Numerous epidemiological studies have demonstrated that consumption of Brassica vegetables such as Brussels sprouts, broccoli and cabbage has been associated with decreased incidence of several types of cancer(Reference Zhang, Munday and Jobson1, Reference Higdon, Delage and Williams2). Brassica vegetables are usually consumed in the processed form. They are subjected to technological and culinary processing, such as cutting, blanching, cooking and fermentation. Due to dietary habits, fermentation of vegetables, mainly white cabbage, has been limited only to some parts of the world(Reference Ciska and Pathak3). Fermented cabbage is very popular in Poland and Germany, but is also consumed in the USA, Canada and Russia.
The most common product among fermented white cabbage is sauerkraut, which results from lactic acid fermentation of shredded and salted white cabbage.
Epidemiological data, although not so numerous as for the other cruciferous vegetables, indicate that consumption of processed cabbage might be related to reduced risk of breast, prostate and colorectal cancer(Reference Higdon, Delage and Williams2). Similar to other vegetables, Brassica contain a number of micronutrients (minerals: Mg, I, K and P; vitamins: C, E, K, α-, β-carotene and folic acid) and phytochemicals such as polyphenols with chemopreventive properties. However, they are unique in that they are rich sources of glucosinolates (GLS). Depending on the type and conditions of processing, GLS undergo either enzymatic hydrolysis or thermal degradation resulting in the formation of biologically active compounds including indoles and isothiocyanates (ITC). The effect of fermentation on GLS degradation products has been poorly recognised(Reference Ciska and Pathak3, Reference Villaluenga-Martinez, Penas and Frias4). The formation of some specific products such as ascorbigen is suggested(Reference Wagner and Rimbach5).
It is generally accepted that the breakdown products of GLS, particularly ITC, act through induction of phase II enzymes(Reference Higdon, Delage and Williams2).
Induction of phase II detoxifying and antioxidant enzymes such as glutathione S-transferase (GST) and NAD(P)H:quinone oxidoreductase 1 (NQO1) is the major defence system in response to cell oxidative and electrophilic insults. Several lines of evidence clearly indicate that a coordinated regulation of the genes encoding phase II enzymes is mediated at the transcriptional level, by an NF-E2-related transcription factor (Nrf2) in part, by a cis-acting element in the promoter, i.e. the antioxidant-responsive element(Reference Keum, Han and Liew6). The induction of phase II enzymes, especially GST, which detoxifies xenobiotics and potential carcinogens by conjugation with glutathione, was closely correlated with the reduction in the number of chemically induced tumours in rodents(Reference Williamson, DuPont and Wanigatunga7). Although very few studies have addressed induction of these enzymes by food components in human subjects or human cells, the study of Bogaards et al. (Reference Bogaards, Verhagen and Willens8) demonstrated that a diet high in Brussels sprouts increased the level of plasma GST α. This GST isoform may inactivate ultimate carcinogenic metabolites of carcinogenic polycyclic aromatic hydrocarbons. Thus, the modulation of these enzymes might be linked to the reduced risk of cancer among cabbage consumers.
The aim of the present study was to investigate the effect of long-term feeding (28 d) with cabbage and sauerkraut juices on the GST and NOQ1 expressions and activities in the rat liver and kidney. In order to explore the mechanism of the possible modulation of their induction, the influence of cabbage juices on the activation of Nrf2 was also examined, and comparison with the effects of pure GLS degradation products, indole-3-carbinol (I3C) and phenethyl isothiocyanate (PEITC), was made.
Experimental methods
Chemicals
I3C, glutathione, 1-chloro-2,4-dinitrobenzene, 2,6-dichlorophenolindophenol, dicoumarol, NADPH, dithiothreitol, sucrose, bovine serum albumin, PEITC and Tris were purchased from Sigma Chemicals Company (St Louis, MO, USA). Primary antibodies against β-actin and NQO1 and secondary antibodies were supplied by Santa Cruz Biotechnology (Santa Cruz, CA, USA). Primary antibodies against GST α, GST π and GST μ, purified standards of GST π and secondary antibodies were supplied by Oxford Biomedical Research (Oxford, MI, USA). Primary and secondary antibodies against GST θ were obtained from LabAs Limited (Tartu, Estonia). All antibodies used in these experiments were specific for their respective proteins, and according to the information provided by suppliers, there was no cross-reactivity within the isozymes of the same family. Rainbow-coloured protein molecular weight marker was purchased from Amersham Pharmacia Biotechnology (Piscataway, NJ, USA). All other chemicals were commercial products of the highest purity available.
