The year is 2021, more than 70 years after the first systematic attempt to investigate dietary intake in the Mediterranean region, undertaken by the Rockefeller Foundation at the initiative of the Greek government in 1948. Leland Allbaugh(Reference Allbaugh1) was commissioned by the foundation to undertake an epidemiological study in Crete to determine how best to raise the standard of living of the population. From 7-d food intake records obtained from over 500 people in 128 households, it was found that plant foods (cereals, pulses, nuts, potatoes, vegetables and fruits) accounted for 61 % of total energy intake (TEI), animal foods (meat, eggs, fish and dairy products) accounted for 7 % of TEI; table fats and oils accounted for 29 % of energy, of which 78 % came from olive oil (OO). Total fats accounted for 38 % of the TEI. However, even from the years before the 1960s, there was not one but different food styles in the Mediterranean region due to differences in soil, farming techniques and cooking procedures. For example, whole fat content varies from less than 30 % of TEI in the traditional diet of southern Italy to about 40 % in Crete, where the diet is rich not only in OO but also in n-3 fatty acids from oily fish. The diet is still different in the southern Mediterranean countries (Algeria, Egypt, Israel, Lebanon, Libyan Arab Jamahiriya, Morocco, Syria and Tunisia). What was common to Mediterranean countries was outdoor physical activity, conviviality and seasonality, now included in the most recent versions of the Mediterranean Diet (MedDiet) pyramid. Recall that in ancient Greek, ‘δίαιτα’ meant ‘lifestyle’. The purpose of this review is to describe the history of research on the relationship of the MedDiet with cardiovascular health since its inception in the early 1950s. We will also try to describe how the MedDiet concept has evolved since that time, to ask whether there is a single MedDiet or many MedDiets, and how this concept can be applied today in the Mediterranean region and beyond.
In the early 1950s, Ancel Keys, founder in 1939 of the Laboratory of Physiological Hygiene at the University of Minnesota School of Public Health(2), was impressed by new reports of an apparent ‘epidemic’ of heart attacks among executives in the USA, as well as reports of declining and then increasing heart-related deaths in northern Europe during and after World War II. He undertook a study of healthy executives in Minnesota: The Minnesota Business and Professional Men study(3). This was the first systematic prospective cohort study to identify factors predictive of heart attack in healthy participants. The study focused on a single socio-economic class group and sought to describe only differences within that group. The primary objective was to compare the characteristics of CHD development in men who developed CHD and those who did not. The investigators examined a group of approximately 300 men aged 45–55 years and 200 men younger than 45 years. After determining eligibility to participate, the fifty most overweight, fifty most underweight and fifty most physically active men were selected to participate. A random draw selected the remaining 150 subjects. The subjects were first examined in the fall of 1947; the final number of participants was 281. These subjects underwent annual physical examinations during the 15 years of the study and again in 1983(Reference Keys, Taylor and Blackburn4–Reference Keys6). The major factor associated with CHD at the 15-year follow-up was serum cholesterol. This observation was the basis for several other studies by A. Keys. One of the pitfalls of this study was the very low statistical power.
First steps in the research
The real story of the relationship between the MedDiet and health begins in 1951, when Ancel Keys, then a visiting professor at Oxford, was invited FAO (Food and Agriculture Organization of the United Nations) after the end of the Second World War(Reference Moro7). Keys, at that time, was a very famous nutritionist and physiologist because he had invented Ration K used by the US Army to feed thousands of soldiers during the Second World War(Reference Oransky8), and because he had undertaken the very important study ‘The Biology of Human Starvation’(Reference Keys, Brožek and Henschel9). Keys was invited to lead the inaugural session and found himself with all the colleagues talking about nutritional deficiencies. He asked a question, that in the USA they had the opposite problem, because at that time they had a great scientific conundrum: the 50 % of American adult men in 1951 would die of a heart attack, the causes of which were still unknown. The only one to comment was Gino Bergami of the University Hospital of Naples. As Ancel Keys writes in his private recollections(Reference Keys10), Professor Bergami told him that there were no cases of CVD or heart attack in Naples, if not very rare. He did not know why either, but it might have been worth investigating in that direction. Keys resumed his trip from Rome, returned to Oxford and decided to send a telegram to Bergami to tell him whether the data he had provided were correct. Bergami replied that the data were correct, but that he could test it himself. Keys told him that he would come with his wife Margaret, a biologist at the Mayo Foundation. The couple arrived in Naples in 1952 and began an initial screening of the Neapolitan male population equivalent to that studied in the USA (adult men between 39 and 59 years of age). They confirmed the very low rate of myocardial infarction (MI), except in the small class of rich people.
Ancel and Margaret Keys took plasma samples from Neapolitan steel mill workers and compared their plasma cholesterol with that of wealthy Neapolitans and men from Minnesota. Plasma cholesterol was significantly lower by 40–50 mg/l in Neapolitan workers eating 20 % less fat than in wealthy Neapolitans and men living in Minnesota (eating 40 % fat)(Reference Keys, Fidanza and Scardi11). They began to observe what the workers ate, and the first evidence was that they ate meat only once a week, on Sunday night. This was the meat of a traditional pasta sauce, called ragout, which was, and still is, obligatory on Sunday noon when families gather around the table. As E. Moro(Reference Moro7) writes: ‘the Keys began to take note of what the common people ate: lots of vegetables, legumes, broccoli, all kinds of fruit, unrefined grains, dairy products, but very little fish and meat. Neapolitans loved soups, minestre and minestrone, made with vegetables, OO and a little pasta. The Keys found bean soup, pumpkin soup, green pea soup, zucchini soup. Those who could afford pasta would add a few spoonfuls of so-called short pasta, not long spaghetti, but small pasta. If there was no pasta, they used rice, hard bread, or what’s called biscotto or fresella – a whole grain bread, baked twice to make it very dry, so it could be stored for long periods of time’. The Keys quickly came to the conclusion that food was probably the key factor(Reference Moro7). Keys repeated the observation in Madrid: poor men who got 22 % of their energy content from fat had plasma cholesterol levels 40–50 mg/l lower than wealthy men with diets similar to those of the Minnesota men (40 % fat)(Reference Keys, Vivanco and Minon12). In suburban London men eating 35 % fat, plasma cholesterol was similar to that of American men(Reference Keys and Keys13,Reference Keys14) . Keys therefore concluded that there was a relationship between fat intake and plasma cholesterol. These data suggested that plasma cholesterol concentration was primarily related to the fat content of the diet. Next, Keys showed a curvilinear relationship between death rates from degenerative heart disease and % fat in the diet (10–40 % TEI) based on six countries (Japan, Italy, England, Australia, Canada and the USA)(Reference Keys15). Deaths from CHD were ten times higher in the USA than in Japan. He published his data and his hypothesis of a relationship between diet and plasma cholesterol and between plasma cholesterol and CHD deaths in 1953 at a symposium in New York. In another paper in 1953, he discussed the type of fatty acids, the role of physical activity and the lower incidence of CHD in women, and proposed that these observations be used as a basis for further studies(Reference Keys14). In the years 1957–1959, Keys et al. undertook, in Minnesota, several comprehensive studies in groups of men fed diets containing various kinds and amounts of fats and observed that serum cholesterol was positively related to the intake of SFA, inversely to PUFA and that MUFA were neutral(Reference Keys, Anderson and Grande16–Reference Keys, Anderson and Grande18). These data were later confirmed by Hegsted et al. in 1965 in Boston(Reference Hegsted, McGandy and Myers19). Keys observations, and those of other scientists over the course of early 1950s, of contrasted differences in eating patterns, nutrient intake, blood cholesterol and CHD statistics in Italy, Spain, Yugoslavia, South Africa and Japan by social or occupational class were the basis of the conceptual idea that dietary factors (especially fatty acids), plasma cholesterol and deaths and CHD were linked(Reference Blackburn, Kromhout, Menotti and Blackburn20).
