According to the WHO, globally more than 300 000 deaths during the first 28 d of life were attributable to birth defects in 2015, with neural tube defects (NTD) being one of the most serious of these defects(1). Also in 2015, the Global Burden of Disease ranked birth defects as the number 5 cause globally of deaths under the age of 5 years, an increase from number 7 in 1990(2). Deaths caused by birth defects most often occur early in life, and thus the burden of years of life lost due to premature mortality is severe(2,3) . Micronutrient requirements increase substantially throughout pregnancy but are especially important during the first trimester when organogenesis mainly occurs(Reference Gernand, Schulze and Stewart4). Nutritional status pre- and post-conception affects the growth and development of the fetus, and deficiencies increase the risk of birth defects(Reference Ross, Caballero and Cousins5). Folate deficiency is one of the most common micronutrient deficiencies among women in reproductive age(Reference Gernand, Schulze and Stewart4), along with Fe(Reference Borch-Iohnsen, Pedersen and Henriksen6), iodine(Reference Henjum, Abel and Meltzer7) and vitamin D(Reference Haugen, Brantsaeter and Alexander8) in the Nordic countries. In Norway, intake of dietary folate(9,Reference von Ruesten, Brantsaeter and Haugen10) and folic acid supplements(Reference Nilsen, Vollset and Gjessing11) is low. In the early 1990s, two randomised controlled studies demonstrated that periconceptional folic acid supplementation prevented the recurrence and occurrence of NTD(12,Reference Czeizel and Dudas13) . Because of these studies, many authorities worldwide implemented policies to fortify flour with folic acid, starting in the USA from 1998(14). To date, more than sixty countries have mandatory folic acid fortification programmes(15). In Norway, there is no mandatory folic acid fortification and women planning to conceive have since 1998 been advised to take a daily supplement of 0·4 mg folic acid from 1 month before conception and until the 12th week of gestation to reduce the risk of NTD(16). Several observational studies and randomised controlled trials have explored the associations of folic acid and multivitamins with other birth defects, such as congenital heart defects(Reference Botto, Olney and Erickson17), limb reduction defects(Reference Werler, Hayes and Louik18), urinary tract defects, as well as other structural developmental anomalies(Reference Botto, Olney and Erickson17). However, the literature is inconclusive(Reference De-Regil, Pena-Rosas and Fernandez-Gaxiola19,Reference Øyen, Olsen and Basit20) , and the role of folate remains unclear for birth defects other than NTD. To our knowledge, associations between folic acid supplements and major birth defect categories have not previously been investigated in nationwide cohorts in the Nordic countries.
We conducted a prospective study using data from the nationwide, population-based Medical Birth Registry of Norway (MBRN) in the period 1999–2013. First, we investigated the prevalence of birth defects among all births, including live births, stillbirths and termination of pregnancies due to fetal anomaly (TOPFA). Second, as our main objective, we compared the risks of organ-specific major birth defects in live- and stillborn infants of mothers who did or did not use folic acid and/or multivitamin supplements.
Methods
Data source
The MBRN contains data on all live births and stillbirths in Norway from 16 weeks’ gestation onwards since 1967. From 1999, information on women’s use of folic acid and multivitamin supplements before and during pregnancy has been included, along with smoking habits and ultrasound dating. Notification of births is compulsory and includes information on the parents (e.g. socio-demographic markers, maternal health status before and during pregnancy, maternal smoking and use of folic acid/multivitamins and medication), the delivery (e.g. operative vaginal delivery, caesarean section, complications during delivery) and the condition of the infant (e.g. plurality, birth weight, gestational age, birth defects, neonatal diseases). Data are collected during pregnancy and completed after delivery by the attending midwife, and during the stay at the delivery unit until discharge. Additionally, the MBRN also receives notifications from the neonatal intensive care units for all infants transferred to such units after birth. This routine has been in place since 1999 and has improved the registration of birth defects. Also from 1999, all TOPFA have routinely been added to the birth registry to increase the ascertainment of birth defects. The present study used data from the MBRN for the years 1999–2013.