Animals and treatments
Male Wistar rats (6 weeks of age), provided by University of Medical Sciences, Department of Toxicology Breeding Facility (Poznań, Poland), were housed in polycarbonate cages (30 × 20 × 25 cm; ≤ four rats/cage), containing hardwood chip bedding. Commercial rat food (Altromin GmbH, Lage, Germany) and distilled water were available without restriction. Six animals (average body weight 250 g) were used for each of twenty-one experimental groups characterised in Tables 1–6. The animals were treated by oral administration (intragastric) with cabbage and sauerkraut juices (1·25 ml/kg body weight), I3C (100 mg/kg body weight) or PEITC (100 mg/kg body weight) for 4, 10 and 30 d.
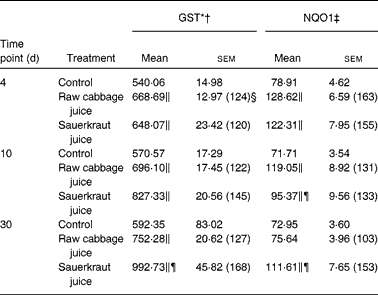
Table 1 Effect of raw cabbage juice and sauerkraut juice on the activities of phase II enzymes in the rat liver
(Mean values with their standard errors, n 6)
GST, glutathione S-transferase; NQO1, NAD(P)H:quinone oxidoreductase 1.
* Each assay was run in triplicate.
† GST is expressed in nmol 1-chloro-2,4-dinitrobenzene conjugated formed/min per mg.
‡ NQO1 is expressed in nmol 2,6-dichloroindophenol reduced/min per mg.
§ Percentage of control.
∥ Mean values were significantly different from the control group (P < 0·05).
¶ Mean values were significantly different from the raw cabbage juice group (P < 0·05).
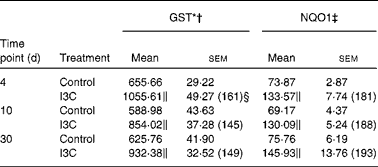
Table 2 Effect of indole-3-carbinol (I3C) on the activities of phase II enzymes in the rat liver
(Mean values with their standard errors, n 6)
GST, glutathione S-transferase; NQO1, NAD(P)H:quinone oxidoreductase 1.
* Each assay was run in triplicate.
† GST is expressed in nmol 1-chloro-2,4-dinitrobenzene conjugated formed/min per mg.
‡ NQO1 is expressed in nmol 2,6-dichloroindophenol reduced/min per mg.
§ Percentage of control.
∥ Mean values were significantly different from the control group (P < 0·05).
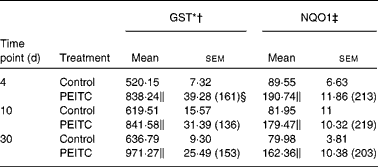
Table 3 Effect of phenethyl isothiocyanate (PEITC) on the activities of phase II enzymes in the rat liver
(Mean values with their standard errors, n 6)
GST, glutathione S-transferase; NQO1, NAD(P)H:quinone oxidoreductase 1.
* Each assay was run in triplicate.
† GST is expressed in nmol 1-chloro-2,4-dinitrobenzene conjugated formed/min per mg.
‡ NQO1 is expressed in nmol 2,6-dichloroindophenol reduced/min per mg.
§ Percentage of control.
∥ Mean values were significantly different from the control group (P < 0·05).
