Vitamin D deficiency in pregnancy is a common occurrence globally( Reference Saraf, Morton and Camargo 1 ). Adequate vitamin D intake during pregnancy is required to address the ongoing demand for Ca for fetal growth and development( Reference Ponsonby, Lucas and Lewis 2 ). In addition, vitamin D has been associated with a number of neonatal outcomes including neonatal birth length and weight( Reference Pérez-López, Pasupuleti and Mezones-Holguin 3 , Reference De-Regil, Palacios and Lombardo 4 ). Pérez-López et al.( Reference Pérez-López, Pasupuleti and Mezones-Holguin 3 ) conducted a systematic review and meta-analysis of randomised controlled trials, which examined vitamin D supplementation during pregnancy with various neonatal outcomes. They observed that neonatal birth weight and birth length were significantly greater in the vitamin D intervention groups than in the placebo group. However, evidence surrounding a possible beneficial relation between vitamin D and birth weight is conflicting. Harvey et al.( Reference Harvey, Holroyd and Ntani 5 ) carried out a comprehensive systematic review on the relationship of vitamin D with maternal and neonatal health outcomes. They observed a modest relationship between maternal 25OHD status and offspring birth weight and bone mass in observational studies( Reference Harvey, Holroyd and Ntani 5 ). Observational studies that directly measured maternal serum 25OHD reported no association of serum 25OHD with low birth weight( Reference Gernand, Simhan and Klebanoff 6 ). The lack of clear evidence suggests a need for larger observational and potentially intervention trials to further investigate this relation.
It has been hypothesised that destruction of β-cells in the development of type 1 diabetes may occur before birth( Reference Lindberg, Ivarsson and Lernmark 7 ) and, if correct, then early identification of environmental determinants in utero, which could affect β-cell function, is of particular relevance. Preliminary reports have suggested that vitamin D deficiency is associated with decreased β-cell function( Reference Chiu, Chu and Go 8 , Reference Kayaniyil, Retnakaran and Harris 9 ), and as neonates derive vitamin D from their mother it is crucial to confirm or refute these findings.
The aim of this study was to investigate the associations of maternal total 25-hydroxy vitamin D (25OHD) with neonatal anthropometrics and markers of neonatal glycaemia in the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study at the Belfast centre.
Methods
The methods for the HAPO study have been published in detail elsewhere( 10 , 11 ). In brief, the HAPO study was a 15-centre multicultural and multinational study designed to examine the association between maternal hyperglycaemia and adverse pregnancy outcomes in singleton pregnancies whose results on oral glucose tolerance testing (OGTT) were below the traditional thresholds for overt diabetes. All pregnant women at a given centre were eligible to participate unless they had one or more of the following exclusion criteria: age younger than 18 years, a plan to undergo delivery at another hospital, an uncertain date of last menstrual period and no ultrasonographic estimation between 6 and 24 weeks of gestational age, inability to complete the oral glucose-tolerance test within 32 weeks of gestation, multiple pregnancy, conception by means of gonadotropin ovulation induction or in vitro fertilisation, glucose testing before recruitment or a diagnosis of diabetes during the current pregnancy, diagnosis of diabetes before the current pregnancy and requiring treatment with medication, participation in another study that could interfere with the HAPO study, infection with the HIV or hepatitis B or C virus, previous participation in the HAPO study or inability to converse in the languages used on centre forms without the aid of an interpreter.