The Seven Countries Study
To confirm this hypothesis, Keys and colleagues conducted the Seven Countries Study (SCS), which included 12 763 healthy men aged 40–59 years between 1958 and 1964. They were enrolled in one of sixteen cohorts from seven countries. Eleven cohorts consisted of men living in rural areas of Finland, Greece, Italy, Yugoslavia and Japan, two of railroad employees living in the USA and Italy, one of men working in a large cooperative in Serbia, one of university professors in Belgrade and one of men living in a small market town in the Netherlands(21,Reference Menotti, Kromhout, Menotti and Blackburn22) . Details of the cohorts and their characteristics are presented in Table 1. The SCS was an ecological study, which means that only data from average populations, not individual data, were used to establish correlations. After a 15-year follow-up, the rate of CHD was thirty-two times higher in eastern Finland than in Crete(Reference Keys, Menotti and Karvonen23). Differences in mean age, blood pressure, serum cholesterol and smoking explained 80 % of the variance in CHD deaths between cohorts. All death rates were positively related to the percentage of TEI from SFA on the one hand and negatively related to the percentage of MUFA, oleic acid and the MUFA/SFA ratio on the other hand. No association was found with PUFA intake. Deaths from all causes and CHD were low in cohorts in which OO was the major dietary fat. The age-standardised CHD mortality rate per 10 000 persons over 15 years was 2·3 times lower in the rural Mediterranean European cohorts (Dalmatia, Monte Giorgio, Crevalcore, Crete and Corfu) than in their rural non-Mediterranean European counterparts (Eastern and Western Finland, Suwonia and Velika Krsna). These nine cohorts were composed of rural Europeans, including farmers and men in other occupations common to European villages, that is, builders, truck drivers, local merchants and a few government officials. These cohorts differed little in physical activity, family structure and smoking, but they differed in diet. Five cohorts were Mediterranean: OO provided 15–30 % of total dietary energy, with wine providing an average of 8–20 % of energy content. In contrast, the four rural cohorts from Finland and Yugoslavia consumed mostly fat from milk and meat, and alcoholic beverages were beer and distilled liquor. Mediterranean people ate more fresh fruit and vegetables than people from other regions. Thus, after 15 years of follow-up, the SCS strongly suggested by ecological analysis that the diet consumed in the Mediterranean European cohorts may be protective at least for CHD deaths. After analysis of the 15-year follow-up results, Keys and his wife published a book in 1975 entitled ‘How to eat well and stay well. The Mediterranean way’(Reference Keys and Keys24). This was the first time the term ‘Mediterranean Diet’ was used and described. It should be noted that the term ‘Mediterranean way’ was used instead of MedDiet because ‘diet’ was considered restrictive in the opinion of potential readers, and because ‘way’ was more in line with the Mediterranean lifestyle, which is not limited to diet alone.
Table 1. The sixteen cohorts of the Seven Countries Studies. ‘The cohorts were chosen to represent cultures with apparent contrasts in lifestyle, eating habits, risk factors levels, and, presumably, incidence of and mortality from CHD, through the latter was unknown’ (adapted from ref. [Reference Menotti, Kromhout, Menotti and Blackburn22])

A further analysis of SCS, after 25-year follow-up, brought a confirmation of the negative correlation between characteristics of diet consumed in Mediterranean cohorts and CHD deaths(Reference Kromhout, Menotti and Bloemberg25). After a 25-year follow-up, 47 % of men included in SCS died. Population death rates from CHD showed large differences, ranging from 268 per 1000 in East Finland to 25 per 1000 in Crete(Reference Menotti, Kromhout and Blackburn26) (Table 2). Dietary samples had been taken at the beginning of the study and analysed 25 years later in 1987 from equivalent food composites(Reference Menotti, Kromhout and Blackburn26). As reported by Menotti and Puddu(Reference Menotti and Puddu27), baseline dietary data showed large differences in saturated fat intake across cohorts, which was high in Finland, the Netherlands and the USA, and low in Southern European cohorts especially the Mediterranean ones and in Japan. The Finnish cohorts were characterised by a high consumption of milk, potatoes, edible fat and pastries; a similar pattern, but with lower intakes, was seen in the Netherlands; meat, pastries and fruit consumption was common in USA; cereals and wine consumption was typical in Italy; bread consumption was high in the Yugoslavian cohorts except in Belgrade, with a large amount of vegetables and fish in Dalmatia; OO and fruit consumption was high in Greece; fish, rice and soya products were typical of the Japanese cohorts. Nutrients analysis revealed a very huge difference in the amount of SFA consumed between cohorts at entry in the study (3·8 % of TEI in Japan cohort v. 22·7 % in East Finland). The average intake of all major SFA as a whole was strongly positively associated with CHD deaths (r 0·88; P < 0·001) as well as individually lauric, myristic, palmitic, stearic acids (r 0·81–0·86; all P < 0·001). C18:1 T trans-fatty acid was also associated with CHD mortality (r 0·78; P < 0·001). Association with dietary cholesterol was weaker (r 0·55; P < 0·05). In multivariate analysis models, only SFA, antioxidants, flavonoids and smoking were independently associated with CHD mortality rates; SFA were the major determinant explaining 73 % of the total variance. Multivariate analysis selected butter, lard + margarine and meat as significant predictors of CHD mortality rate (r 2 0·922). Analyses of eighteen food groups, rather than nutrients, showed that a factor score extracted by factor analysis closely correlated with 25-year CHD mortality across the sixteen study cohorts using ‘ecologic’ comparisons(Reference Menotti, Kromhout and Blackburn26). This study established the beginnings of the demonstration of the potentially protective role of the MedDiet. Later, in 2004, Fidanza et al. (Reference Fidanza, Alberti and Lanti28) produced the Mediterranean Adequacy Index (MAI), computed it systematically for all of the cohorts of the Seven Countries Study, and related it to the 25-year CHD death rates (Table 3) (Fig. 1). The MAI was computed by dividing the sum of the total energy percentages of the food groups typical of a Reference MedDiet (bread, cereals, legumes, potatoes, vegetables, flesh fruit, nuts, fish, wine, vegetable oils) by the sum of the total energy percentages of the food groups that are much less typical of the Reference MedDiet (milk, cheese, meat, eggs, animal fats and margarines, sweet beverages, cakes/pie/cookies, sugar). The higher the MAI, the greater the amount of energy derived from the typical Mediterranean foods. As described by Fidanza et al. (Reference Fidanza, Alberti and Lanti28), their choice of the two groups of foods was based on the data from the pilot dietary survey conducted, in 1960, in the Southern Italian population sample of Nicotera. Fidanza considered that diet as the Reference Italian Mediterranean Diet(Reference Fidanza29). The components of the diet in Nicotera(Reference Fidanza, Alberti and Lanti28), expressed as percentage of TEI, were as follows: a high cereal intake (50–59 %), virgin OO (13–17 %), vegetables (2·2–3·6 %), potatoes (2·3–4·4 %), legumes (3–6 %), a moderate fruit intake (2·6–3·6 %, including nuts representing about 3 % of the weight of all fruit), fish (1·6–2·0 %) and red wine (1–6 %). Meat (2·6–5·0 %), dairy products (2–4 %), eggs and animal fats were rarely eaten. Of interest, Hertog et al. (Reference Hertog, Kromhout and Aravanis30) found a significant (r 2 0·25, P = 0·048) negative correlation between flavonoids intake at baseline in the sixteen cohorts and age-adjusted CHD deaths after 25 years of follow-up (Fig. 2). Flavonoids intake was not correlated with other causes of deaths. At the same time, SFA intake was positively correlated with CHD mortality (r 2 0·66, P < 0·001) (Fig. 3) but not total mortality (r 2 0·22, P = 0·068). Anecdotally, Nicotera was a poor rural area in the south, perched on a spur in the mountains overlooking the Tyrrhenian Sea about 60 km north of Reggio Calabria, near the toe of Italy. In 1960, Nicotera was the first pilot village of the areas of the Seven Countries Study, but because of a shortage of money and then because of its similarity with the two rural areas in Greece, it was not finally included in the SCS.