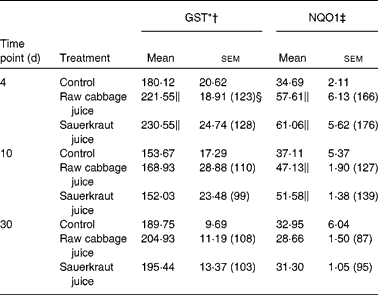
Table 4 Effect of raw cabbage juice and sauerkraut juice on the activities of phase II enzymes in rat kidney
(Mean values with their standard errors, n 6)
GST, glutathione S-transferase; NQO1, NAD(P)H:quinone oxidoreductase 1.
* Each assay was run in triplicate.
† GST is expressed in nmol 1-chloro-2,4-dinitrobenzene conjugated formed/min per mg.
‡ NQO1 is expressed in nmol 2,6-dichloroindophenol reduced/min per mg.
§ Percentage of control.
∥ Mean values were significantly different from the control group (P < 0·05).
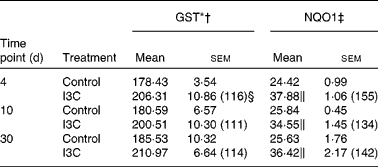
Table 5 Effect of indole-3-carbinol (I3C) on the activities of phase II enzymes in rat kidney
(Mean values with their standard errors, n 6)
GST, glutathione S-transferase; NQO1, NAD(P)H:quinone oxidoreductase 1.
* Each assay was run in triplicate.
† GST is expressed in nmol 1-chloro-2,4-dinitrobenzene conjugated formed/min per mg.
‡ NQO1 is expressed in nmol 2,6-dichloroindophenol reduced/min per mg.
§ Percentage of control.
∥ Mean values were significantly different from the control group (P < 0·05).
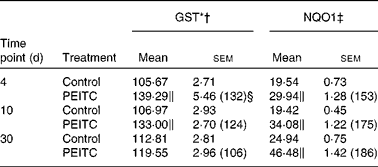
Table 6 Effect of phenethyl isothiocyanate (PEITC) on the activities of phase II enzymes in rat kidney
(Mean values with their standard errors, n 6)
GST, glutathione S-transferase; NQO1, NAD(P)H:quinone oxidoreductase 1.
* Each assay was run in triplicate.
† GST is expressed in nmol 1-chloro-2,4-dinitrobenzene conjugated formed/min per mg.
‡ NQO1 is expressed in nmol 2,6-dichloroindophenol reduced/min per mg.
§ Percentage of control.
∥ Mean values were significantly different from the control group (P < 0·05).
The doses were selected based on the protocols of Zhang & Malejka-Giganti(Reference Zhang and Malejka-Giganti9,) and Adam-Rodwell et al. (Reference Adam-Rodwell, Morse and Stoner10). Control groups of animals received water, or 20 % ethanol in olive oil, or olive oil, respectively. All animals were killed 24 h after the last treatment. Ketamine (intramuscular injection, 130 mg/kg body weight) was used as an anaesthetic.
All procedures were carried out according to the European guidelines for the care and use of laboratory animals and were approved by the Regional Ethics Committee (no. 36/2008).
Preparation of juices
Fresh white cabbage was purchased in a wholesale shop supplying the area of Gdańsk in vegetables. After removal of outer leaves, cabbage heads were cut in a shredder into approximately 2 mm thick strips. The part of shredded cabbage was blended, and the juice was squeezed out from the pulps. The rest of the shredded cabbage was subjected to a fermentation process. The shredded cabbage was mixed with 20 g/kg of NaCl and fermented in a traditional stoneware pot for 2 weeks. The juice standardisation was performed according to previously published protocols(Reference Kusznierewicz, Śmiechowska and Bartoszek11). In fresh juice, the average content of ITC groups and I3C was 0·15 and 2·74 μmol/l, respectively. In sauerkraut juice, the content of I3C was below the level of detection, while the level of ITC was not determined.
Preparation of cytosolic fractions
At 24 h after the last treatment, the rats were killed by decapitation, and the liver and kidneys were removed. The tissues were rinsed in the ice-cold buffered 0·2 m-sucrose (pH 7·5) and homogenised in the same medium. For the determination of enzyme activities, the cytosolic fraction was prepared by differential centrifugation, and protein concentrations were determined as described previously(Reference Krajka-Kuźniak, Szaefer and Baer-Dubowska12).