Each participant underwent a standard 75-g OGTT between 24 and 32 weeks’ gestation (average 28 weeks), with sampling of plasma glucose fasting and at 1 and 2 h. OGTT results were blinded to the clinician responsible for the care of the pregnant woman unless the fasting plasma glucose level exceeded 5·8 mmol/l or the 2-h post-load level exceeded 11·1 mmol/l. Additional blood samples were collected concurrently for storage and future biomarker analysis. A number of standardised questionnaires were used to determine information about the mother, including age at OGTT, pre-pregnancy BMI, family history of diabetes, parity and years in education. Maternal height, weight and blood pressure were measured at the OGTT. The number of cigarettes smoked during pregnancy per day and the number of alcoholic drinks taken during pregnancy per day were collected using a standardised questionnaire at the time of the OGTT. This information was used to derive smoking status (≥1 cigarette/d) and alcohol use during pregnancy (≥1 drink/d). In addition, Belfast centre participants at their OGTT visit completed a semi-quantitative validated FFQ, which was used to assess usual dietary intake( Reference Rogers, Emmett and Baker 12 ). Mean dietary vitamin D intake was calculated from the FFQ using the nutritional software package Q-Builder (Questionnaire Design System), version 2.0 (Tinuviel Software), which uses UK food composition tables to quantify nutrient intakes( 13 ). Quantification of dietary intake of vitamin D was based on food sources alone, as the FFQ was not designed to ascertain the quantification of vitamin D entering the diet via food fortification or vitamin supplementation.
A random blood sample was also collected between 34 and 37 weeks’ gestation to identify women with undiagnosed diabetes in late gestation. If the plasma glucose equalled or exceeded 8·9 nmol/l or was ≤2·5 nmol/l, the result was unblinded to the medical caregivers responsible for the pregnant woman.
Cord blood specimens were collected at delivery for the analysis of serum C-peptide and plasma glucose. Outcome measures included delivery method (including any adverse outcomes such as shoulder dystocia and birth injury), birth weight, birth length, head circumference and neonatal skinfold thickness measurements. All neonatal anthropometric measurements were obtained within 72 h by trained HAPO personnel, and a detailed description has been published elsewhere( 11 ). Birth weight was obtained without a nappy using a calibrated electronic scale. Length was measured on a standardised plastic length board constructed for use in the HAPO Study. Head circumference was measured across the occipital fontanel (standard plastic measuring tape). Skinfold thickness was measured with skinfold calipers (Harpenden; Baty). Triceps, subscapular and flank skinfold thicknesses were measured twice, and if results differed by more than 0·5 mm a third measurement was made.
Overall, 23 316 blinded participants successfully completed the study. Of the participating 1677 women from the Belfast centre, thirty-seven women were removed from the study owing to glucose intolerance and being unblinded (n 1640 (98 %)). A further twenty-eight women were of non-white European ethnicity and were removed from the analysis owing to the relationship between GDM and ethnicity( Reference Schwartz, Nachum and Green 14 ) (n 1612 (96 %)). Of these 1612 women, six had antepartum fetal deaths and two had neonatal deaths. Serological samples for the measurement of vitamin D were available for 1585 women.
Laboratory analysis
25OHD2/D3 and 3-epi-25hydroxyvitamin D2/D3 (3-epi-25OHD2/D3) in serum samples were measured using a liquid chromatography tandem-MS (LC-MS/MS) method (Waters® Xevo TQ-S® & ACQUITY UPLC; Waters Corporation).
For 25OHD2/D3, calibration was achieved using commercially available 25OHD2/D3 bi-level (level I and level II) serum controls (Chromsystems) diluted in horse serum (Sigma-Aldrich Co. Ltd). Low-, medium- and high-quality control (QC) samples were prepared by diluting 25OHD2/D3 level I and II serum controls in horse serum. For 3-epi-25OHD2/D3, calibration was achieved using commercially available 3-epi-25OHD2/D3 (Sigma-Aldrich Co. Ltd) diluted in methanol (Fisher Scientific) to make a stock solution of 270 nmol/l. Extra low-, low-, medium- and high-QC samples were prepared by diluting 3-epi-25OHD2/D3 stock solution in horse serum (Invitrogen Life Technologies). The final concentrations of the extra low-, low-, medium- and high-QC samples were 8·438, 16·875, 67·5 and 135 nmol/l, respectively, for both 3-epi-25OHD2 and 3-epi-25OHD3.
