Independent risk factors for mortality amongst African patients starting antiretroviral therapy (ART) include anaemia( Reference O'Brien, Kupka and Msamanga 1 ), a failure to increase Hb within the first few months of ART( Reference Giganti, Limbada and Mwango 2 ), and malnutrition represented by a BMI of < 18·5 kg/m2( Reference Koethe, Lukusa and Giganti 3 – Reference Paton, Sangeetha and Earnest 5 ). Heightened systemic inflammation is a hallmark of both untreated and treated HIV infection( Reference Nasi, Pinti and Mussini 6 ), and higher levels of persistent inflammation, despite treatment with ART( Reference Armitage, Stacey and Giannoulatou 7 ), confer an increased risk of morbidity and mortality in HIV patients( Reference Koethe, Blevins and Nyirenda 8 , Reference Kuller, Tracy and Belloso 9 ). Redistribution of Fe during HIV infection can lead to increased Fe sequestration in macrophages with an accompanying decline in Fe available for tissue supply and erythropoiesis( Reference Armitage, Stacey and Giannoulatou 7 , Reference Wisaksana, Sumantri and Indrati 10 ). This disordered Fe metabolism has been associated with rapid progression of HIV( Reference Chang, Bayeva and Taiwo 11 – Reference Gordeuk, Delanghe and Langlois 13 ), exacerbation of co-infections( Reference De Monyé, Karcher and Boelaert 14 ), especially tuberculosis( Reference Minchella, Armitage and Darboe 15 , Reference McDermid, Hennig and van der Sande 16 ), and early death( Reference Gordeuk, Onojobi and Schneider 17 , Reference Mcdermid, Loeff, Van and Jaye 18 ). Although ART is increasingly available, initiation of ART is associated with a high mortality rate: 17 % of patients starting ART in sub-Saharan Africa die within 1 year, and the majority within the first 3 months( Reference Gupta, Nadkarni and Yang 19 ). The detrimental effects of anaemia, Fe redistribution, malnutrition, and inflammation on early ART mortality have been well-documented. In sub-Saharan Africa the HIV disease burden remains vast( 20 ), with a third of adults starting ART being malnourished in some African countries( Reference Koethe, Lukusa and Giganti 3 , Reference Liu, Spiegelman and Semu 21 ). The control of anaemia and normalisation of Fe metabolism within this population remains, therefore, a critical strategy for improving patient survival.
Markers other than Hb are required to assess Fe status. Serum ferritin can be used as a marker of body stores of Fe( Reference Finch, Bellotti and Stray 22 ), and soluble transferrin receptor (sTfR) to estimate tissue Fe demand( Reference Cook, Skikne and Baynes 23 ). The determination of Fe status in the presence of inflammation is notoriously challenging( 24 ), with no internationally agreed methodology as yet( 25 ). It is well known that inflammation alters many markers of Fe status, including increasing serum ferritin as an acute phase protein( 25 ). sTfR is less affected by the inflammatory response, and can therefore be used to distinguish anaemia of inflammation (with elevated serum ferritin and normal-to-elevated sTfR) from Fe deficiency anaemia (with low serum ferritin and high sTfR)( 25 , Reference Gibson 26 ). However, Fe deficiency and inflammation often co-exist, complicating assessment of Fe status and needs. Measurement of C-reactive protein (CRP) and α1-acid glycoprotein – acute phase proteins – can assist interpretation of the Fe biomarkers in order to separate those patients with inflammation-induced Fe sequestration from those who are both sequestering Fe and Fe-deficient( Reference Thurnham, McCabe and Haldar 27 ). Hepcidin, a peptide hormone which regulates iron metabolism by controlling the absorption of dietary Fe, Fe transport, and Fe distribution among different cell types, can be measured to help elucidate the complex interplay between anaemia, Fe status and immunity( Reference Drakesmith and Prentice 28 ).