Preparation of nuclear extracts from the rat liver
The cytosolic and nuclear extracts from the rat liver were prepared by using a Nuclear/Cytosol Fractionation Kit (BioVision Research, Mount View, CA, USA).
Enzyme activity assay
The activities of cytosolic NQO1 (assayed with NADPH as the electron donor and 2,6-dichlorophenolindophenol as the electron acceptor) and GST, using 1-chloro-2,4-dinitrobenzene as a substrate, were determined using protocols described in our earlier studies(Reference Krajka-Kuźniak, Szaefer and Baer-Dubowska12).
Protein immunoblotting
Cytosolic proteins (20–100 μg) were separated on 10 or 12 % SDS-PAGE slab gels, and the proteins were transferred to nitrocellulose membranes (Immobilon-P; Millipore, Bedford, MA, USA)(Reference Krajka-Kuźniak, Kaczmarek and Baer-Dubowska13). After blocking with 10 % skimmed milk, the proteins were probed with rabbit anti-human GST α, goat anti-rat GST μ, rabbit anti-human GST π, human anti-mouse GST θ, goat anti-human NQO1, rabbit anti-mouse Nrf2 and rabbit anti-mouse β-actin antibodies. The β-actin protein was used as an internal control. As the secondary antibodies in the staining reaction, the alkaline phosphatase-labelled anti-goat IgG, anti-mouse IgG or anti-rabbit IgG were used. The amount of the immunoreactive product in each lane was determined by densitometric scanning using a BioRad GS 710 Image Densitometer (BioRad Laboratories, Hercules, CA, USA). The values were calculated as relative absorbance units (RQ)/mg protein.
Statistical analysis
Statistical analysis was performed by one-way ANOVA. Statistical significance between the experimental groups and their respective controls was assessed by Tukey's post hoc test at P < 0·05.
Results
Effect of raw cabbage and sauerkraut juices, indole-3-carbinol and phenethyl isothiocyanate on phase II enzyme activity in the rat liver and kidney
The effects of intragastric treatment with cabbage juices on GST and NQO1 in the rat liver are summarised in Table 1. Feeding with raw cabbage juice increased the activity of both enzymes except the 30 d time point. The sauerkraut juice, however, enhanced more significantly the activity of these enzymes in all time points of treatment. The most significant difference was noted in the case of GST and NQO1 after 30 d of feeding. At this time point, a 68 and 53 % increase in the GST and NQO1 activity, respectively, was observed, while raw cabbage juice did not change the activity of NQO1 and only slightly changed (by 27 %) GST activity. The increase in the GST activity by sauerkraut was similar to that observed as a result of treatment with I3C (Table 2) and PEITC (Table 3). The NQO1 activity, however, was less affected by sauerkraut juice in comparison with these two compounds. Statistical analysis showed the significant effect of sauerkraut juice on NQO1 activity after 10 and 30 d, while GST was affected only after 30 d of treatment (Table 1).
In the kidney, the increase in GST activity was observed after 4 d of raw cabbage (23 %) and sauerkraut juices (28 %) and the activity of NQO1 on the 10th day of feeding with both juice (27 and 37 %) treatments (Table 4).
I3C (Table 5) significantly increased the activity of NQO1 at all time points. The most effective inducer of NQO1 in this tissue was PEITC, which increased the activity of this enzyme by 86 % on the 30th day of treatment with this compound. The increased (24–32 %) activity of GST was also observed only as a result of 4 and 10 d treatment with PEITC (Table 6).
Effect of raw cabbage and sauerkraut juices, indole-3-carbinol and phenethyl isothiocyanate on glutathione S-transferase isozymes and NAD(P)H:quinone oxidoreductase 1 protein levels in the rat liver and kidney
Figs. 1, 3 show the immunoblots of GST isozymes and NQO1 in the liver of rats treated with cabbage juice and I3C or PEITC, respectively. On the basis of our(Reference Krajka-Kuźniak, Kaczmarek and Baer-Dubowska13) and other(Reference Nijhoff, Groen and Peters14) results showing the lack of GST π in the rat liver, only GST α, μ and θ were analysed.