Table 2. Age-standardized 25-year death rates per 1000 from CHD in sixteen cohorts of the Seven Countries Study (from ref. [Reference Menotti, Kromhout and Blackburn26]) (Numbers)
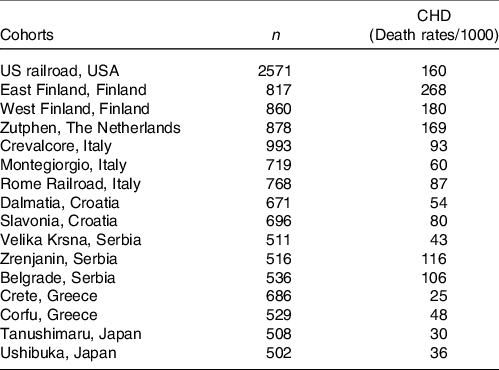
Table 3. Mediterranean Adequacy Index (MAI) of diets consumed by random samples of men and age-standardised 25-year CHD death rates per 1000 in sixteen cohorts of the Seven Countries Study (from ref. [Reference Fidanza, Alberti and Lanti28]) (Numbers)
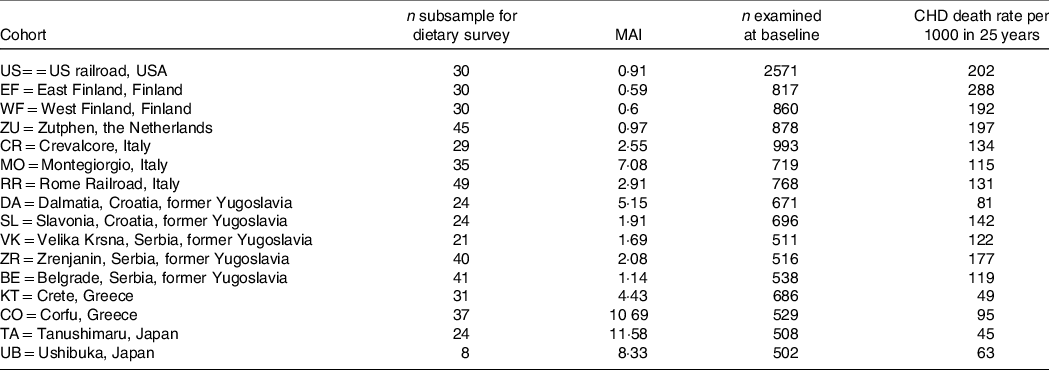
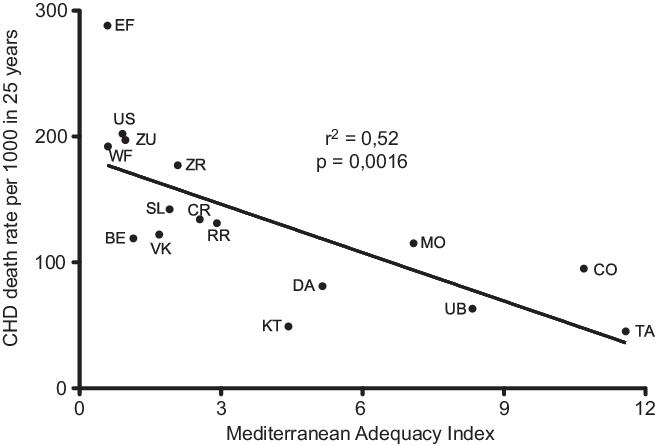
Fig. 1. Correlation of Mediterranean Adequacy Index with CHD rate per 1000 after 25-year follow-up in the sixteen cohorts of the Seven Countries Study. (EF = East Finland, Finland; WF = West Finland, Finland; Zu = Zutphen, The Netherlands; US = US Railroad, USA; BE = Belgrade, Serbia (former Yugoslavia); ZR = Zrenjanin, Serbia (former-Yugoslavia); CR = Crevalcore, Italy; VK = Velika Krsna, Serbia (former Yugoslavia); RR = Rome Railroad, Italy; SL = Slavonia, Croatia (former Yugoslavia); DA = Dalmatia, Croatia (former Yugoslavia); KT = Crete, Greece; CO = Corfu, Greece; MO = Montegiorgio, Italy; UB = Ushibuka, Japan; TA = Tanushimaru, Japan) (reconstructed from data of ref. [Reference Fidanza, Alberti and Lanti28]).
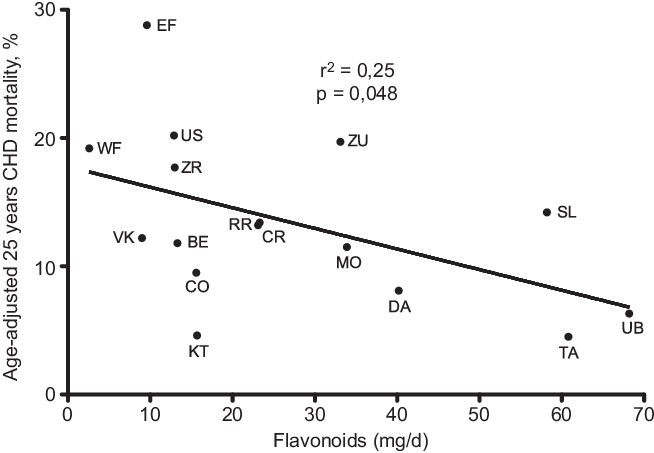
Fig. 2. Correlation of flavonoids with age-adjusted 25 years CHD mortality after 25-year follow-up in the sixteen cohorts of the Seven Countries Study. (EF = East Finland, Finland; WF = West Finland, Finland; Zu = Zutphen, The Netherlands; US = US Railroad, USA; BE = Belgrade, Serbia (former Yugoslavia); ZR = Zrenjanin, Serbia (former-Yugoslavia); CR = Crevalcore, Italy; VK = Velika Krsna, Serbia (former Yugoslavia); RR = Rome Railroad, Italy; SL = Slavonia, Croatia (former Yugoslavia); DA = Dalmatia, Croatia (former Yugoslavia); KT = Crete, Greece; CO = Corfu, Greece; MO = Montegiorgio, Italy; UB = Ushibuka, Japan; TA = Tanushimaru, Japan) (reconstructed from data of ref. [Reference Hertog, Kromhout and Aravanis30]).
Lastly, after 50 years of follow-up(Reference Kromhout, Menotti and Alberti-Fidanza31), the strong negative correlation between MAI at entry in the study and CHD mortality (r –0·91) was confirmed as well as the very strong positive correlation (r 0·92; P < 0·05) of SFA intake with CHD mortality. It was not possible to establish at individual level a correlation between dietary FA and death or CHD.
Data from SCS, beyond its great merits, necessitate some comments. It was an ecologic study, which intrinsically did not establish individual correlations as others cohorts did, such as Nurses Health Study or Health Professionals’ Follow-up Study by example(Reference Delarue32); correlations (positive with SFA and negative with MAI) do not prove a link of causality; Japanese cohorts from SCS displayed an even lower incidence of CHD mortality than Mediterranean cohorts, whereas their diets have common but also different food patterns. As written by Menotti and Puddu(Reference Menotti and Puddu27) in their review describing how the SCS contributed to the definition and development of the MedDiet concept: ‘despite the size, the uniqueness and wealth of findings, the Seven Countries Study has particular limits, mainly too few cohorts and statistical units (16 cohorts) available for ecological analysis and the lack of dietary data on all individuals at baseline, rather on sampled families and individuals at entry examination. The choice of the countries studied has been questioned and was based on the need to compare cultures contrasting widely in diet, as well as the availability of resources and of local collaborators experienced and capable of joining such a long and extensive project’. However, concerning the ‘ecologic’ aspect of SCS, its results were confirmed by another ecologic study performed in forty countries, including the seven ones of SCS(Reference Artaud-Wild, Connor and Sexton33). That study found the same positive correlation between cholesterol plus saturated fat intake and CHD mortality (r 0·78), as well as the same correlation coefficients of group foods as those found in SCS.