There are currently many unanswered questions about: the range and characteristics of disordered Fe metabolism among malnourished, HIV-infected adults; the preferred Fe-related biomarkers for assessing health risks; the effect of oral Fe supplementation on Fe status and health. The common assumption that low Hb requires therapeutic correction through Fe supplementation may be erroneous in HIV, since supplementation can exacerbate the risk of co-infections, and hasten disease progression. Paradoxically it is high serum ferritin that predicts a worse outcome, despite the association of anaemia and mortality( Reference Mcdermid, Loeff, Van and Jaye 18 ). Controversy therefore remains as to what extent Fe supplementation amongst HIV patients affects infection rates and mortality( Reference Esan, van Hensbroek and Nkhoma 29 ). Some interventions supplementing Fe to HIV-positive adults have reduced anaemia without increasing viral load( Reference Olsen, Mwaniki and Krarup 30 , Reference Semba, Ricketts and Mehta 31 ); longer-term outcomes were not assessed. This creates a therapeutic dilemma for the clinician as to how HIV-infected patients with anaemia should be treated. Given the clear link between anaemia and early ART mortality, the existing knowledge gaps jeopardise the health and survival of thousands of malnourished HIV/AIDS patients.
The present study uses a clinical trial of a malnourished group of adults starting ART, to answer three main research questions. Firstly, what effect does a nutritional intervention including Fe have on Fe status? Secondly, does any impact depend on the baseline Fe status of patients? Thirdly, does inflammation have an independent effect on changes in Fe status? We hypothesised that the nutritional intervention would improve Fe status, indicated by an increase in Hb accompanied by no change or a slight decrease in sTfR; effects on sTfR would depend on whether the anaemia was due primarily to chronic disease which has little effect on sTfR, or to Fe deficiency which results in increased sTfR( 25 ). We expected overall serum ferritin results to be harder to predict: decreasing in the correction of anaemia of inflammation, but increasing in the correction of Fe deficiency. We speculated that failing to normalise systemic inflammation after starting ART would attenuate any improvements.
Subjects and methods
Study design
The study analyses information on Fe status and inflammation from a randomised, double blind, controlled phase-III clinical trial in Lusaka, Zambia and Mwanza, Tanzania – the Nutritional Support for Africans Starting Antiretroviral Therapy (NUSTART) trial (registered in the Pan-African Clinical Trials Register as PACTR201106000300631). Details of the trial are described in full elsewhere( Reference Filteau, PrayGod and Kasonka 32 , Reference Rehman, Woodd and PrayGod 33 ). In brief, the NUSTART trial was conducted between August 2011 and December 2013 to assess the effect of a fortified lipid-based nutrient supplement (LNS; prepared by Nutriset) on survival of malnourished patients starting ART. The present paper focuses on two secondary outcomes: markers of Fe status and inflammation. A total of 1815 patients were recruited at the two sites, using the inclusion criteria of age >18 years, BMI < 18·5 kg/m2, CD4 count < 350 cells/μl or stage 3 or 4 AIDS, ART-naïve apart from those who received ART during standard prevention of mother-to-child transmission regimens, and informed consent. Self-reported pregnancy was an exclusion criterion.
The trial intervention was based on established protocols for managing severe malnutrition in young children involving two phases aimed at stabilisation and then rehabilitation( 34 ). Fig. 1 summarises the NUSTART design. The first phase took place between referral and 2 weeks post-ART initiation. Participants were randomised to receive vitamins and minerals, without Fe as is done for malnourished children, in low-energy (30 g containing approximately 628 kJ (150 kcal)/d) LNS (low-dose LNS with added vitamins and minerals (LNS-VM)) in the intervention group v. LNS without the vitamins and minerals (low-dose control LNS) in the control group. This phase aimed to stabilise metabolism before trying to promote weight gain during the second phase. The second phase involved a 4-week intervention, starting 2 weeks after ART initiation and continuing until 6 weeks post-ART. Participants in the intervention group received a higher-energy (250 g containing approximately 5858 kJ (1400 kcal)/d) LNS containing the same added vitamins and minerals as in phase 1 plus Fe as sulphate (high-dose LNS-VM). The control group received the high-dose LNS without the added vitamins, minerals, or Fe (high-dose control LNS). Vitamin and mineral levels in both the high- and low-dose LNS-VM were mostly set at three times the UK recommended nutrient intakes for adult women( 35 ) with the exception of Fe which was only in the second stage, high-dose LNS and only at one recommended nutrient intake (14·7 mg/d) (nutritional composition details in online Supplementary material Table S1).

Fig. 1 Overview of the Nutritional Support for Africans Starting trial design. LNS, lipid-based nutrient supplement; ART, antiretroviral therapy; LNS-VM, LNS with added vitamin and mineral mix.