Fig. 1 Expression of glutathione S-transferase (GST) isozymes and NAD(P)H:quinone oxidoreductase 1 (NQO1) in the rat liver treated with cabbage juices. (a) A representative immunoblot from two independent experiments. (b) Data presented as percentage of control groups (means with their standard errors) from two separate experiments run in triplicate. * Mean values were significantly different from the control group (P < 0·05). Lane 1, molecular weight marker; lane 2, control (4 d); lane 3, raw cabbage juice (RCJ; 4 d, □); lane 4, sauerkraut juice (SJ; 4 d, ![]() ); lane 5, control (10 d); lane 6, RCJ (10 d,
); lane 5, control (10 d); lane 6, RCJ (10 d, ![]() ); lane 7, SJ (10 d,
); lane 7, SJ (10 d, ![]() ); lane 8, control (30 d); lane 9, RCJ (30 d,
); lane 8, control (30 d); lane 9, RCJ (30 d, ![]() ); lane 10, SJ (30 d,
); lane 10, SJ (30 d, ![]() ).
).

Fig. 2 Expression of glutathione S-transferase (GST) isozymes and NAD(P)H:quinone oxidoreductase 1 (NQO1) in the rat liver treated with indole-3-carbinol (I3C). (a) A representative immunoblot from two independent experiments. (b) Data presented as percentage of control groups (means with their standard errors) from two separate experiments run in triplicate. * Mean values were significantly different from the control group (P < 0·05). Lane 1, molecular weight marker; lane 2, control (4 d); lane 3, I3C (4 d, □); lane 4, control (10 d); lane 5, I3C (10 d, ![]() ); lane 6, control (30 d); lane 7, I3C (30 d,
); lane 6, control (30 d); lane 7, I3C (30 d, ![]() ).
).

Fig. 3 Expression of glutathione S-transferase (GST) isozymes and NAD(P)H:quinone oxidoreductase 1 (NQO1) in the rat liver treated with phenethyl isothiocyanate (PEITC). (a) A representative immunoblot from two independent experiments. (b) Data presented as percentage of control groups (means with their standard errors) from two separate experiments run in triplicate. * Mean values were significantly different from the control group (P < 0·05). Lane 1, molecular weight marker; lane 2, control (4 d); lane 3, PEITC (4 d, □); lane 4, control (10 d); lane 5, PEITC (10 d, ![]() ); lane 6, control (30 d); lane 7, PEITC (30 d,
); lane 6, control (30 d); lane 7, PEITC (30 d, ![]() ).
).
The quantitative analysis (Figs. 1, 3) of the immunoblots revealed that both cabbage and sauerkraut juices similarly, as with I3C and PEITC, increased the level of GST μ and to a lesser extent GST α. However, the effect of cabbage juices was observed only after 30 d of feeding, while I3C and PEITC increased the levels of both GST isozymes at all time points examined. Hepatic GST θ was not affected by any treatment regimen.
In concert with the enhanced activity of NQO1, I3C and PEITC increased the enzyme protein level. Sauerkraut juice increased the NQO1 level only on the 4th day of treatment.
In the kidney (Figs. 4–6), neither cabbage juices nor I3C or PEITC affected the expression of GST isozymes and NQO1. The only exception was the reduced level of GST θ observed on the 30th day of feeding with both raw cabbage and sauerkraut juices (Fig. 4).

Fig. 4 Expression of glutathione S-transferase (GST) isozymes and NAD(P)H:quinone oxidoreductase 1 (NQO1) in rat kidney treated with cabbage juices. (a) A representative immunoblot from two independent experiments. (b) Data presented as percentage of control groups (means with their standard errors) from two separate experiments run in triplicate. * Mean values were significantly different from the control group (P < 0·05). Lane 1, molecular weight marker; lane 2, control (4 d); lane 3, raw cabbage juice (RCJ; 4 d, □); lane 4, sauerkraut juice (SJ; 4 d, ![]() ); lane 5, control (10 d); lane 6, RCJ (10 d,
); lane 5, control (10 d); lane 6, RCJ (10 d, ![]() ); lane 7, SJ (10 d,
); lane 7, SJ (10 d, ![]() ); lane 8, control (30 d); lane 9, RCJ (30 d,
); lane 8, control (30 d); lane 9, RCJ (30 d, ![]() ); lane 10, SJ (30 d,
); lane 10, SJ (30 d, ![]() ).