Keys in his comments of SCS results(Reference Keys, Menotti and Karvonen23) was always cautious to indicate that the correlations between dietary fat and deaths and CHD were not a demonstration of a causal relationship: ‘It is not claimed that the differences in death rates are caused by the dietary differences; interrelations with other unidentified variables could be involved. But if the relationships reported here actually depend on differences among the cohorts in other characteristics, it would seem that the dietary characteristics must be closely related to those other unidentified variables’.
However, during the following years until very recently, SFA have been considered as a risk factor for CHD(Reference Delarue32). Several meta-analysis published in the last 10 years did not confirm the individual relationship between SFA and plant PUFA with CHD. However, substitution studies replacing 5 % of SFA with plant PUFA or whole grain showed a decreased CHD risk, so that SFA cannot be considered as ‘neutral’ towards CHD risk. After 45-year follow-up in Seven Countries Study, 92·9 % of participants had died. The age of death from CHD was 80·9 years in Crete v. 68·0 years in East Finland: a 12·9 years difference(Reference Menotti, Puddu and Tolonen34); the ratios of fatty acids, vegetable oils, vegetable foods and the MAI were inversely related to all-cause mortality rates(Reference Kromhout, Menotti and Alberti-Fidanza31,Reference Menotti, Kromhout and Puddu35) . This suggested that the protective MUFA and PUFA, vegetable oils and vegetable foods are indicators of healthful diets as operationalised in the MAI diet score. A study from Greece showed using a 10-point MedDiet scale that after a follow-up of 44 months, the adherence to a traditional MedDiet was associated with a 25 % reduction in total mortality (relative risk (RR): 0·75; 95 % CI 0·64, 0·87). This was observed for both CHD mortality (RR: 0·67; 95 % CI 0·47, 0·94) and cancer mortality (RR: 0·76; 95 % CI 0·59, 0·98)(Reference Trichopoulou, Costacou and Bamia36).
Trials and observational studies after the Seven Country Study and up to date
The Lyon Diet Heart study(Reference de Lorgeril, Salen and Martin37) was a secondary prevention French single-blind trial. Patients with previous MI were separated in two groups. The experimental group (n 302) received a Mediterranean-type diet: more bread, more root vegetables and green vegetables, more fish, less meat (beef, lamb and pork to be replaced with poultry, no day without fruit. Butter and cream had to be replaced with a rapeseed oil-based margarine whose composition was comparable to OO with 15 % SFA, 48% oleic acid but 5–4% 18:1 trans, 16·4 % LA and 4·8% ALA). Seasoning oils were rapeseed and OO exclusively. Moderate wine consumption was allowed at meals. The control group (n 303) received a low-fat step 1 diet of National Cholesterol Education Program for secondary prevention. The National Cholesterol Education Program diet recommends less than 30% of energy from total fat, less than 10% from saturated fat and less than 300 mg/d of cholesterol. The RR for the major primary end points: cardiac deaths and cardiac deaths + non-fatal MI was 0·35 (95% CI 0·15, 0·83; P = 0·01) and 0·28 (95% CI 0·15, 0·53; P < 0·001), respectively.
The Indo-Mediterranean Diet Heart Study(Reference Singh, Dubnov and Niaz38) was both a primary (patients with surrogate markers of CV risk) and secondary prevention trial (patients with an history of CHD) with a 2-year follow-up. All patients were randomly allocated to two groups: one receiving an Indo-Mediterranean Diet (n 499) and the other as control a low-fat step 1 National Cholesterol Education Program diet (n 501). The Indo-Mediterranean Diet was rich in whole grains, fruits, vegetables, walnuts and almonds (250–300 g/d of fruit, 125–150 g/d of vegetables and 25–50 g/d of walnuts or almonds). The experimental group was also encouraged to eat 400–500 g/d of whole grains, legumes, rice, maize and wheat, as well as mustard seed or soyabean oil, in three to four servings per day. The RR for non-fatal MI, fatal MI, sudden cardiac death and total cardiac end points was 0·47 (95 % CI 0·28, 0·79), 0·67 (95 % CI 0·31, 1·42), 0·33 (95 % CI 0·13, 0·86) and 0·48 (95 % CI 0·33, 0·71) respectively. However, the Lancet published in 2015 an expression of concern(Reference Horton39) because of lack of clarity about original data; other concerns have been addressed by Martínez-González(Reference Martínez-González, Gea and Ruiz-Canela40) considering it was reasonable to consider Indo-Mediterranean Diet study as discredited, even though it has been included in many reviews and meta-analysis.
The Global Secondary Prevention strategies to Limit event recurrence after MI (GOSPEL study)(Reference Giannuzzi, Temporelli and Maggioni41,Reference Giannuzzi, Temporelli and Marchioli42) was a multicentre randomised controlled trial (RCT) study carried out in seventy-eight Italian cardiac rehabilitation centres. After completion of an initial cardiac rehabilitation programme, patients with recent (< 3 months) MI were randomised to either a long-lasting (over 3 years) multifactorial continued educational and behavioural programme (intensive approach) or usual care (control) group. The intensive approach was multimodal including a MedDiet, lifestyle recommendations, control of all CV risk factors plus CV medications. For patients in the control group, a letter was sent to the primary care physician recommending secondary prevention goals. There was no attempt by the research cardiologist to aid in lifestyle adherence or in up-titration of cardioprotective medications. Thus, MedDiet was not the sole factor differentiating the two experimental groups. The combination of CV mortality, non-fatal MI, non-fatal stroke and hospitalisation for angina pectoris, heart failure or urgent revascularisation procedure was the primary end point. Other end points were major CV events, major cardiac and cerebrovascular events, lifestyle habits and drug prescriptions. The intensive intervention did not decrease the composite primary end point (RR = 0·88; 95 % CI 0·74, 1·04), but decreased CV mortality plus non-fatal MI and stroke (RR = 0·67; 95 % CI 0·47, 0·95), cardiac death plus non-fatal MI (RR = 0·64; 95 % CI 0·43, 0·94) and non-fatal MI (RR = 0·52; 95 % CI 0·31, 0·86).
The Prevencion con Dieta Mediterranea (PREDIMED) Study(Reference Estruch, Ros and Salas-Salvadó43) is a RCT assessing the effects of a MedDiet for primary CV prevention in 7447 participants with a high CV risk (median follow-up: 4·8 years). They were assigned to a MedDiet supplemented with extra-virgin OO or a MedDiet supplemented with mixed nuts, or a control diet (advice to reduce dietary fat) (Table 4). After a median follow-up of 4·8 years, the participants assigned to a MedDiet with extra-virgin OO and those assigned to a MedDiet with nuts increased their consumption of extra-virgin OO (to 50 and 32 g/d, respectively) and nuts (to 0·9 and 6 servings per week, respectively). The primary end point was a major CV event (MI, stroke or CV death). The RR for the primary end point was 0·70 (95 % CI 0·55, 0·89). The RR for the secondary end points was 0·58 (95 % CI 0·42, 0·82) for stroke, 0·80 (95 % CI 0·53, 1·21) for MI and 0·80 (95 % CI 0·51, 1·24) for CV deaths. Thus, the RR for the composite end point was reduced by 30 %, but when separating the secondary end points, it was significant for stroke only with a reduction of 42 % of risk.
Table 4. Experimental (Mediterranean) diet used during PREDIMED study (adapted from ref. [Reference Psaltopoulou, Sergentanis and Panagiotakos44])
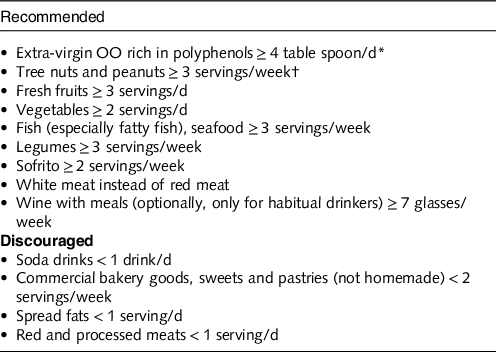
* In the group assigned to the Mediterranean diet with extra-virgin OO, the goal was to consume 50 g (approximately 4 tbsp) or more per day of the polyphenol-rich OO supplied.