The interval between referral for ART and starting ART was based on the individual patient's readiness to start life-long drug treatment and practices of the different clinics from which the study recruited patients for investigation. Study personnel were not involved in deciding when to initiate ART; the duration of phase 1 reflects routine practices in these clinics at the time. The median interval between referral for ART and starting ART for both arms was 21 d, (interquartile range 15–30). During this period, the routine medications that the study participants continued to take were: Tenofovir (TDF)/Emtricitabine (FTC)/Efavirenz (EVF) (55·4 %); Zidovudine (AZT)/Lamivudine (3TC)/Nevirapine (NVP) (16·0 %); AZT/3TC/EVF (9·2 %); TDF/FTC/NVP (4·3 %); another (3·6 %); a small group of 11·5 % of the population had no ART regimen information( Reference Filteau, PrayGod and Kasonka 32 ).
The Data Safety and Monitoring Board (DSMB) statistician conducted the randomisation using sixteen computer-generated blocks stratified by site. The contents of the LNS packets were assigned an allocation code (letters A to H), known only to the DSMB statistician and Nutriset, which were linked to study ID numbers using a randomisation code. This randomisation code was only known to the DSMB statistician and site-based pharmacists, none of whom had direct patient contact. The LNS and LNS-VM packets were delivered by Nutriset in lots assigned by allocation code. Clinic pharmacists labelled packets with study ID numbers as packets were dispensed. Clinic nurses (with no access to the allocation or randomisation code) then recruited eligible participants to the study using sequential ID numbers.
Blood collection
Patients were seen weekly from referral for ART until the ART initiation visit, and then at 2, 6, 8, and 12 weeks after starting ART. They were asked at each follow-up visit whether they were taking Fe supplements in addition to the study supplement. Hb and serum CRP were measured for all patients, whilst serum ferritin and sTfR were analysed for a fifth of the patients, referred to as the Fe marker subsample, and chosen systematically for every patient ID divisible by 5. Hb was measured at recruitment and at 6 weeks post-ART. Fe markers and CRP were measured in serum from the recruitment and 6 weeks post-ART samples which were stored at − 80°C until batch analysis. The flow of participants included in the Fe marker subsample from identification to analysis at baseline and week 6 is shown in Fig. 2.

Fig. 2 Flow of subsample participants from identification to analysis of iron markers at baseline and week 6 post-antiretroviral therapy (ART). * Inclusion criteria: >18 years, BMI < 18·5 kg/m2, CD4 count < 350/μl or stage 3 or 4 AIDS, ART-naïve apart from those who received ART during standard prevention of mother-to-child transmission regimens, and informed consent. Self-reported pregnancy was an exclusion criterion. † Note Hb and C-reactive protein (CRP) were collected from all patients at recruitment and 6 weeks post-ART. The flow diagram for the whole sample is published elsewhere( Reference Filteau, PrayGod and Kasonka 32 ). Available samples at baseline: CRP, n 1762; Hb, n 1670. Available samples at week 6: CRP, n 863; Hb, n 826. ID, identification number; LNS, lipid-based nutrient supplement; LNS-VM, LNS with added vitamin and mineral mix; sTfR, soluble transferrin receptor.
Iron and inflammatory marker analysis
Fingerstick capillary blood samples from all patients were used for analysis of Hb levels by a portable haemoglobinometer (Hemocue®). Anaemia severity cut-offs of the present study followed standard WHO categorisations( 36 ). Mild anaemia was defined as Hb < 120 g/l for women and < 130 g/l for men. Moderate and severe anaemia categories used the same cut-offs for both sexes, defined as < 110 and < 80 g/l respectively.
Serum ferritin was measured by ELISA (AssayPro Human Ferritin ELISA Kit, catalogue no. EF2003-1). The intra-assay and inter-assay CV were 2 and 7 % in Mwanza and 5 and 23 % in Lusaka respectively. sTfR was measured by ELISA (Quantikine® IVD® Human sTfR Immunoassay, Ref DTFR1; R&D Systems, Inc.). The intra-assay and inter-assay CV were 2 and 4 % in Mwanza and 3 and 13 % in Lusaka respectively. Serum CRP was analysed by ELISA (AssayPro). The intra-assay and inter-assay CV were 3 and 37 % in Mwanza and 6 and 32 % in Lusaka respectively. For all analyses, values over the upper range of the standard curve were set to the top standard multiplied by the dilution factor. For all assays plates with poor precision were re-run.