Ethical statement
The present study was approved by the regional Medical Ethics Committee of Western Norway, REK 2010/3310/REK vest.
Classification of birth defects
All infants in Norway undergo a paediatric examination at the maternity ward before discharge or at the neonatal ward if the child is transferred to such units after birth. Birth defects were coded by the International Classification of Diseases-10 and the International Classification of Diseases-10-British Pediatric Association (addition by the British Pediatric Association). We used the European Surveillance of Congenital Anomalies (EUROCAT) classification system to define eleven organ-specific major birth defect categories (subtypes in parentheses)(21). These categories were nervous system (NTD), eye, ear–face–neck, cardiovascular system (severe cardiovascular defects), respiratory system, oral clefts, digestive system (oesophageal atresia with or without tracheaoesophageal fistula; anorectal atresia and stenosis; other digestive defects), abdominal wall (gastroschisis; omphalocele), urinary system (hydronephrosis; remaining urinary defects), genital organs (hypospadias) and limb (limb reduction defects; talipes equinovarus; polydactyly; syndactyly) (online Supplementary Table S1). In the present study, we excluded individuals with teratogenic syndromes, chromosomal anomalies, and genetic syndromes and microdeletions (n 1747) because major birth defects associated with these groups in most cases have known causes unrelated to the exposure(21) (Fig. 1). Very few hip anomalies are recognised as birth defects, and hip anomalies were thus not counted as cases (online Supplementary Table S1 for International Classification of Diseases codes). Births with multiple NTD were classified according to the most severe defect; for example, anencephaly accompanied with encephalocele or spina bifida was coded as anencephaly, and encephalocele with spina bifida was coded as encephalocele. Cases with one or more birth defect(s) within the same organ-specific group were classified as isolated if other organs were unaffected. In keeping with the EUROCAT definition, sequences were assigned to the primary anomaly, for example, clubfoot or pulmonary hypoplasia associated with bilateral renal agenesis was classified as isolated bilateral renal agenesis, and oral cleft associated with anencephaly was classified as isolated anencephaly(Reference Garne, Dolk and Loane22). Multiple births differ from single births regarding birth defects(Reference Glinianaia, Rankin and Wright23), and therefore, we ran separate analyses with singletons. However, our main analyses included singletons and multiple births.

Fig. 1. Study population. TOPFA, termination of pregnancies due to fetal anomaly.
Folic acid and multivitamin supplementation
Information on supplement use is collected during pregnancy and transferred to the birth notification form, where it is registered into the following four checkboxes: ‘Folic acid before pregnancy’, ‘Folic acid during pregnancy’, ‘Multivitamins before pregnancy’ and ‘Multivitamins during pregnancy’. The data that were available to us only had information on these four checkboxes (multivitamin and/or folic acid before and/or during pregnancy), without a specific option for those who had not used supplements (one checkbox for ‘no use’). By default, all those who had not reported use were therefore set to non-users. No use of supplements (folic acid or multivitamins) at any time was used as the reference group in all analyses (all of the four checkboxes left unmarked). Infants, where the mother used supplements (folic acid and/or multivitamins) before and during pregnancy, were classified as exposed. This also included infants where mothers only used supplements before pregnancy (1·1 %). The exposure variable had the following categories: no supplement use, supplement use before and during pregnancy or only before and supplement use during pregnancy only. In order to study use of folic acid separately from use of multivitamins, or the two of them combined, we created three subcategories: use of multivitamins only (before and during pregnancy), use of folic acid only (before and during pregnancy) and use of both folic acid and multivitamins (before and during pregnancy). Folic acid tablets available over the counter in Norway during the study period contained 0·4 mg of folic acid, while the maximum limit of folic acid in multivitamins was 0·2 mg(24).