† For participants assigned to the Mediterranean diet with nuts, the recommended consumption was one daily serving (30 g, composed of 15 g of walnuts, 7·5 g of almonds and 7·5 g of hazelnuts).
Several meta-analyses have been published about the effect of adherence to a MedDiet on cardiovascular health (Table 5). Psaltopoulou et al. (Reference Psaltopoulou, Sergentanis and Panagiotakos44), in 2013, performed a meta-analysis of eleven cohorts. A high MedDiet score (MDS) was associated with a 29 % reduction of stroke incidence (RR = 0·71; 95 % CI 0·57, 0·89). Sofi et al. (Reference Sofi, Macchi and Abbate45), in 2013, performed a meta-analysis of prospective cohort studies (4 172 412 subjects) assessing the association of adherence to a MedDiet with CVD incidence and/or mortality risk. A two-point increase in adherence score to the MedDiet was associated with a 10 % reduction of CV risk (RR = 0·90; 95 % CI 0·87, 0·92). Kontogianni and Panagiotakos(Reference Kontogianni and Panagiotakos46), in 2014, performed an update of meta-analysis (fourteen cohorts) of association of a high MDS with stroke and found a 42 % reduction of stroke incidence (RR = 0·58; 95 % CI 0·68, 0·79). Grosso et al. (Reference Grosso, Marventano and Yang47) performed, in 2015, a meta-analysis of prospective studies and cohorts comparing adherence to a MedDiet with CHD events and CHD deaths, CHD incidence was reported in 4 prospective studies (RR = 0·72; 95 % CI 0·60, 0·86) and MI incidence in 3 (RR = 0·67; 95 % CI 0·54, 0·83). After pooling the four RCT cited above (Lyon Heart Study, Indo-Mediterranean Study, GOSPEL Study, PREDIMED study), MI incidence, stroke incidence, CVD mortality and composite end points were all significantly decreased by 40, 36, 41 and 45 %, respectively. Liyanage et al. (Reference Liyanage, Ninomiya and Wang48), in 2016, included six RCT comparing MedDiet to a control diet (10 950 participants). Three RCT, including 9052 participants and 477 events, reported major CV events (RR = 0·63; 95 % CI 0·53, 0·75; P < 0·001). Rosato et al. (Reference Rosato, Temple and La Vecchia49), in 2017 (published in 2019), included twenty-nine observational studies. Comparing the highest to the lowest MDS, they found for total CVD (eleven studies) a RR = 0·81 (95 % CI 0·74, 0·88), and for CHD/MI (eleven studies) a RR = 0·70 (95 % CI 0·62, 0·80). Dinu et al. (Reference Dinu, Pagliai and Casini50), in 2017, published an umbrella review of meta-analyses of observational studies and randomised trials. They included thirteen meta-analyses of observational studies and sixteen meta-analyses of RCT investigating the association between the adherence to MedDiet and thirty-seven different health outcomes (12 800 000 participants). The meta-analysis of cohorts showed for CVD incidence a RR = 0·67 (95 % CI 0·58, 0·57), for CVD mortality a RR = 0·75 (95 % CI 0·68, 0·83), for CHD incidence a RR = 0·72 (95 % CI 0·60,0·86), for MI incidence a RR = 0·67 (95 % CI 0·54, 0·83) and for stroke a RR = 0·76 (95 % CI 0·60, 0·96). Martínez-González et al. (Reference Martínez-González, Hershey and Zazpe51), in 2017, performed a meta-analysis of twenty-seven cohorts plus RCT. They found that each two-point increment in MDS was associated with an 11 % reduction in the CVD risk (RR = 0·89; 95 % CI 0·86, 0·91). Eleftheriou et al. (Reference Eleftheriou, Benetou and Trichopoulou52), in 2018, performed an updated meta-analysis of prospective cohort studies to quantify the association of adherence to MedDiet with all-cause mortality. Thirty studies were included. The RR for the study-specific highest/lowest MDS was 0·79 (95 % CI 0·77, 0·81). Galbete et al. (Reference Galbete, Schwingshackl and Schwedhelm53), in 2018, performed an umbrella review of meta-analysis of cohort studies, evaluating the association of MedDiet (using adherence to a MedDiet score) with type 2 diabetes, CVD, cancer and cognitive-related diseases. They included twenty-seven meta-analyses based on seventy primary studies. Regarding CVD end points, they found twelve meta-analyses including a total of thirty-one primary studies. A two-point increase in adherence to the MDS was associated with a 28 % lower risk of CHD (RR = 0·72; 95 % CI 0·60, 0·86), MI (RR = 0·67; 95 % CI 0·54, 0·83) and a 26 % lower risk of acute MI (RR = 0·74; 95 % CI 0·66, 0·83). Rees K et al.(Reference Rees, Takeda and Martin54), in 2019, performed a Cochrane meta-analysis of RCT aiming to study the effects of Mediterranean style diet in primary prevention in subjects at high CV risk and in secondary prevention. The meta-analysis included for primary prevention nine trials (1337 participants) comparing a Mediterranean dietary intervention v. no intervention or minimal intervention for primary prevention and thirteen trials comparing MedDiet to another diet (8687 participants), and for secondary prevention two trials (706 participants) comparing MedDiet to usual care and six trials (1731 participants). The authors observed a great heterogeneity and high risk of bias. The author’s conclusion is that the quality of evidence for the modest benefits on CVD risk factors in primary prevention is low or moderate, with a small number of studies reporting minimal harms and that there is a paucity of evidence for secondary prevention. Chen et al. (Reference Chen, Neelakantan and Martín-Calvo55), in 2019, undertook a meta-analysis of twenty prospective studies evaluating the association of MedDiet with stroke. Of the twenty included studies, six were from the USA, two from Eastern Asia, five from Mediterranean countries (Italy, Greece and Spain), six from non-Mediterranean European countries, in addition to one from both five Mediterranean and non-Mediterranean European countries. Participants in three studies were high-risk individuals who had diabetes or multiple other CVD risk factors. The stroke risk was 16 % lower when comparing, for all studies, the highest MDS to the lowest (RR = 0·84; 95 % CI 0·80, 0·88). The RR was 0·84 (95 % CI 0·80, 0·88) for studies from non-Mediterranean regions and 0·74 (95 % CI 0·60, 0·92) for studies from the Mediterranean regions. A systematic overview by Saulle et al. (Reference Saulle, Lia and De Giusti56), in 2019, included sixteen narrative reviews, nine systematic reviews and six systematic reviews with meta-analyses. The authors stated that MedDiet may be a useful means of preventing stroke, especially the six meta-analyses highlighted that high adherence to MedDiet was protective against stroke, with a RR ranging from 0·64 (95 % CI 0·48, 0·88) to 0·90 (95 % CI 0·87, 0·93). Becerra-Tomás et al., in 2020(Reference Becerra-Tomás, Blanco Mejía and Viguiliouk57), undertook a meta-analysis of three RCT and thirty-eight cohorts aiming to evaluate the effect of MedDiet on the prevention of CVD incidence and mortality. Meta-analyses of RCT revealed a beneficial effect of the MedDiet on total CVD incidence (RR: 0·62; 95 % CI 0·50, 0·78) and total MI incidence (RR: 0·65; 95 % CI 0·49, 0·88).