Sample size
The original trial sample size was powered on the primary outcome (mortality). Our ‘one in five’ subsample for the Fe markers was sufficient to detect an inter-group difference of 0·35 standard deviation using 90 % power.
Data analysis
Continuous variables were assessed for normality using normal probability plots and visual inspection of histograms. Hb and sTfR approximated a normal distribution and remained on the linear scale. Serum CRP and serum ferritin were skewed to the right and transformed to natural logs. We considered using correction factors for ferritin derived from the methodology suggested by Thurnham et al. ( Reference Thurnham, McCabe and Haldar 27 ); however, our population was extremely malnourished, and exhibited high levels of inflammation with very deranged Fe metabolism so it was unclear whether correction factors derived from populations less ill were appropriate. We decided instead to simply adjust for CRP in regression analyses, as has been done elsewhere( Reference Friis, Range and Braendgaard Kristensen 37 ).
We compared baseline characteristics of those in the smaller subsample containing data on sTfR and serum ferritin (n 353) with those not in the subsample (n 1462) to assess the generalisability applicable to the whole sample. The χ2 test was used to compare proportions, independent t tests to compare means of normally distributed data, and the Wilcoxon–Mann–Whitney test to compare medians of non-parametric data. Sample sizes of all further analyses were set by the number of available samples at 6 weeks post-ART.
A variable was created to summarise the frequency of taking Fe supplements in addition to the study supplement over the follow-up period; this was categorised as ‘never consumed’ (62 %), ‘reported consumed at one follow-up’ (19 %), and ‘reported consumed at two or more follow-up visits’ (19 %). We assessed the within-subject changes in markers of Fe status and inflammation between baseline and week 6 by intervention arm using paired t tests.
For our first objective assessing the effect of the intervention on Fe marker status at week 6 we used multivariable linear regression. The first model adjusted only for the baseline value of the Fe marker being assessed, the second model additionally adjusted for serum CRP at week 6 given our hypothesis that inflammation would affect Fe markers, and the third model further adjusted for sex, site, age, baseline BMI and CD4 count, being on TB treatment at recruitment, taking Fe supplements in addition to the study supplement, and length of time taken from recruitment to starting ART. TB treatment at recruitment was coded yes or no and taking Fe supplements in addition to the study supplement was a categorical variable.
For our second objective, we repeated the third (fully adjusted) model analysis stratified by baseline values of the Fe markers to determine whether these modified the effect of the intervention. We used a binary Hb category: normal Hb and mild anaemia v. those with moderate and severe anaemia. Due to lack of internationally agreed cut-offs for serum ferritin and sTfR, and the specific context of our malnourished sample with heightened systemic inflammation, we divided these variables into two groups using the median value to create binary categories. We chose binary categories rather than continuous measures since we felt this would provide a more accessible way of interpreting overall trends that may have physiological significance. The test for interaction between the baseline Fe marker category and intervention arm used a likelihood ratio test between the multivariable linear regression models with and without the interaction term.
For our third objective, we assessed the extent to which inflammation was driving the changes in our Fe markers independent of the intervention. We investigated interrelations among the Fe markers and serum CRP using Pearson correlation matrices. We then created a multiple linear regression model exploring the association between change in Fe marker from baseline to week 6 with change in serum CRP over the same timeframe, adjusting for trial arm, sex, site and being on tuberculosis treatment at recruitment as binary variables; taking Fe supplements in addition to the study supplement as a categorical variable; and baseline BMI, age, CD4 count and length of time taken from recruitment to starting ART as continuous variables.
Stata version 13.1 (StataCorp) was used for all analyses.
Ethical considerations
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki. All NUSTART trial procedures, including the collection and analysis of the Fe markers, were approved by the ethics committee of the London School of Hygiene and Tropical Medicine, the University of Zambia Biomedical Research Ethics Committee (reference no. 009-01-11), and the National Institute for Medical Research, Tanzania. Written informed consent or thumbprint was obtained from all patients before enrolment.