A liquid–liquid extraction method was used to extract serum samples for 25OHD2/D3 and 3-epi-25OHD2/D3. Hexadeuterated 25OHD3 (d6-25OHD3; internal standard; Synthetica AS) and trideuterated 3-epi-25OHD3 (d3-3-epi-25OHD3; internal standard, Sigma-Aldrich Co. Ltd) were added to calibrator, QC and participant’s serum samples to correct for variability during sample preparation.
The extracted sample (20 µl) was injected onto an Agilent Zorbax SB-CN column (2·1×50 mm; 1·8 µm particle size). A Waters Xevo TQ-S Tandem Quadrupole Mass Spectrometer was used to quantify the amount of 25OHD and 3-epi-25OHD in samples. Instrument analysis time was 17·5 min/sample.
The inter-assay CV of the method for 25OHD2 and 25OHD3 were 4·4 and 3·4 % at a concentration of 16·1 nmol/l, respectively, whereas the intra-assay CV were 2·7 and 2·3 %, respectively. The inter-assay CV of the method for 3-epi-25OHD2 and 3-epi-25OHD3 were 2·3 and 2·6 % at a concentration of 8·4 nmol/l, respectively, whereas the intra-assay CV were 5·5 and 4·5 %, respectively. The quality and accuracy of serum 25OHD analysis using the LC-MS/MS method in our laboratory was monitored on an ongoing basis by participation in the Vitamin D External Quality Assessment Scheme (Charing Cross Hospital); however, this scheme was for total serum 25OHD and does not take into consideration 3-epi-25OHD. Commercially available QC samples (Chromsysytems) were extracted and analysed in parallel to the serum samples, and were strategically placed close to the beginning, middle and end of the analysis on the LC–MS/MS instrument; in addition, a number of patient samples were also routinely re-analysed on a daily basis to ensure accuracy and precision of the method.
The measurement of all maternal and cord glucose samples were recorded at the HAPO Central Laboratory. Aliquots of maternal fasting, 1- and 2-h OGTT specimens and serum cord were analysed for glucose using a chemical analyser (Vitros 750; OrthoClinical Diagnostics) by an oxidase/peroxidase method. Cord c-peptide was measured only in non-haemolysed samples by a two-way immunometric assay on an Autodelfia instrument (PerkinElmer).
Statistical analysis
Statistical analysis in the Belfast HAPO cohort was carried out using SPSS version 21 (IBM Corp.). Homoeostatic model assessment of insulin resistance (HOMA-IR) and homoeostatic model assessment-β (HOMA-β) were calculated using the HOMA2 calculator( Reference Levy, Matthews and Hermans 15 ).
Birth weight, birth height and head circumference were converted to standard deviation scores (SDS) using the 1990 British Growth Standard, which takes into consideration the offspring sex and gestational age( Reference Cole, Freeman and Preece 16 ). Neonates born before 36 weeks’ gestation were removed for analysis with fat mass, as the equation does not apply to those neonates born <36 weeks’ gestation. Season of maternal OGTT was defined as winter/spring (November, December, January, February, March and April) or summer/fall (May, June, July, August, September and October) for regression analysis. Total 25OHD comprised 25OHD2 and 25OHD3, of which 25OHD3 is the main constituent. Total 25OHD was split into quintiles for certain analysis (≤25 nmol/l, 25·01–49·9 nmol/l, 50–74·9 nmol/l, 75–99·9 nmol/l and ≥100 nmol/l).
Variables were examined for a normal distribution using normality plots, and serum 25OHD, cord HOMA-IR and cord HOMA-β were logarithmically transformed to the base 2 because their distributions were positively skewed. Pearson’s correlation coefficient was used to assess the association between total 25OHD concentrations and continuous variables. Independent-samples t tests and one-way ANOVA were used to compare total 25OHD concentrations between groups defined by variables with two and three or more categories, respectively.