).

Fig. 5 Expression of glutathione S-transferase (GST) isozymes and NAD(P)H:quinone oxidoreductase 1 (NQO1) in rat kidney treated with indole-3-carbinol (I3C). (a) A representative immunoblot from two independent experiments. (b) Data presented as percentage of control groups (means with their standard errors) from two separate experiments run in triplicate. Lane 1, molecular weight marker; lane 2, control (4 d); lane 3, I3C (4 d, □); lane 4, control (10 d); lane 5, I3C (10 d, ![]() ); lane 6, control (30 d); lane 7, I3C (30 d,
); lane 6, control (30 d); lane 7, I3C (30 d, ![]() ).
).

Fig. 6 Expression of glutathione S-transferase (GST) isozymes and NAD(P)H:quinone oxidoreductase 1 (NQO1) in rat kidney treated with phenethyl isothiocyanate (PEITC). (a) A representative immunoblot from two independent experiments. (b) Data presented as percentage of control groups (means with their standard errors) from two separate experiments run in triplicate. Lane 1, molecular weight marker or standard for GST α; lane 2, control (4 d); lane 3, PEITC (4 d, □); lane 4, control (10 d); lane 5, PEITC (10 d, ![]() ); lane 6, control (30 d); lane 7, PEITC (30 d,
); lane 6, control (30 d); lane 7, PEITC (30 d, ![]() ).
).
Activation of NF-E2-related transcription factor in the rat liver by cabbage juice, indole-3-carbinol and phenethyl isothiocyanate
As shown in Fig. 7, the treatment with both cabbage and sauerkraut juices or I3C and PEITC led to the translocation of Nrf2 protein from the cytosol to the nucleus in the cells of the rat liver, increasing its level in the nuclear fraction. Quantitative analysis (Fig. 7(b)) revealed a significant increase in the amount of Nrf2 in the nucleus only on the 30th day of feeding with raw cabbage juice. Activation of Nrf2 by I3C and PEITC was observed at all time points studied, resulting in a 21–35 % increase of this protein in the nucleus (Fig. 7(b)).

Fig. 7 Expression of NF-E2-related transcription factor (Nrf2) in nuclear fractions from the rat liver treated with cabbage juices, indole-3-carbinol (I3C) and phenethyl isothiocyanate (PEITC). (a) A representative immunoblot from two independent experiments. (b) Data presented as percentage of control groups (means with their standard errors) from two separate experiments run in triplicate. * Mean values were significantly different from the control group (P < 0·05). Lane 1, molecular weight marker; lane 2, control (4 d); lane 3, raw cabbage juice (RCJ), I3C and PEITC (4 d, □); lane 4, sauerkraut juice (SJ; 4 d, ![]() ); lane 5, control (10 d); lane 6, RCJ, I3C and PEITC (10 d,
); lane 5, control (10 d); lane 6, RCJ, I3C and PEITC (10 d, ![]() ); lane 7, SJ (10 d,
); lane 7, SJ (10 d, ![]() ); lane 8, control (30 d); lane 9, RCJ, I3C and PEITC (30 d,
); lane 8, control (30 d); lane 9, RCJ, I3C and PEITC (30 d, ![]() ); lane 10, SJ (30 d,
); lane 10, SJ (30 d, ![]() ).
).
Discussion
Epidemiological studies have demonstrated that consumption of Brassica vegetables is associated with a lower incidence of cancers(Reference Morimitsu, Nakagawa and Hayashi15).