Although the MBRN registers birth defects in terminated pregnancies, spontaneous abortions from 16 gestational weeks, and still- and live births from 22 gestational weeks, information on supplement use has not been registered for TOPFA. Consequently, our main analyses focused on the relation between vitamin supplementation and birth defects among live births and stillbirths from 16 gestational weeks.
Statistical analyses
We used a log-binomial regression model to calculate the relative risks (RR) with 95 % CI for the relationship between maternal periconceptional use of supplements and the risk of infant birth defects, with the infant as the observation unit. To account for non-independent data, we performed clustered regression analyses using mothers’ identification number as the cluster variable in all analyses. We also explored stratification of the study sample by year of birth (1999–2005 v. 2006–2013). The adjusted relative risk (aRR) models accounted for a priori selected potential confounders, including year of birth, maternal age (<20, 20–24 (reference), 25–29, 30–34, 35–39, ≥40 years), marital status (married (reference), cohabiting, single, other), parity (0 (reference), 1, ≥2 previous births), maternal smoking habits at the beginning of pregnancy (non-smoker (reference), occasional smoker, daily smoker, missing data), pregestational diabetes (no (reference), yes) and maternal epilepsy (no (reference), yes). RR were reported with and without adjustment for these potential confounders. In a sensitivity analysis, we assessed multiple gestations and in vitro fertilisation (IVF) related to supplement use and birth defects. All analyses were performed using Stata version 16.0 (StataCorp).
Results
During the years 1999–2013, 888 294 (99·0 %) live-born infants, 6633 (0·7 %) stillborn infants and 2135 (0·2 %) TOPFA were registered in the MBRN (Fig. 1). Table 1 shows the distributions of birth defects among live births, stillbirths and terminated pregnancies (n 20 517). Among live-born, stillborn and TOPFA, heart defects were the most common subtype of birth defects (85·6/10 000) followed by birth defects in the limbs (40·6/10 000), the genital organs (25·9/10 000), urinary system (24·3/10 000), nervous system (17·9/10 000), oral clefts (17·8/10 000), digestive system (14·5/10 000), respiratory system (6·3/10 000), abdominal wall system (5·7/10 000), eye (2·7/10 000) and ear–face–neck (2·4/10 000). Among non-syndromic fetuses with birth defects, the percentage of TOPFA was highest for those with NTD (67·1 %), nervous system defects in general (51·4 %) and omphalocele (37·2 %). In contrast, the percentage of TOPFA was lowest for those with genital defects (1·2 %), oral clefts (2·4 %), eye defects (4·1 %) and heart defects (4·4 %).
Table 1. Major birth defects among 888 294 live births (birth defects, n 18 117), 6633 stillbirths (birth defects, n 265), and 2135 pregnancy terminations due to fetal anomaly (TOPFA), Norway 1999–2013
(Relative risks and 95 % confidence intervals; numbers and percentages)
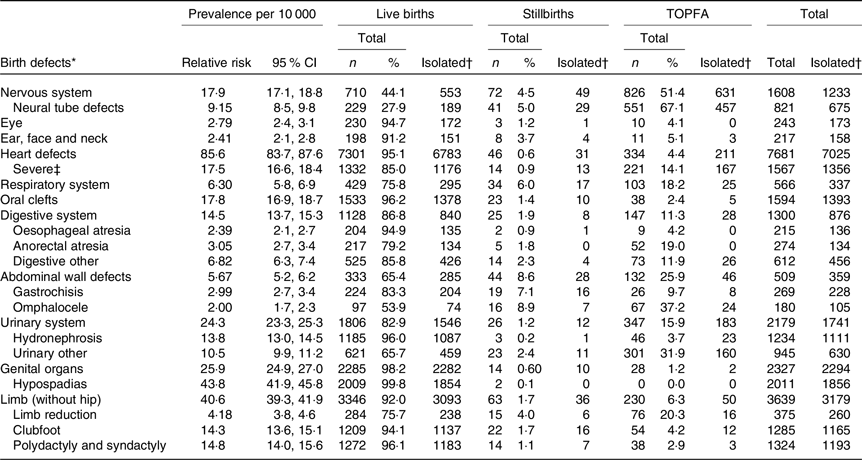
* Birth defects were classified according to the EUROCAT (European Surveillance of Congenital Anomalies) classification system, https://eu-rd-platform.jrc.ec.europa.eu/sites/default/files/appendices.pdf (hip anomalies not counted, chromosomal anomalies, genetic syndromes and microdeletions and teratogenic syndromes excluded).