Results
Detailed baseline characteristics of the 1815 patients recruited are published elsewhere( Reference Filteau, PrayGod and Kasonka 32 ). In summary, one-third had BMI < 16 kg/m2 and mean age was 35·8 (sd 9·4) years. Only 10 % of the patients were without anaemia at baseline, with two-thirds categorised as either moderately or severely anaemic. Table 1 shows the baseline characteristics for the subsample assessed for Fe markers (n 353). Mean baseline Hb was lower amongst those in the Fe marker subsample compared to those not included (93 (sd 23) v. 96 (sd 23) g/l, P= 0·012). Median serum CRP was higher amongst those in the subsample compared to those not included: 71 (interquartile range 18–160) v. 57 (interquartile range 13–155) mg/l, P= 0·004). Patient baseline characteristics in the Fe subsample were very similar in the two treatment arms (Table 1), as was the case for the whole sample( Reference Filteau, PrayGod and Kasonka 32 ).
Table 1 Baseline characteristics of the iron marker subsample by trial arm and overall summaries of those included in and excluded from the iron marker subsample (Number of participants and percentages; mean values and standard deviations; median values and interquartile ranges (IQR))

LNS-VM, lipid-based nutritional supplement with added vitamins and minerals; LNS, lipid-based nutritional supplement without added vitamins and minerals; CRP, C-reactive protein; TB, tuberculosis; ART, antiretroviral therapy; sTfR, soluble transferrin receptor; N/A, not applicable.
* χ2 test to compare proportions, independent t tests to compare means of normally distributed data, and the Wilcoxon–Mann–Whitney test to compare medians of non-parametric data.
† Normal defined as ≥ 120 g/l for women and ≥ 130 g/l for men. Mild anaemia defined as Hb < 120 g/l for women and < 130 g/l for men. Moderate and severe anaemia categories defined as < 110 and < 80 g/l respectively for both sexes.
In the control group from baseline to week 6 post-ART, patients gained a mean of 3 g/l Hb (P= 0·029, n 369), decreased their serum ferritin by 100 μg/l (P= 0·021, n 89), increased their sTfR by 4 nmol/l (P= 0·045, n 101), but experienced no overall change in CRP levels (P= 0·08, n 407) (Table 2). The intervention group displayed similar trends: patients gained a mean of 6 g/l Hb (P≤ 0·001, n 383), decreased their serum ferritin by 141 μg/l (P= 0·004, n 76), increased their sTfR by 4 nmol/l (P= 0·030, n 85), and also experienced no overall change in CRP levels (P= 0·36, n 431). There was no effect of the vitamins and minerals added to the intervention LNS on Hb, serum ferritin, sTfR or serum CRP in any of the three statistical models (Table 3). Note that sample sizes in Table 3, which used various adjusted models, were restricted to the patients who had no missing data in all the variables we adjusted for; they differ, therefore, from those seen in Table 2, which used unadjusted data.
Table 2 Overview of changes in iron and inflammatory markers from baseline to week 6 by trial arm, unadjusted (Mean values, geometric mean values and 95 % confidence intervals)
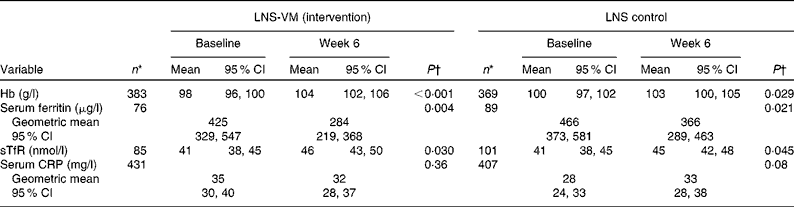
LNS-VM, lipid-based nutrient supplement with added vitamins and minerals; LNS, lipid-based nutrient supplement; sTfR, soluble transferrin receptor; CRP, C-reactive protein.
* Only patients with week 6 data included; therefore sample size lower than that of Table 1.
† Paired t test.
Table 3 Linear regression showing the effect of the intervention on Hb, iron and inflammatory markers at week 6 with regression coefficients (B), 95 % CI and the corresponding P values, using three models of adjustment (Regression coefficients and 95 % confidence intervals)

sTfR, soluble transferrin receptor; CRP, C-reactive protein.
* Adjusted for baseline value of the same dependent variable.
† Adjusted for the baseline value of the same dependent variable and log-CRP at week 6 for the Fe markers.
‡ Adjusted for the baseline value of the same dependent variable, log-CRP at week 6 for the Fe markers, sex, site, age, baseline CD4 count, being on tuberculosis medicine at recruitment, taking Fe supplements in addition to the study supplement, length of time from recruitment to antiretroviral therapy and baseline BMI.