Multiple linear regressions were used to determine the independent association of total 25OHD with a number of neonatal anthropometric measurements, cord HOMA-IR and cord HOMA-β. Analyses were adjusted for a number of variables: season of sampling, maternal age at OGTT, BMI at OGTT, smoker during pregnancy, alcohol user during pregnancy, family history of diabetes, gestational age at delivery, sex of neonate, parity, systolic blood pressure at OGTT, maternal height, fasting plasma glucose (excluded in cord HOMA-IR/β analysis) and maternal education.
Results are presented as means (geometric means if the variable was log transformed) and 95 % CI. Regression coefficients were back-transformed if the dependent variable was logged. A P value ≤0·05 was considered statistically significant.
Ethics
Written informed consent was obtained from all study participants. Ethical approval was obtained from the Northern Ireland Regional Ethics Committee and the research adhered to the tenets of the Declaration of Helsinki.
Results
Neonatal birth weights were obtained in 1605 neonates, and other neonatal anthropometric measurements were obtained in smaller numbers (1507–1601). Cord measurements of insulin resistance and β-cell function were available in 1150 neonates born to Belfast HAPO mothers.
Descriptive statistics for the mother at 28 weeks’ gestation and the neonate are shown in Table 1. The mean age and BMI at the OGTT of participants in the HAPO study at the Belfast centre were 29·7 (sd 5·5) years and 28·3 (sd 4·6) kg/m2, respectively. Women had blood samples taken on average at 29 weeks’ gestation. The prevalence of cigarette smoking and alcohol use during pregnancy was relatively high (24·1 and 26·9 %, respectively). The mean maternal 25OHD concentration was 46·3 (sd 30·3) nmol/l. No 3-epi-25OHD2 was present in participant samples. 3-epi-25OHD3 concentrations were low (2·9 (sd 1·9) nmol/l) and present in 95 % of all participants sampled. Dietary vitamin D as estimated from the FFQ was low (3·3 (sd 2·5) µg/d), and below the recommended nutrient intake of 10 µg/d for pregnant women in the UK( 17 ); however, it should be again noted that the FFQ did not quantify vitamin D from nutritional supplements and fortified foods. The prevalence of vitamin D deficiency was high, with 26·7 % of women having 25OHD concentrations <25 nmol/l. Neonates at the Belfast HAPO Centre were born at an average of 40 weeks’ gestation. The average birth weight was 3402 (sd 517) g and the average birth weight SDS was −0·1 (sd 1·0). The average birth length was 50·8 (sd 2·5) cm and birth length SDS was 0·2 (sd 1·1).
Table 1 Neonatal anthropometric outcomes and biochemical insulin indices in neonates born to Belfast Hyperglycemia and Adverse Pregnancy Outcome mothers (Numbers and percentages; mean values and standard deviations; medians and interquartile ranges (IQR))

25OHD, 25-hydroxy vitamin D; 3-epi-25OHD3, 3-epi-25hydroxyvitamin D3; HAPO, Hyperglycemia and Adverse Pregnancy Outcome Study; HOMA-β, homoeostasis model assessment of β cell function; HOMA-IR, homoeostasis model assessment of insulin resistance; SDS, standard deviation score.
* Neonates born <36 weeks’ gestation were removed from analysis.
Circulating concentrations of maternal 25OHD were not significantly correlated with maternal age or maternal BMI; however, 25OHD was significantly and positively correlated with years of education (P≤0·001) (data not shown). Serum 25OHD was significantly lower in those women who smoked during pregnancy (P≤0·001). There were no significant differences in 25OHD concentrations between alcohol use and non-alcohol use during pregnancy. There was evidence of seasonal variation in 25OHD concentrations. Maternal 25OHD concentrations were lower in the winter/spring (29·3 nmol/l) compared with summer/fall (47·6 nmol/l) (data not shown).