† Cases with one or more birth defect(s) within the same organ-specific group and no birth defects in other organ-specific groups were classified as isolated.
‡ Severe cardiovascular defects included common arterial truncus (International Classification of Diseases-10 code Q200), double-outlet right ventricle (Q201), transposition of great vessels (Q203), single ventricle (Q204), atrioventricular septal defect (Q212), tetralogy of Fallot (Q213), pulmonary valve atresia (Q220), tricuspid stenosis/atresia (Q224), Ebstein’s anomaly (Q225), hypoplastic right heart (Q226), aortic valve stenosis/atresia (Q230), mitral valve anomalies (Q232–233), hypoplastic left heart (Q234), coarctation of aorta (Q251), aortic atresia/interrupted aortic arch (Q252) and total anomalous pulmonary venous return (Q262).
Table 2 shows maternal characteristics for 894 927 live- and stillborn infants according to folic acid and/or multivitamin supplement use before and/or during pregnancy. Mean maternal age at delivery was 29·6 years (sd 5·2, range 13–55 years, data not shown in table). About 42 % were first-time mothers, and more than 90 % of the women were married or cohabiting. Nearly 14 % of the women smoked at the beginning of pregnancy, with a decreasing prevalence over the study period from 22·5 to 6·9 %. Smoking information was missing in more than 16 % of the women overall. The proportion of women with pregestational diabetes and epilepsy was both 0·7 %. During 1999–2013, there was a 3-fold increase in reported supplement use before and during pregnancy from 9·0 to 27·1 %, and more than a 2-fold increase for use during pregnancy only, from 19·5 to 48·6 %. Use of folic acid supplements before and during pregnancy was associated with higher maternal age, marriage/cohabitation, lower parity and non-smoking. Almost half of women with pre-gestational diabetes and more than 30 % of mothers with epilepsy were reported as supplement non-users.