§ Number restricted to the same sample as in the fully adjusted Model 3.
∥ The coefficient shows the effect associated with the intervention on week 6 outcomes in comparison to the control.
¶ Two sample t test.
Table 4 shows the extent to which the intervention effect differed for patients, based on their baseline Fe marker category. The coefficient shows the change in week 6 Fe marker associated with the intervention in comparison to the control within the baseline Fe marker category strata. There was no evidence that the impact of the intervention on Hb at week 6 was affected by baseline Fe marker category (P>0·18 for interaction tests). Amongst those with moderate and severe anaemia at baseline, the intervention was associated with a decrease in 0·40 of log serum ferritin at week 6 (P= 0·023). However, evidence for an overall interaction between the intervention and baseline Hb on log serum ferritin was weak (P= 0·12). There was no evidence of any interaction between the intervention and baseline Fe marker categories on sTfR at week 6 (P>0·52 for interaction tests).
Table 4 Linear regression models showing the effect of the intervention on iron markers at week 6, stratified by baseline iron marker category*

sTfR, soluble transferrin receptor.
* Adjusted for the baseline value of the same dependent variable, log-C-reactive protein at week 6 for the Fe markers, sex, site, age, baseline CD4 count, being on tuberculosis medicine at recruitment, taking Fe supplements in addition to the study supplement, length of time from recruitment to antiretroviral therapy and baseline BMI.
† Hb categories defined as normal ≥ 120 g/l for women and ≥ 130 g/l for men, mild anaemia < 120 g/l for women and < 130 g/l for men, moderate and severe anaemia < 110 and < 80 g/l respectively for both sexes. Serum ferritin median = 752 μg/l. sTfR median = 45 nmol/l.
‡ The coefficient shows the change in week 6 Fe marker associated with the intervention in comparison to the control within the baseline Fe marker category strata.
§ Two sample t test
∥ Likelihood ratio test comparing models with and without the interaction between trial arm and baseline Fe marker category.
At both baseline and week 6, Hb was negatively correlated with serum CRP, serum ferritin was positively associated with serum CRP and there was no correlation between sTfR with serum CRP (Table 5). At both time points sTfR was negatively correlated with Hb and serum ferritin. Serum ferritin was not correlated with Hb at baseline, but showed a weak positive correlation at week 6.
Table 5 Pairwise correlation matrix between iron markers and C-reactive protein (CRP) at baseline and week 6, unadjusted† ‡
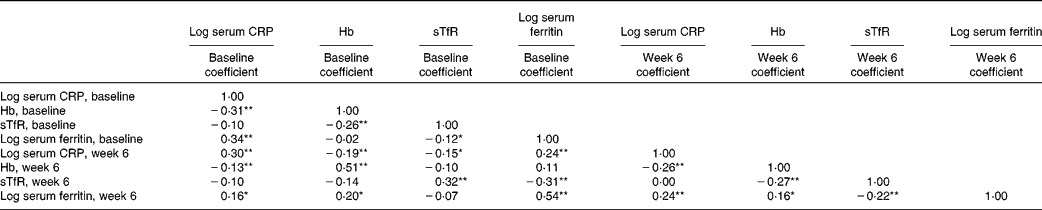
sTfR, soluble transferrin receptor.
* P< 0·05.
** P< 0·01.
† Pearson's correlation.
‡ n: serum CRP baseline (1762), Hb baseline (1670), sTfR baseline (353), serum ferritin baseline (353), serum CRP week 6 (863), Hb week 6 (826), sTfR week 6 (186), serum ferritin week 6 (165). Note that CRP and Hb were available for the whole trial sample, sTfR and ferritin only for the subsample, and sample sizes for the individual variables are determined by the availability of completed week 6 data.
Table 6 shows the associations between changes in serum CRP and changes in Fe markers. A decrease in one-log of serum CRP from baseline to week 6 was associated with an increase of 1·81 g/l of Hb (95 % CI 0·85, 2·76; P< 0·001) and a decrease of 0·11 log of serum ferritin (95 % CI − 0·20, 0·03; P= 0·012) from baseline to week 6. There was no association between the change in serum CRP and the change in sTfR over the same time period (P= 0·78).
