induced arterial occlusion and increased fibrinolysis after a high diet of EPA and DHA( Reference Harker, Kelly and Hanson 77 – Reference Braden, Knapp and Fitzgerald 79 ). Key platelet-related events in atherogenesis such as released CD40 ligand( Reference Aarsetoy, Brugger-Andersen and Hetland 80 ), platelet–monocyte interactions( Reference Din, Harding and Valerio 81 ) and certain markers of inflammation( Reference Moertl, Berger and Hammer 82 ) are also reduced by EPA and DHA. The above-reported reduction in the exposure of phosphatidylserine on platelet outer membranes which serve as a docking site for factors that contribute to the production of thrombin (prothrombin, factor Xa and factor Va( Reference Monroe, Hoffman and Roberts 83 , Reference Tracy, Nesheim and Mann 84 )) may explain the slowed rate of thrombin generation and the reduction in factor V concentration observed in subjects on fish oil( Reference Vanschoonbeek, Feijge and Paquay 85 ). Furthermore, in vitro studies also observed a decrease in fibrin generation in whole blood treated with EPA and DHA, as well as a reduced rate of thrombin formation on treated platelets( Reference Larson, Tormoen and Weaver 86 ). This reduction in platelet-mediated thrombin generation is a newly discovered mechanism by which EPA and DHA may retard haemostasis. Importantly, the delayed, but not eliminated, thrombin activation can still allow for relatively normal haemostasis while also reducing the risk of catastrophic thrombosis. Thus, there are a number of newly appreciated effects of EPA and DHA on these anuclear cells that could reduce the risk of cardiovascular events which warrant further study.
Conclusions
The purpose of the present review was to summarise the effects of n-3 fatty acids on the risk of bleeding in multiple clinical settings and on platelet biology. We found no evidence to contravene the statement in the Food and Drug Administration-approved package inserts for the currently marketed n-3 pharmaceutical products stating that these agents ‘do not produce clinically significant bleeding episodes’. Indeed, in some clinical settings, they appeared to actually reduce the risk of bleeding. n-3 Fatty acids have many effects on platelet biochemistry that together produce a mild (often undetectable by certain testing methods) inhibition of function. Such effects are likely to contribute to the health benefits associated with n-3 fatty acids.
Acknowledgements
This study received no specific grant from any funding agencies in the public, commercial or not-for-profit sectors.
All authors collaborated on drafting the present review. Specifically, J. K. W. contributed to the review of clinical trials; M. K. L. contributed to the review of n-3 fatty acid and platelet biology; W. S. H. initiated the project, assembled the writing team, and oversaw the preparation of the final manuscript.
The authors declare no conflicts of interest.