In the Central and Eastern European diet, the most common Brassica genus is white cabbage and its fermented product, sauerkraut. However, in contrast to other Cruciferae representatives, the anti-carcinogenic activity of white cabbage and sauerkraut has been less extensively studied. Epidemiological migrant studies have shown that consumption of these food items during adolescence was associated with a 72 % reduced risk of breast cancer(Reference Nelson16). The lower risk of prostate and pancreatic cancers was also associated with cabbage consumption(Reference Kristal and Lampe17, Reference Larsson, Hakansson and Naslund18).
The reduced risk of cancer as a result of Brassica vegetable consumption is usually linked to the dietary intake of GLS and their metabolism to cancer-preventive indoles (e.g. I3C) and ITC, such as PEITC. Their content and characteristics depend on a particular Brassica vegetable and its processing. Moreover, experimental studies have suggested the modulation of phase I and, particularly, phase II metabolising enzymes as one mechanism for the GLS association with anticancer protection.
Thus, the focus of the present study was to evaluate the effect of raw cabbage and sauerkraut juices on the induction of two phase II enzymes GST and NQO1.
The results show that raw cabbage and sauerkraut juices, as with I3C and PEITC treatments, increased GST and NQO1 protein levels in the rat liver and subsequently the enzyme activities.
A significant effect was observed as a result of sauerkraut feeding in the rat liver, where a time-dependent induction of GST activity was observed. This effect was comparable with that exerted by pure I3C and PEITC. The latter observation concerning pure ITC is in agreement with the general opinion that GST are induced by these products of GLS degradation and act to conjugate this class of phytochemicals found in Brassica vegetables(Reference Higdon, Delage and Williams2). The level of the GST induction after the treatment with raw cabbage juice was lower and did not depend on the time point of juice intake.
Sauerkraut is the product of lactic acid fermentation of shredded and salted white cabbage. During shredding, glucobrassicin, the most commonly studied GLS, is transformed into I3C by the action of myrosinase. During fermentation, as the pH decreases, this indole reacts non-enzymatically with l-ascorbic acid to yield ascorbigen, which is thought to be the dominant end product of indole GLS in sauerkraut(Reference Ciska and Pathak3, Reference Wagner and Rimbach5).
While the effects of I3C have been extensively studied, only a few experiments focusing on the anti-carcinogenic effects of ascorbigen have been published. The data provided by Stephenson et al. (Reference Stephensen, Bonnesen and Bjeldanes19) and Kravchenko et al. (Reference Kravchenko, Avren'eva and Guseva20) have shown that ascorbigen itself or in combination with the other degradation products (I3C and sulphoraphane) modulates the enzymes involved in xenobiotic metabolism, inducing also GST and NQO1. The interaction of ascorbigen with an aryl hydrocarbon receptor has been suggested(Reference Kravchenko, Avren'eva and Guseva20) as the mechanism of induction.
The present results indicate that although interaction with an aryl hydrocarbon receptor cannot be excluded particularly for I3C, which is a known bifunctional inducer, the induction of GST and NQO1 by cabbage juices occurred rather as a result of Nrf2 activation and binding to the antioxidant-responsive element in nuclear DNA. This assumption is based on the fact that both juices, as well as I3C and PEITC, caused a parallel increase in GST, NQO1 and Nrf2 protein levels.
Nrf2 plays a key role in the transcriptional regulation of GST and NQO1 expression through interaction with antioxidant response element. Under normal physiological conditions, Nrf2 is sequestered in the cytoplasm as an inactive complex with its repressor Keap1(Reference Kong, Owuor and Yu21). Upon stimulation by inducers, Nrf2 dissociates from Keap1 and translocates into the nucleus where it dimerises with some cofactors such as small Maf protein and binds to antioxidant-responsive element. This leads to the activation of a battery of highly specialised proteins, including NQO1 and GST, and other antioxidant enzymes, such as glutamate cysteine ligase, haem oxygenase-1, that efficiently protect mammalian cells from various forms of toxicants and consequently reduce the propensity of tissues and organs to develop malignancies and other ailments(Reference Talalay and Fahey22).