Table 5. Meta-analyses since 2014 of observational studies and RCT reporting adherence to Mediterranean diet in relation to CV health
(Relative risks and 95 % confidence intervals)
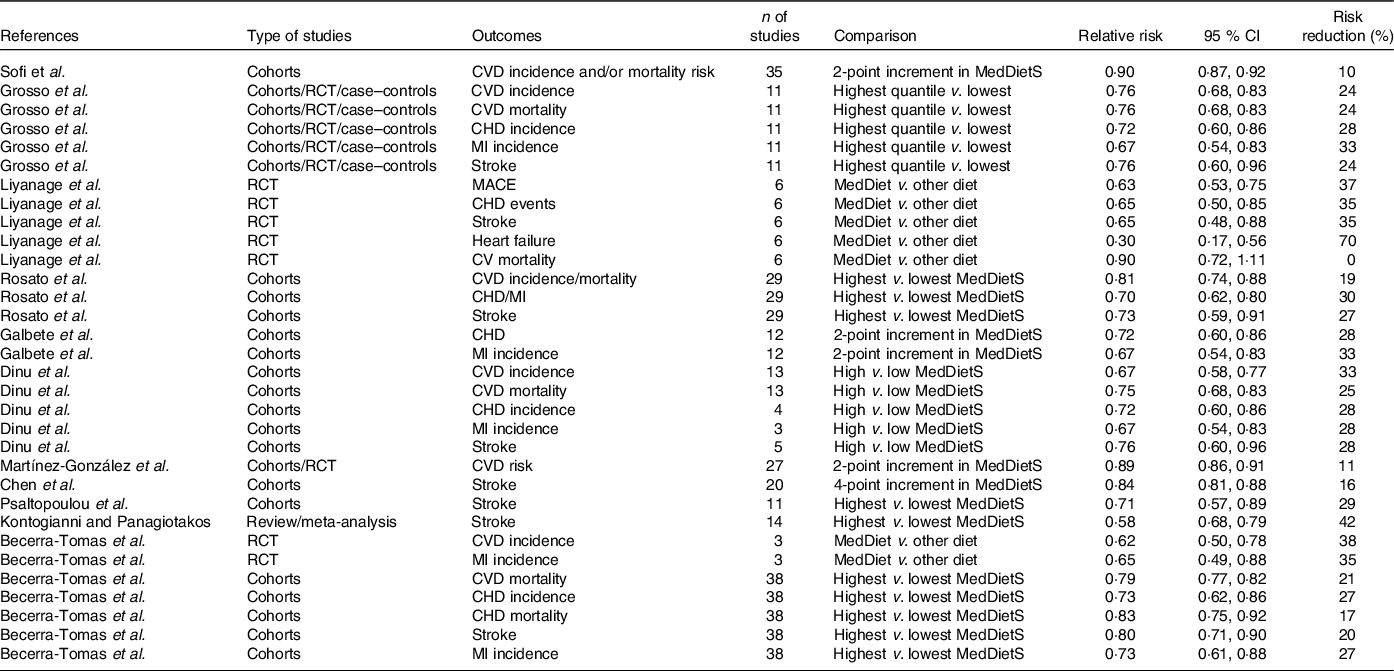
RCT, randomised controlled trials; MI, myocardial infarction; MedDietS, Mediterranean Diet score.
Meta-analyses of the prospective cohort studies, which compared the highest v. lowest categories of MedDiet adherence, revealed an inverse association with total CVD mortality (RR = 0·79; 95 % CI 0·77, 0·82), CHD incidence (RR = 0·73; 95 % CI 0·62, 0·86), CHD mortality (RR = 0·83; 95 % CI 0·75, 0·92), stroke incidence (RR = 0·80; 95 % CI 0·71, 0·90), stroke mortality (RR = 0·87; 95 % CI 0·80, 0·96) and MI incidence (RR = 0·73; 95 % CI 0·61, 0·88). In summary, all meta-analyses conclude that there is a significant reduction of CV risk (CVD, CHD, MI, stroke) (Table 5).
Challenges of adhering to the Mediterranean Diet, indexes of adherence to Mediterranean Diet, transferability to non-Mediterranean countries
Adherence to Mediterranean Diet
Traditional MedDiet was the predominant dietary pattern among populations in the Mediterranean basin before the mid-1960s(Reference Fidanza29,Reference Martínez-González, Hershey and Zazpe51,Reference Trichopoulou, Martínez-González and Tong58) . Despite the health benefits associated with MedDiet, the Western diet has gradually replaced MedDiet in Mediterranean countries, particularly among the poorest. Rubba et al. (Reference Rubba, Mancini, Gentile, Simopoulos and Visioli59), in their review of MedDiet in Italy, report several studies showing that between 1960 and 1996 Italians consumed more milk, cheese, meat, sweetened beverages, cakes/pies/cookies, and less cereals and legumes, especially in the 1980s. However, some positive trends were observed in the 1990s. These changes in the 1980s may have deleterious consequences for blood lipids and blood pressure. Ferro-Luzzi et al. (Reference Ferro-Luzzi, Strazzullo and Scaccini60) conducted a study of forty-eight healthy middle-aged men and women on a MedDiet-type diet in rural Italy. The experimental diet given for 42 d resulted from the partial replacement of usual plant foods (OO, cereals and vegetables) by foods characterised by a high saturated fat content and a low P/S ratio: butter, dairy cream, soft and hard cheeses, and small amounts of meat. The contribution of fat to TEI was increased to 40 %, and the polyunsaturated/saturated (P/S) ratio was reduced from 0·48 to 0·22. Total cholesterol, LDL-cholesterol and apoB were increased (P < 0·001). Another study by the same research group(Reference Strazzullo, Ferro-Luzzi and Siani61), carried out on the same Italian population, showed that decreasing the P:S ratio for 6 weeks to 0·22 increased systolic blood pressure by 2·6 mm Hg in men (P < 0·05) and by 4·2 mm Hg in women (P < 0·001). These results strongly suggest that a change in fat source as part of a MedDiet diet in a free-living population in rural Italy is sufficient to significantly increase some markers of CV risk. Vilarnau et al. (Reference Vilarnau, Stracker and Funtikov62) compared, using the MAI defined by Vidanza et al.(Reference Fidanza, Alberti and Lanti28), MedDiet adherence between 1960 and 2011 in forty-one countries divided into Mediterranean (Mediterranean Europe and Southern Mediterranean) and non-Mediterranean (Central Europe, Northern Europe, Other countries) country groups. The mean MAI and standard deviation values for each period for the forty-one countries were 2·35(s d 1·47), 1·51 (s d 0·88) (P < 0·05) and 1·47 (s d 0·84) (P > 0·05) for periods 1 (1961–1965), 2 (2000–2003) and 3 (2004–2011), respectively. The mean MAI values for the seventeen Mediterranean countries were 3·46 (s d 1·28), 2·03 (s d 0·90) (P < 0·001) and 2·00 (s d 0·93) (P = 0·47) for the same three periods, respectively. The mean MAI values for non-Mediterranean countries (n 24) were 1·57 (s d 1·4), 1·14 (s d 0·67) (P = 0·0053), 1·10 (s d 0·53) (P = 0·26) for the same three time periods, respectively. Between 1960 and 2011, MAI decreased in both Mediterranean Europe (3·43 (s d 1·54) v. 1·53 (s d 0·56); P < 0·001) and the Southern Mediterranean (3·48 (s d 1·01) v. 2·53 (s d 1·00); P = 0·005). In all country groups, the difference between 2000–2003 and 2004–2011 was not significant, reflecting relative stabilisation. The movement away from MedDiet was more pronounced in the Mediterranean Europe, Southern Mediterranean and Central Europe subcategories. The deterioration in MedDiet adherence was more pronounced in Mediterranean Europe than in the Southern Mediterranean, which maintained the highest adherence. It is worth noting that Greece showed the largest decrease in MAI from 5·54 to 1·87 with a drop in ranking from first place to tenth; Egypt maintained a high MAI from 4·81 to 4·36 (from fourth place to first) and Romania and Bulgaria with historically high MedDiet adherence (MAI: 3·89 and 2·68, respectively) showed a large decrease to 1·73 and 1·17, respectively. The average MAI for Central Europe was now around 1 in 2011.