Total 25OHD was split into quintiles (≤25 nmol/l, 25·01–49·99 nmol/l, 50–74·99 nmol/l, 75–99·99 nmol/l and ≥100 nmol/l), and neonatal anthropometric outcomes were compared. There were no significant differences between the quintiles of total 25OHD and birth weight, birth length, neonatal fat mass, neonatal subscapular skinfold, neonatal flank skinfold thickness and neonatal triceps’ skinfold thickness (data not shown). In addition, no significant differences were observed between quintiles of total 25OHD and markers of neonatal glycaemia (data not shown).
Table 2 shows the associations between maternal 25OHD at 28 weeks’ gestation and neonatal anthropometric measurements adjusted for confounders. The regression analysis found that the doubling of maternal 25OHD gave rise to a birth weight higher by 0·05 and birth length SDS higher by 0·07 (both, P=0·03). No associations were found for neonatal measures of skinfold thickness with maternal 25OHD (Table 2).
Table 2 Unadjusted and adjusted associations of maternal 25-hydroxy vitamin D (25OHD) at 28 weeks’ gestation with neonatal anthropometric measurements (Coefficients and 95 % confidence intervals)
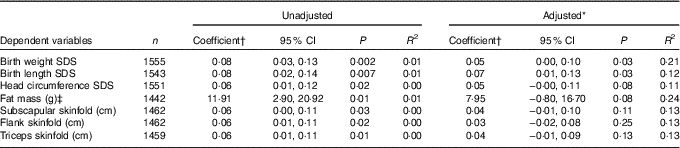
SDS, Standard deviation score; OGTT, oral glucose tolerance testing.
* Adjusted for season of sampling, maternal age at OGTT, maternal BMI at OGTT, smoker during pregnancy, alcohol use during pregnancy, family history of diabetes, gestational age at delivery, sex of neonate, parity, systolic blood pressure at OGTT, maternal height, fasting plasma glucose and maternal education.
† Regression coefficients represent the additive effect on the dependent variable associated with a doubling in maternal serum 25OHD level (owing to 25OHD being logged to the base 2).
‡ Neonates born <36 weeks’ gestation were excluded from analysis.
No significant associations were observed between maternal 25OHD concentrations at 28 weeks’ gestation and cord HOMA-IR and cord HOMA-β in both the unadjusted analysis and adjusted analysis (Table 3).
Table 3 Summary table of the relationship of maternal 25-hydroxyvitamin D (25OHD) at 28 weeks’ gestation to markers of neonatal glycaemia (Coefficients and 95 % confidence intervals)
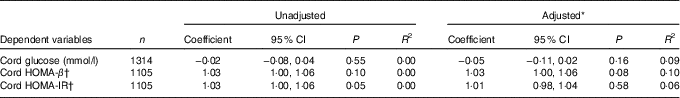
HOMA-β, homoeostatic model of assessment-β; HOMA-IR, homoeostatic model of assessment-insulin resistance; OGTT, oral glucose tolerance testing.
* Adjusted for season of sampling, maternal age at OGTT, maternal BMI at OGTT, smoker during pregnancy, alcohol use during pregnancy, family history of diabetes, gestational age at delivery, sex of neonate, parity, systolic blood pressure at OGTT, maternal height and maternal education.
† Regression coefficients have been anti-logged to represent the multiplicative effect on the dependent variable associated with a doubling in maternal serum 25OHD level (owing to 25OHD being logged to the base 2).
Discussion
Maternal vitamin D deficiency was common in participants in the Belfast HAPO study. After adjustment for confounding variables, maternal serum 25OHD was associated only with neonatal birth weight SDS and birth length SDS. However, the contribution of maternal 25OHD to these neonatal anthropometric outcomes appears to be limited. No associations were observed with maternal 25OHD and markers of neonatal β-cell function and insulin resistance.