The results of the present study showing that treatment of rats with cabbage juices, I3C and PEITC caused the translocation of the Nrf2-active subunit from the cytosol to the nucleus provide an additional argument for the involvement of this transcription factor in the induction of GST and NQO1.
On the other hand, several lines of evidence suggest the linkage between the aryl hydrocarbon receptor and Nrf2 batteries, achieved probably by multiple mechanisms, including Nrf2 as a target gene of the aryl hydrocarbon receptor(Reference Köhle and Bock23).
Among the GST isozymes, treatment with cabbage juices affected the most the hepatic GST μ and to a lesser extent GST α. The increased level of these isozymes might be beneficial in protecting against reactive oxygen species and products of lipid peroxidation, such as 4-hydroxynonenal(Reference Yang, Sharma and Sharma24). Moreover, GST μ has shown a high activity towards most polycyclic aromatic hydrocarbon epoxides(Reference Tang, Sheu and Wong25). This activity may be particularly interesting, since it was coordinated with the elevated expression and activity of NQO1. This enzyme is a cytosolic flavoprotein that catalyses metabolic detoxification of quinones, including benzo[a]pyrene quinones, by converting them into hydroquinones via a single-step two-electron reduction, thus avoiding the formation of deleterious reactive semiquinones(Reference Keum, Han and Liew6).
The results of recent studies with NQO1− / − and NQO2− / − mice have suggested that NQO1, together with NQO2, protects against polycyclic aromatic hydrocarbon-induced skin carcinogenesis(Reference Shen, Barrios and Jaiswal26). One possible mechanism of this protection, which might be applicable also to other tissues, is the fact that NQO interact and protect p53 protein, the key regulator of the control of cell growth, from 20S proteasome degradation. This leads to stability and transient activation of p53 and cellular protection through the cell cycle arrest, senescence or apoptosis(Reference Gong, Kole and Iskander27).
In contrast to the liver, cabbage juices did not affect the renal GST and NQO1 expressions, except GST θ. The low level of GST θ in the kidney demonstrates the poor constitutive expression of GST θ class in this tissue. Moreover, the alterations in the GST θ protein level did not result in changes in GST enzyme activity towards the classical substrate 1-chloro-2,4-dinitrobenzene, used in the present study, since 1-chloro-2,4-dinitrobenzene is not a substrate for GST θ(Reference van Lieshout, Bedaf and Pieter28).
GST θ may act as an activator of halogenated compounds. Moreover, there was an increased risk of kidney and liver tumours in human subjects with the GSTT-1-positive genotype, following exposure to halogenated solvents. Thus, the GSTT-1 genotype is supposed to confer decreased or increased risk of cancer in relation to the source of exposure. The level of GSTT-1 subunit within tissues is an important determinant of susceptibility to the carcinogenic effects of dihaloalkanes. Cabbage juice, by reducing the GST θ, may protect against nephrocarcinogenicity of chlorinated hydrocarbons, especially taking into account that the initial GST-dependent bioactivation step of nephrocarcinogenesis by chlorinated hydrocarbons may take place in the kidney itself, and the liver is not the main site of their initial conjugation(Reference Sherratt, Manson and Thomson29). This indicates that extrahepatic induction of detoxifying enzymes requires higher concentrations of active compounds than those occurring in cabbage juices.
Thus, the results of the present and earlier studies(Reference Baer-Dubowska, Szaefer and Krajka-Kuźniak30) indicate that induction of the key detoxifying enzymes by cabbage juices, particularly sauerkraut, may be responsible for their chemopreventive activity demonstrated by epidemiological studies and in animal models. The final effects, however, might be organ or tissue dependent.
Acknowledgements
The present study was supported by the Ministry of Science and Higher Education of Poland, grant 2 PO6T 085 26. The authors declare no conflict of interest. V. K.-K. helped in planning and performing the animal experiments, Western blot analysis and writing the first draft of the manuscript; H. S. helped in planning and performing the animal experiments, enzymes' activity assay and writing the first draft of the manuscript; A. B. helped in preparing and analysing of cabbage juices; W. B.-D. helped in planning the experiments and writing the manuscript.