The socio-economic determinants of MedDiet adherence loss were reported in the results of the Italian MOLI-SANI study(Reference Bonaccio, Di Castelnuovo and Costanzo63–Reference Bonaccio, Di Castelnuovo and Bonanni66). This single-centre population-based study collected information on 25 000 people living in the Molise region. The results showed that adherence to MedDiet was related to material resources, nutritional knowledge, exposure to mass media and education level(Reference Bonaccio, Di Castelnuovo and Costanzo63–Reference Bonaccio, Di Castelnuovo and Bonanni66). The higher these parameters, the greater the adherence to MedDiet. Interestingly, a dramatic drop from over 30 % to 18 % high adherence was observed in 2007, during the economic crisis, compared with 2005–2006. This strongly suggests that a decrease in material resources probably explains this decline. Comparing the 2005–2006 period with the 2007–2010 period (after the crisis), the authors observed that adherence was not associated with socio-economic determinants, but with age, sex, higher education and living in urban areas. In the 2007–2010 period, socio-economic determinants played a major role. Household wealth, education, rurality and occupation became associated with greater adherence. The proposed explanation was the increased cost of Mediterranean food products and/or reduced purchasing power of wages(Reference Bonaccio, Di Castelnuovo and Bonanni66). Such observations have been made in several Mediterranean and non-Mediterranean countries(Reference Bonaccio, Bes-Rastrollo and de Gaetano67,Reference Darmon and Drewnowski68) .
Indexes of adherence to Mediterranean Diet since 1995
The main utility of these indexes is their ability to assess MedDiet adherence in various study populations, and to relate it to disease or mortality risk in many countries, including non-Mediterranean countries. Several scoring systems have been defined to operationally assess the (mainly health) effects of MedDiet. Historically, the first was the MDS defined by Trichopoulou et al. (Reference Trichopoulou, Kouris-Blazos and Wahlqvist69) to assess the association between MedDiet adherence and mortality in a population of elderly people in Greece. The MDS (0–9 points) consisted of eight components: six beneficial components (monounsaturated/saturated fat ratio, vegetables, fruits and nuts, legumes, fish, cereals), two harmful components (meat/meat products, dairy products) and moderate alcohol consumption (5–25 g/d for women, 10–50 g/d for men). One point was assigned to positively weighted items if consumption was superior or equal to the sex-specific median, and one assigned to negatively weighted items if consumption was inferior to the sex-specific median. The MDS was further modified by the same authors to include fish consumption as a beneficial component(Reference Bonaccio, Di Castelnuovo and Costanzo65). The MDS has been used in many studies since its definition. The MAI defined by Fidanza et al. (Reference Fidanza, Alberti and Lanti28) for the Seven Countries Study report after 25 years of follow-up has been reported above. The PREDIMED Screener score (0–14 points) was defined by Estruch et al. (Reference Estruch, Ros and Salas-Salvadó43) for their RCT aiming to evaluate the primary prevention effects of MedDiet on CHD events in a high CV risk population (Table 4).
Several other indexes of adherence have emerged over the years until recently to study the association of MedDiet adherence with health outcomes. Searching PubMed through October 2014, Hernandez-Ruiz et al. (Reference Hernández-Ruiz, García-Villanova and Guerra Hernández70), in their review, found twenty-two indexes with differences regarding the number of components (7–28), scoring (0, 1, 2, 3, 4, 5, 8 or 10, if adherent), range (0–100) and type of components (foods, food groups, nutrients and/or lifestyle factors). Fruits and vegetables were the most common beneficial components, and meats were the most common detrimental components. Moderate alcohol consumption was common to all indexes and was considered positive, but its definition differed among indexes: 10–20 g/d, or 5–25 g/d in women and 10–50 g/d in men, or 0 g/d in women and up to 10 g/d in men. Another difference between indexes was the scoring system and the cut-off points (in medians, terciles or established portions). Milà-Villarroel et al. (Reference Milà-Villarroel, Bach-Faig and Puig71) evaluated the reliability of ten MedDiet adherence indexes, including the MDS, MAI and PREDIMED scores. They found that all ten indexes satisfactorily assessed MedDiet adherence, but that there was a lack of internal consistency among the indexes, arguing for standardisation. Sofi et al. (Reference Sofi, Macchi and Abbate45) in their meta-analysis of twenty-seven cohorts, addressing the association of MedDiet with health status, reported all selected cut-offs for different MedDiet adherence indexes. Because of the wide distribution of median consumption of some food groups (legume consumption ranged from 2 to 75 g/d), the data were log-transformed. The median (or mean) values of food group consumption comprising the MedDiet adherence score were weighted according to the number of subjects enrolled in each study. Then, the mean value of all weighted medians and the 2 standard deviations for each food group were calculated. Finally, they rounded the resulting numbers close to the 2 standard deviation values for each measure, obtaining three consumption categories for each food group. This highlights the difficulty and the value of harmonising the adherence indexes to allow for better comparability between studies.
Transferability of Mediterranean Diet to non-Mediterranean countries
Despite health benefits associated with the MedDiet, adhering to it can be challenging. Globalisation, economic, urban and technology-driven developments have led to a significant shift towards Western diet, including in low-income countries which show an increase in the prevalence of CVD. As discussed above, the MOLI-SANI(Reference Bonaccio, Di Castelnuovo and Costanzo63–Reference Bonaccio, Bes-Rastrollo and de Gaetano67) study has shown that socio-economic factors, nutrition knowledge and education were important contributors to the loss of adherence to MedDiet. This was confirmed in the UK Fenland study carried out in 12 417 British adults(Reference Tong, Imamura and Monsivais72). At cohort level, participants who had a high MedDiet adherence had higher dietary cost associated with consumption of vegetables, legumes, fruits, nuts, fish, eggs, cereals and OO. On the other hand, high MedDiet adherence was also associated with a lower diet cost related to the consumption of red meat, processed meat, potatoes, alcoholic beverages and sweets, with the biggest negative cost difference attributed to red meat consumption. Socio-economic status partially explained the observed association between MedDiet adherence and dietary cost, and significant interaction was observed between MedDiet adherence and test site, education, income and occupation. Adopting the MedDiet in non-Mediterranean countries entails difficulties relevant to cultural differences, changing established behaviours, lack of education or increased cost.
Replacing a Western-style diet with a Mediterranean-style diet has shown promising results in studies that investigated CV and cognitive effects(Reference Tsofliou, Theodoridis, Arvanitidou, Preedy and Watson73). The effect of a Mediterranean dietary pattern in secondary prevention (median follow-up 3·7 years) was studied in a population of 15 482 patients (67 (sd 9) years) from thirty-nine countries with a stable CHD(Reference Stewart, Wallentin and Benatar74). MedDiet adherence was calculated for increasing consumption of whole grains, fruits, vegetables, legumes, fish and alcohol, and for less meat, and a Western diet adherence for increasing consumption of refined grains, sweets and deserts, sugared drinks and deep-fried foods. Major Adverse Cardiac Events (MACE) occurred in 7·3 % of 2885 subjects with a MedDiet adherence score ≥ 15, in 10·5 % of 4018 subjects with a score 13–14 and in 10·8 % of 8579 subjects with a score ≤ 12. A one unit increase in the score > 12 was associated with lower MACE after adjusting for all covariates (hazard ratio: 0·95; 95 % CI 0·91, 0·98; P = 0·002). There was no association between Western diet adherence and MACE. A study carried out in Hong Kong(Reference Lau, Wong and Chan75) prospectively followed up 274 consecutive patients with stable CHD. After a mean follow-up of 77 (sd 12) months, 16·1 % of the patients developed a MACE. Patients who developed a MACE had a lower MedDiet adherence score compared with those who did not (P < 0·01). Multivariate Cox regression analysis identified a higher MedDiet score to be protective against stroke (hazard ratio: 0·48; 95 % CI 0·24, 0·94; P = 0·03). Such a relationship was independent to other confounding factors. There was no association between MedDiet adherence score and risk of acute coronary syndrome and CV mortality. The European Prospective Investigation of Cancer-Norfolk cohort(Reference Tong, Wareham and Khaw76) recruited 25 639 men and women, aged 40–79 years with no prior MI or stroke, in eastern England, and followed up 12 years. The MedDiet adherence score used, based on the Mediterranean dietary pyramid, was associated with a lower incidence of the CV outcomes (RR = 0·95; 95 % CI 0·92, 0·97 per one standard deviation for incident CVD and 0·91; 95 % CI 0·87, 0·96 for CVD mortality). The Northern Manhattan Study(Reference Gardener, Wright and Gu77) is a US primary/secondary prevention multi-ethnic study which included 2568 participants aged 68·6 (sd 10·3) years (mean follow-up of 9·0 (sd 3·5) years). The MedDiet adherence score was that proposed by Trichopoulou et al. (Reference Trichopoulou, Kouris-Blazos and Wahlqvist69). The MedDiet adherence score was inversely associated with the risk of the composite outcome of ischaemic stroke, MI or vascular death (P-trend = 0·04) and with vascular death (P-trend = 0·02). Moderate and high MedDiet adherence score was marginally associated with decreased risk of MI. There was no association with ischaemic stroke. These long-term prospective cohort studies, among others, clearly demonstrate that a Mediterranean dietary pattern is associated with lower CV events in primary and/or secondary prevention in people living in non-Mediterranean countries. Thus, it can be concluded that MedDiet CV benefits are transferable to non-Mediterranean populations.