There is conflicting evidence regarding the relationship between vitamin D and neonatal and maternal outcomes. A number of studies have found associations of maternal vitamin D status with a number of neonatal outcomes, including birth weight( Reference Gernand, Simhan and Caritas 18 ), pre-term birth( Reference De-Regil, Palacios and Lombardo 4 ), asthma and allergies( Reference Miller, Turner and Spidery-Cornish 19 ). The conflicting results are most likely owing to low numbers of participants in the studies, and failure to control for relevant confounding variables. Well-designed longitudinal observational studies are required to investigate thoroughly maternal–neonatal associations with vitamin D. These might in turn point to the need for large-scale vitamin D intervention trials to investigate the association of 25OHD with maternal and neonatal outcomes. In the current study, maternal 25OHD was significantly associated with birth weight SDS in adjusted analysis. Further, there was a lack of association between maternal 25OHD with measures of neonatal adiposity. One small study reported greater neonatal fat mass with higher cord 25OHD concentrations( Reference Josephson, Fein glass and Pacemaker 20 ) and that cord 25OHD was directly related to maternal 25OHD concentrations( Reference Josefson, Reisetter and Scholtens 21 ). Although we did not measure cord 25OHD, our data pertain to a much larger cohort of mother–neonate pairs, and our results have been adjusted for a considerable number of confounding variables. The lack of significant associations in the present study between maternal 25OHD and neonatal adiposity is also concordant with the results from a sub-sample of a North American HAPO cohort, which also found no significant association between cord 25OHD and neonatal adiposity( Reference Josefson, Reisetter and Scholtens 21 ).
Previous studies that have examined the association between maternal vitamin D and birth weight have reached conflicting conclusions. In an observational study, Gernand et al. assessed the 25OHD concentrations of 2146 pregnant women at 26 weeks’ gestation and reported mean maternal 25OHD concentrations of 51·3 nmol/L similar to findings in the current study (46·3 nmol/l). The authors observed a non-linear relationship between 25OHD concentrations and birth weight. When adjusted for several confounders (trimester at maternal blood draw, maternal race (white/other), pre-pregnancy BMI, height, smoking, season and study site), maternal 25OHD concentrations higher by 1 nmol/l were associated with mean neonatal birth weight higher by 3·6 g( Reference Gernand, Simhan and Klebanoff 6 ). A prospective cohort study performed in a number of cities in Spain among 2382 pregnant women during the early second trimester reported a median circulating 25OHD level of 73·4 nmol/l. No association was found between maternal 25OHD concentrations and neonatal birth weight in either the unadjusted or adjusted analysis. In addition, maternal 25OHD was not associated with low birth weight (<2500 g)( Reference Rodriguez, García-Esteban and Basterretxea 22 ). It is possible that the association of vitamin D with low birth weight is only observed in vitamin-D-deficient mothers. A prospective study by Chen and colleagues in 3658 pregnant women reported a significant positive correlation between maternal 25OHD and birth weight (r 0·477; P<0·001), with evidence of a threshold of 100 nmol/l for maternal 25OHD concentrations below which vitamin D was an important predictor of neonatal birth weight but above which there was no association( Reference Chen, Fu and Hao 23 ). The evidence, therefore, regarding the relationship between vitamin D and birth weight is inconclusive. There are data that suggest an association between maternal glucose and fatty acid metabolism and therefore the transfer of energy to the fetus, and consequently an increase in neonatal birth weight( Reference Gernand, Simhan and Klebanoff 6 ). The relationship with vitamin D and birth weight could also be simply explained by a healthy maternal diet, which in turn increases 25OHD concentrations and gives rise to an increased birth weight in the neonate.