Table 6 Multivariable linear regression model showing the effect of a one-log decrease in C-reactive protein (CRP) on change in iron markers from baseline to week 6*
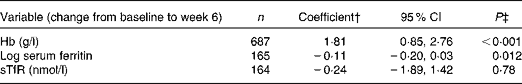
sTfR, soluble transferrin receptor.
* Adjusted for trial arm, sex, site, age, baseline CD4 count, being on tuberculosis medicine at recruitment, taking Fe supplements in addition to the study supplement, length of time from recruitment to antiretroviral therapy and baseline BMI.
† The coefficient represents the change in Fe marker from baseline to week 6 associated with a one-log decrease in CRP from baseline to week 6.
‡ Two sample t test score result.
Discussion
We hypothesised that the two-stage nutritional intervention involving a stabilisation phase followed by the provision of Fe together with other micronutrients would help reverse anaemia of chronic disease and improve Fe deficiency anaemia among malnourished, HIV-infected adults in sub-Saharan Africa. Contrary to expectations, the present results show that the intervention with fortified LNS-VM made no overall difference to Hb or any Fe markers. Furthermore, there was no obvious subgroup, defined by baseline anaemia, serum ferritin or sTfR, which demonstrated any clinically meaningful improvement from the intervention. Although there was weak evidence to suggest that the effect of the intervention on serum ferritin at week 6 was dependent upon baseline levels of Hb, the reduction in log serum ferritin was small and there was no concomitant improvement in Hb or reduction in sTfR in this subgroup, suggesting that the finding was of no clinical significance.
In unadjusted correlation analyses between the Fe markers and CRP, it was not surprising that serum ferritin, being a positive acute phase protein, was positively correlated with CRP at both time points. The negative correlation of sTfR with Hb and serum ferritin was also to be expected, due to sTfR being a marker of tissue Fe deficiency and, more specifically, the requirement of Fe for erythropoiesis( Reference Cook, Skikne and Baynes 23 ). The linear regression model exploring the relationship between serum CRP and Hb suggested that reducing systemic inflammation between baseline and week 6 was associated with an increase of Hb over that time period. Fe metabolism involves a series of complex, tightly regulated mechanisms to ensure homeostasis, especially during infection or inflammation. Chief amongst these is the need to maintain Fe tightly protected in order to avoid oxidative damage and to limit its availability to pathogens( Reference Drakesmith and Prentice 28 ). During HIV infection the chronic inflammation causes a hepcidin-mediated redistribution of Fe within the body, a process that becomes more pronounced as the HIV stage progresses( Reference O'Brien, Kupka and Msamanga 1 , Reference Savarino, Pescarmona and Boelaert 38 ). Upregulated hepcidin inactivates ferroportin (the only Fe-efflux channel in cells) causing decreased intestinal Fe absorption as well as sequestration of Fe in macrophages( Reference Drakesmith and Prentice 28 ), thus blocking erythropoiesis. This leads to anaemia, and possibly it creates a niche for intra-cellular pathogens such as mycobacteria( Reference De Monyé, Karcher and Boelaert 14 , Reference al-Khafaji, Kralovic and Smith 39 ). The present results suggest that to reverse anaemia and normalise Fe redistribution, the source of the innate immune activation first needs to be identified and addressed, and only then, after systemic inflammation has been brought under control, will an Fe-containing nutritional intervention be likely to have an impact.
Irrespective of whether the LNS was fortified with vitamins and minerals or not, it appeared that ART plus LNS improved Hb levels and reduced serum ferritin. ART has been associated with a reduction in prevalence of anaemia in other studies( Reference Semba, Shah and Klein 40 – Reference Moore and Forney 42 ), although some ART drugs, e.g. zidovudine( Reference Kiragga, Castelnuovo and Nakanjako 43 ) which was prescribed to 26 % of NUSTART patients( Reference Rehman, Woodd and PrayGod 33 ), have increased anaemia in some patients. However, in the NUSTART context the overall mean improvement of Hb and reduction of serum ferritin was modest. For there to have been enough of a functional improvement in the distribution and use of Fe in the body, we would have expected sTfR to remain stable at least, if not drop, and yet in this context sTfR levels increased slightly. Irrespective of whether LNS was fortified with vitamins and minerals or not, the combination of LNS and ART for 6 weeks does not appear to improve sufficiently the Fe profile of our patients or reduce their systemic inflammation.