However, what is also important is the ability of populations to adhere to a Mediterranean dietary pattern over the long term, which may involve major changes in food choices. By applying evidence-based knowledge and policies, based on the new MedDiet pyramid(Reference Serra-Majem, Tomaino and Dernini78) (Fig. 4), governments can facilitate lifestyle changes and promote all its sustainable aspects(Reference Dernini, Berry and Serra-Majem79). Several national dietary guidelines promote the Mediterranean food model and sustainability. The FAO of the United Nations has focused on diet-based dietary guidelines with key messages for many countries(80).
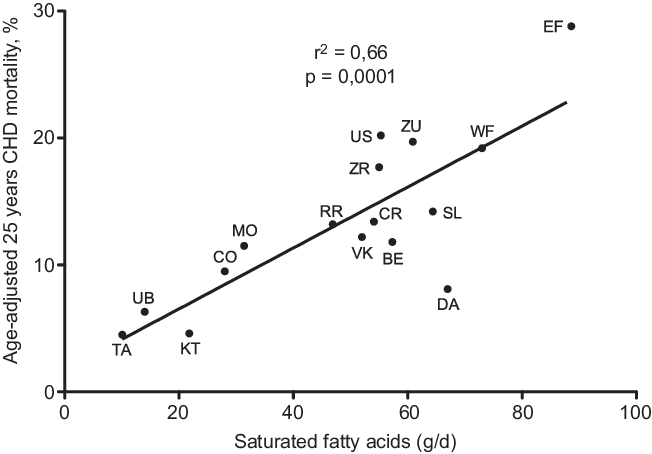
Fig. 3. Correlation of SFA intake with age-adjusted 25 years CHD mortality after 25-year follow-up in the sixteen cohorts of the Seven Countries Study. (EF = East Finland, Finland; WF = West Finland, Finland; Zu = Zutphen, The Netherlands; US = US Railroad, USA; BE = Belgrade, Serbia (former Yugoslavia); ZR = Zrenjanin, Serbia (former-Yugoslavia); CR = Crevalcore, Italy; VK = Velika Krsna, Serbia (former Yugoslavia); RR = Rome Railroad, Italy; SL = Slavonia, Croatia (former Yugoslavia); DA = Dalmatia, Croatia (former Yugoslavia); KT = Crete, Greece; CO = Corfu, Greece; MO = Montegiorgio, Italy; UB = Ushibuka, Japan; TA = Tanushimaru, Japan) (reconstructed from data of ref. [Reference Hertog, Kromhout and Aravanis30]).

Fig. 4. New pyramid for a sustainable Mediterranean Diet (from ref. [80]).
Food preparation should be better adapted to the Mediterranean culinary style to improve nutrient intake, including in packaged foods. In addition, a label for traditional Mediterranean foods and recipes could help promote the Mediterranean food model and help counteract advertising of unhealthy packaged and prepared foods. The use of the various means of communication and advertising available will help to promote health awareness and health-promoting food patterns. Education of children and adults has a major role to play in increasing knowledge about nutrition and its contribution to health, including lifestyle as proposed in the new MedDiet pyramid. Educating medical students on the health benefits of the Mediterranean dietary pattern and its personalised promotion to patients is a major issue for prevention. It is important to explain that the Mediterranean food model is not as costly as commonly thought, as the higher cost of fruits and legumes is almost entirely offset by the lower meat consumption. In addition, respecting seasonality and local production minimises costs and is more sustainable. At the first MedDiet World Conference held in Milan in 2016, the International MedDiet Foundation illustrated MedDiet as a sustainable and human-centred food model. This was followed by the second World Conference on Revitalising the MedDiet held in Palermo in 2019(81). The four sustainable benefits of MedDiet were highlighted in the review by Dernini et al. (Reference Dernini, Berry and Serra-Majem79): (a) major health and nutrition benefits, (b) low environmental impact and biodiversity richness, (c) high socio-cultural food values and (d) positive local economic returns. As written by Mastorakou et al. (Reference Mastorakou, Rabaeus and Salen82): ‘Because MedDiet is a plant-based diet with low consumption of animal products, it has a smaller water footprint and less greenhouse gas emissions and energy consumption’. MedDiet is a biodiverse diet because it uses a wide range of grains, fruits and vegetables that are not only cultivated but can also be wild; in the latter case, they are accompanied by specific local and traditional knowledge about their use. The seasonality of MedDiet’s plant products is another important factor that contributes to its biodiversity. The third advantage concerns the socio-cultural values of food; MedDiet populations have had many religious and cultural traditions and differences throughout their history, with values such as family and communal meals, all of which are valued and contribute to MedDiet being considered an intangible cultural heritage of humanity. The fourth and final benefit of MedDiet is the positive local economic return. MedDiet encourages sustainable development or rural areas producing local and traditional food products. As a result, it can reduce dependence on external food imports. To achieve this, local producers must be empowered, supported and protected, and typical Mediterranean food products must be properly labelled, identified and promoted.
Perspectives and conclusion
Most of the data showing a protective effect of MedDiet on CHD come from observational studies, confirmed by several meta-analyses. However, there is a paucity of randomised clinical trials (RCT) confirming the protective effect of MedDiet, with two landmark trials: in primary prevention, the PREDIMED study, and in secondary prevention, the Lyon Heart Study. The former proved a significant protective effect towards stroke but not towards CHD events; the latter used a margarine rich in α-linolenic acid as the main source of fat. Several studies are underway: Rees et al.(Reference Rees, Takeda and Martin54) found seven ones, which will provide new data. It is also important to continue to revitalise MedDiet in Mediterranean countries, following up on the conferences in Milan 2016 and Palermo 2019. The ongoing dialogues of the Independent Food Systems Summit for the future of sustainable food systems in the Mediterranean will bring concrete proposals in this direction. Outside the Mediterranean region, it is necessary to continue working with all actors/stakeholders on the transferability of the characteristics of the Mediterranean food model, at least in countries where globalisation has been responsible for the westernisation of diet and lifestyle. Epidemiological, clinical and basic scientific data demonstrating both the protective effects on CV health and the sustainability of MedDiet are now available, although they need to be completed.
The initial work of Ancel Keys in Minnesota, Italy and Spain in the mid-1950s was the impetus for the Seven Countries Study, which demonstrated that the traditional diet consumed in rural Mediterranean countries was associated with lower mortality and incidence of CHD. In 1975, the book by A. Keys and his wife popularised what they called in the title ‘The Mediterranean Way’ and detailed within the book the characteristics of the MedDiet and culinary recipes. The numerous observational and cohort studies and a few (too few) RCT have highlighted, among many others not discussed here, the CV benefits of this dietary pattern, confirming the observations of the Seven Countries Study. The observation that the rural Mediterranean cohorts included in the Seven Countries Study had almost half of the total deaths and incidence over 15 years and beyond has led anyway to many other studies and to a general recommendation in Europe(Reference Raygor and Khera83) and in the USA(Reference Carson, Lichtenstein and Anderson84) that the MedDiet should be considered the best dietary pattern for CHD prevention among other benefits.
Acknowledgements
Jacques Delarue is the sole author and takes responsibility for the whole content of this paper.
The author did not receive any financial support fo this paper.
The author has no conflict of interest.