A significant association was observed in the current study between maternal 25OHD concentrations and birth length SDS. This is in line with a large mother–neonate study in the USA (n 2473)( Reference Eckhardt, Gernand and Roth 24 ). In this latter study, maternal 25OHD at 26 weeks’ gestation was 58·9 nmol/l, and a significant positive association was observed between 25OHD and birth length z score in adjusted analysis. The authors also observed that this association was sustained from birth to 12 months( Reference Eckhardt, Gernand and Roth 24 ), which would suggest that skeletal growth deficits in infants with low maternal 25OHD may be difficult to recover. This is concordant with a previous study that suggested that maternal vitamin D status is associated with reduced bone mass accrual during childhood up to 9 years of age( Reference Javaid, Crozier and Harvey 25 ). The effect of maternal vitamin D on birth length, and perhaps bone mass, could be linked to the maternal–fetal transfer of Ca during pregnancy. Javaid and colleagues observed that umbilical–venous concentrations of Ca were significantly associated with bone mass accrual in offspring( Reference Javaid, Crozier and Harvey 25 ). The main role of vitamin D in pregnancy is to escalate Ca absorption and placental Ca transport( Reference Olmos-Ortiz, Avila and Durand-Carbajal 26 ). It is possible that vitamin D deficiency may reduce the capacity of maternal transfer of Ca to the fetus and therefore decrease bone mass and bone length.
25OHD concentrations above the threshold of deficiency have been associated with improved β-cell function in various population groups( Reference Chiu, Chu and Go 8 , Reference Kayaniyil, Retnakaran and Harris 9 ); however, this was not observed in the current study. Vitamin D actions are mediated by vitamin D receptors (VDR), which undergo several processes to activate metabolic regulatory factors, including insulin. VDR are present in β-cells, which suggest that vitamin D may have a direct involvement in regulating β-cell function( Reference Moore, Bowser and Fausnacht 27 ). Information on the impact of maternal vitamin D deficiency on the incidence of type 1 diabetes in the offspring is limited. A meta-analysis on the association between maternal vitamin D intake and the risk of type 1 diabetes was conducted by Dong et al. Three studies were included in the analysis and the pooled OR was 0·95 (95 % CI 0·66, 1·36), indicating no association between maternal intake of vitamin D and risk of type 1 diabetes in the offspring( Reference Dong, Zhang and Chen 28 ). Owing to limited literature on the impact of maternal vitamin D concentration on neonatal β-cell function, it is difficult to assess whether further investigation is warranted regarding maternal vitamin D and the risk of diabetes in the offspring.
The strengths of this study include the large number of participants, the rigorous nature of the methodology, the detailed neonatal end points and the methodology for vitamin D measurement during pregnancy. In addition, exploration of the association between maternal vitamin D and neonatal outcomes was controlled for relevant confounding variables. The limitations include the observational nature of the study and the use of HOMA equations to assess β-cell function and insulin resistance. Information on cord HOMA-β and HOMA-IR was only available for 75 % of the total study population, which reduced the sample size for analysis of cord HOMA-β and HOMA-IR. In addition, we did not measure 25OHD in cord blood, which would have provided further information on the relation of maternal 25OHD to neonatal adiposity.
In future studies, we plan to examine the associations of maternal 25OHD on offspring bone mass and bone length in childhood years. In summary, the present study has shown a positive association between maternal 25OHD and birth weight SDS and birth length SDS controlled for confounding variables; however, the contribution of maternal 25OHD in regression models appears to be limited. No other significant associations were found for other measures of neonatal anthropometry. In general, it would seem wise to encourage pregnant women to maintain 25OHD concentrations ≥25 nmol/l for skeletal health and to prevent the neonate being vitamin D deficient.
Acknowledgements
We would like to acknowledge the HAPO Study Cooperative Group and the staff of the HAPO Study.
The HAPO study was funded by grants from the National Institute of Child Health and Human Development and the National Institute of Diabetes and Digestive and Kidney Diseases (RO1-HD34242 and RO1- HD34243) and Diabetes UK (RD04/ 0002756), which supported the enrolment and collection of data on participants. None of the funders had a role in the design, analysis or writing of this article.
D. R. M. designed the project; C. C. and A. M. G. conducted the research; C. C., C. C. P., I. S. Y. and D. R. M. analysed data; C. C., V. A. H. and D. R. M. wrote the paper. D. R. M. has primary responsibility for the final content. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.