The present study has several limitations. Patients in the subsample had lower baseline Hb and were more inflamed compared to those not in the subsample. This suggests that the subsample patients were slightly sicker than those not included, and restricts the possibility of extending the results to the whole sample. Budget limitations precluded analysis of Fe markers in the full cohort and analysis of results at other time points, for example, at the end of phase 1, as well as assessment of other potentially interesting markers such as hepcidin or α1-acid glycoprotein. Since there was no control group not receiving LNS (for ethical reasons), we were unable to separate the overall impact of ART and LNS on our outcomes.
The level of Fe fortification of the LNS during stage two was modest (one recommended nutrient intake) in comparison to higher levels (usually three recommended nutrient intakes) of other micronutrients. This was a conservative approach to avoid potentially increasing the risks associated with higher serum ferritin stores. It would appear that the level of Fe included in the fortified LNS was safe in this regard, since there was no overall increase in serum ferritin from the intervention. That said, we would recommend that Fe dosage within fortified LNS not be increased in future research amongst similar populations before investigating the impact this modest fortification level has, once inflammation has been successfully controlled. Further research is required: firstly, to determine whether non-nutritional interventions designed to reduce systemic inflammation are sufficient to correct anaemia of inflammation in HIV; secondly, to assess whether a product with a different nutrient composition may also assist this process; and thirdly, to quantify the level of improvement in inflammation necessary before a nutritional intervention will improve Fe deficiency anaemia.
Conclusion
Our large clinical trial of Fe supplementation as part of a nutritional intervention showed no appreciable effect on Hb and Fe metabolism, even when the majority of patients were anaemic at baseline. HIV-related inflammation resulting in disordered Fe metabolism appears to severely attenuate the potential impact of receiving dietary Fe in an intervention. Given the clear associations between anaemia, disordered Fe metabolism and mortality amongst HIV-positive patients starting ART, it is of critical importance that strategies to reduce the level of systemic inflammation (going beyond the provision of ART) are investigated. Without the ability to control inflammation, it would appear that the impact of a type of nutritional intervention similar to the one used in the present study would remain severely restricted.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114515001920
Acknowledgements
The authors are grateful to the European and Developing Countries Clinical Trials Partnership for funding the study, and to Nutriset, Malaunay, France, for preparing the trial intervention supplements. We thank the NUSTART patients for consenting to participate in the study.
The work was conducted by the NUSTART study team which includes: Suzanne Filteau, the principal investigator; Aase Bengaard Andersen, John Changalucha, Henrik Friis, Douglas C. Heimburger, Lackson Kasonka and Paul Kelly, senior investigators; John R. Koethe, Daniela Manno, Natasha Larke, Andrea M. Rehman and Susannah Woodd, statisticians and other senior research fellows; David Thurnham and Andrew Tomkins, the steering group; George PrayGod, the Mwanza trial manager; Molly Chisenga and Joshua Siame, the Lusaka trial managers; Jeremiah Kidola, Denna Michael, Kelvin Musa, Charles Masilingi, Elizabeth Fue, Eva Masesa and Neema Mpandachalo, the Mwanza senior clinic team; Anne Kanunga, Likando Munalula, Brenda Kapinda and Nellie Sikanyika, the Lusaka senior clinic team; Julius Mngara, George Ogweno, Piu Ikigo, Mutinta Muchimba, Memory Samwinga, Ellen Besa, Leo Beacroft, Harry Black and Celeste Gregg Smith, laboratory technicians; Caroline Chisenga, Marlene Hebie, Derek Munkombwe and Gemma Sampson, postgraduate students; Yolanda Fernandez, Gunda Wandore, Aswile Jonas, Hildah Banda Mabuda and Wakwoya Adugna, administrators and data managers; Stephen Makandilo, Mwangana Mubita and Jessy Mulenga, pharmacists. We are grateful also to nurses, data entry clerks, drivers and other support staff at both the NUSTART sites.
The European and Developing Countries Clinical Trials Partnership (grant no. IP.2009.33011.004) funded the present study, but had no role in the design, analysis or writing of the present article.
The authors' contributions are as follows: S. F., P. K. and H. F. designed the study; P. J. performed the data analyses with input from A. M. R. and S. W.; P. J. wrote the first draft of the manuscript. All the authors were involved in the interpretation of results and the editing of the final version of the manuscript. All the authors read and approved the final version of the manuscript.
The authors declare no conflicts of interest.