Table 2. Maternal characteristics of 894 927 live- and stillborn infants according to folic acid and/or multivitamin supplement use before* and during pregnancy, and during† pregnancy only, Norway 1999–2013
(Numbers and percentages)
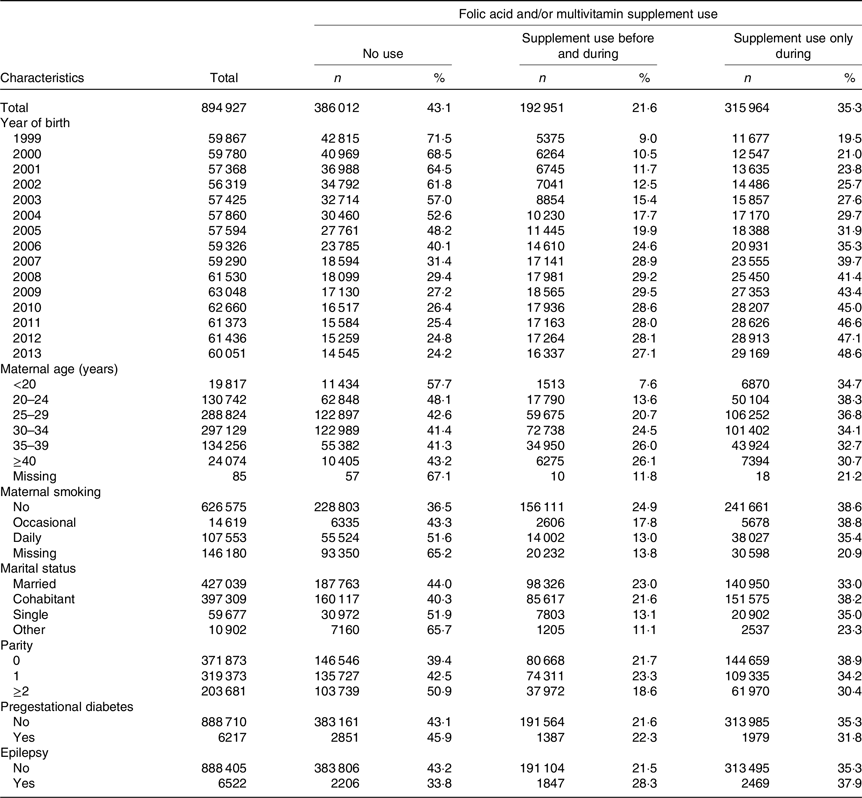
* Folic acid and/or multivitamin supplement use before and during pregnancy (including 1·1 % of the infants where mothers only used supplements before pregnancy.
† Folic acid and/or multivitamin supplement use during pregnancy only.
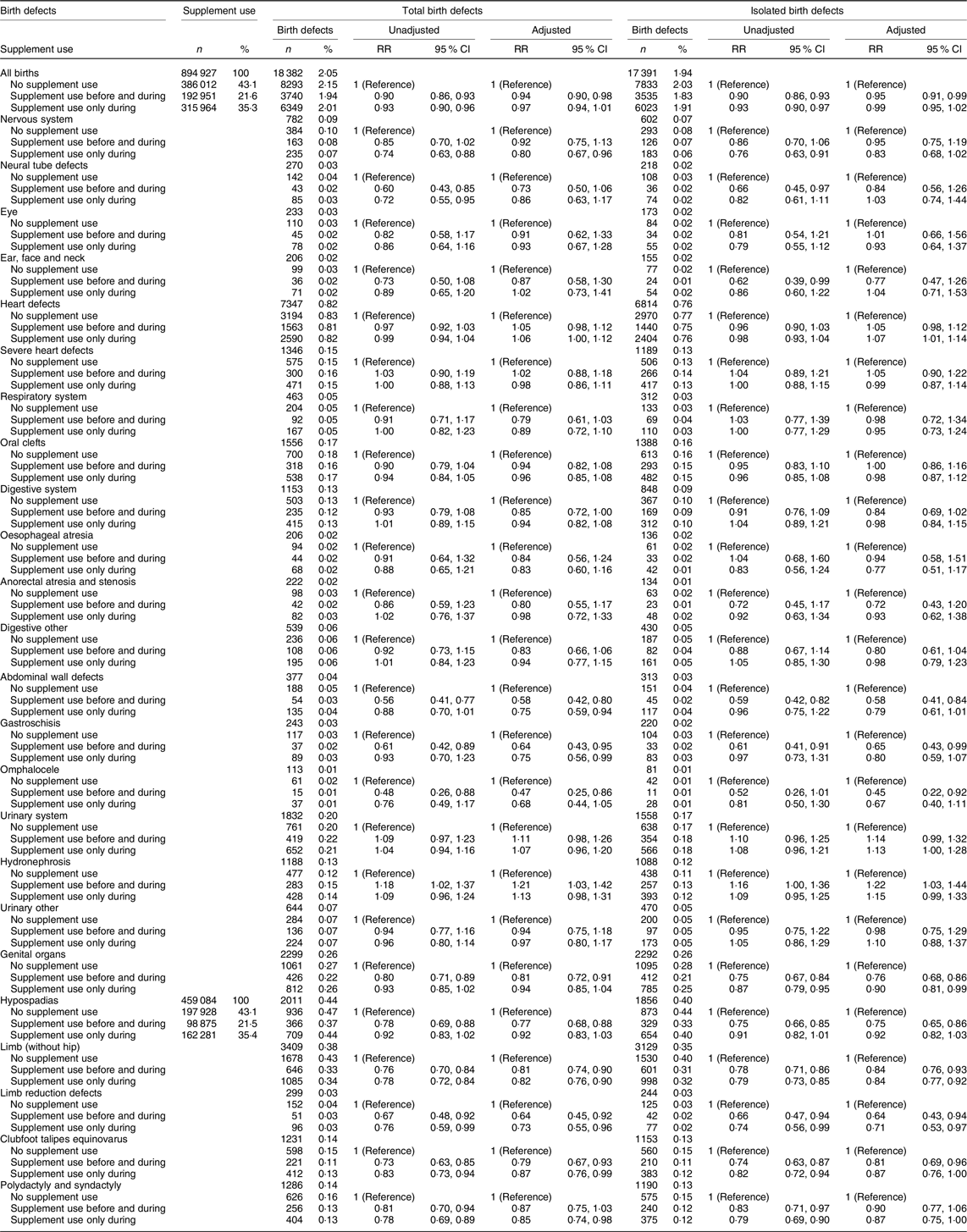
When we explored the associations between supplement use and birth defects, we included live-born and stillborn infants in the study population (n 894 927). Among them, 18 382 infants had birth defects (2·1 %) and the majority of these infants had isolated defects (n 17 391, 94·6 %). Among live-born infants, 18 117 were registered with birth defects (2·0 %), and among stillbirths, the number was 265 (4·0 %). Table 3 shows the association between supplement use and infant birth defects, with separate columns for isolated birth defects. Among infants of mothers who used supplements, aRR was 0·94 (95 % CI 0·91, 0·98) and 0·95 (95 % CI 0·91, 0·99) for total and isolated birth defects overall, respectively. Analysis stratified by year of birth suggested that the inverse associations may be stronger in the second time period (2006–2013) than in the first time period (1999–2005) (online Supplementary Table S2). Supplement use was associated with reduced risk of abdominal wall defects (total aRR 0·58, 95 % CI 0·42, 0·80 and isolated aRR 0·58, 95 % CI 0·41, 0·84). Results for abdominal wall defects were similar when the analysis was limited to folic acid only (Table 4) compared with no supplement use (total aRR 0·50, 95 % CI 0·29, 0·84 and isolated aRR 0·45, 95 % CI 0·24, 0·83). Protective associations were observed for both total and isolated gastroschisis and omphalocele.
Table 3. Unadjusted and adjusted* relative risks† (RR) for birth defects‡ among 888 294 live births (birth defects, n 18 117) and 6633 stillbirths (birth defects, n 265) by folic acid and/or multivitamin supplement§ use, shown for eleven organ-specific birth defect groups with additional subgroups, based on EUROCAT|| definitions, Norway 1999–2013
(Relative risks and 95 % confidence intervals; numbers and percentages)
* Adjusted for the following: year of birth, maternal age, marital status, parity, maternal smoking habits at the beginning of pregnancy, pregestational diabetes and maternal epilepsy.
† Relative risks compared with infants of supplement non-users (Reference group), relative risk calculated as the ratio of birth prevalence in the exposed group and the birth prevalence in the unexposed group.
‡ Cases with one or more birth defect(s) within the same organ-specific group and no other organ affected were classified as isolated.
§ Vitamin supplement use: folic acid and/or multivitamins before and during pregnancy (including 1.1 % of the infants where mothers only used vitamins before pregnancy) and during pregnancy only.
|| EUROCAT: European Surveillance of Congenital Anomalies (https://eu-rd-platform.jrc.ec.europa.eu/sites/default/files/appendices.pdf).
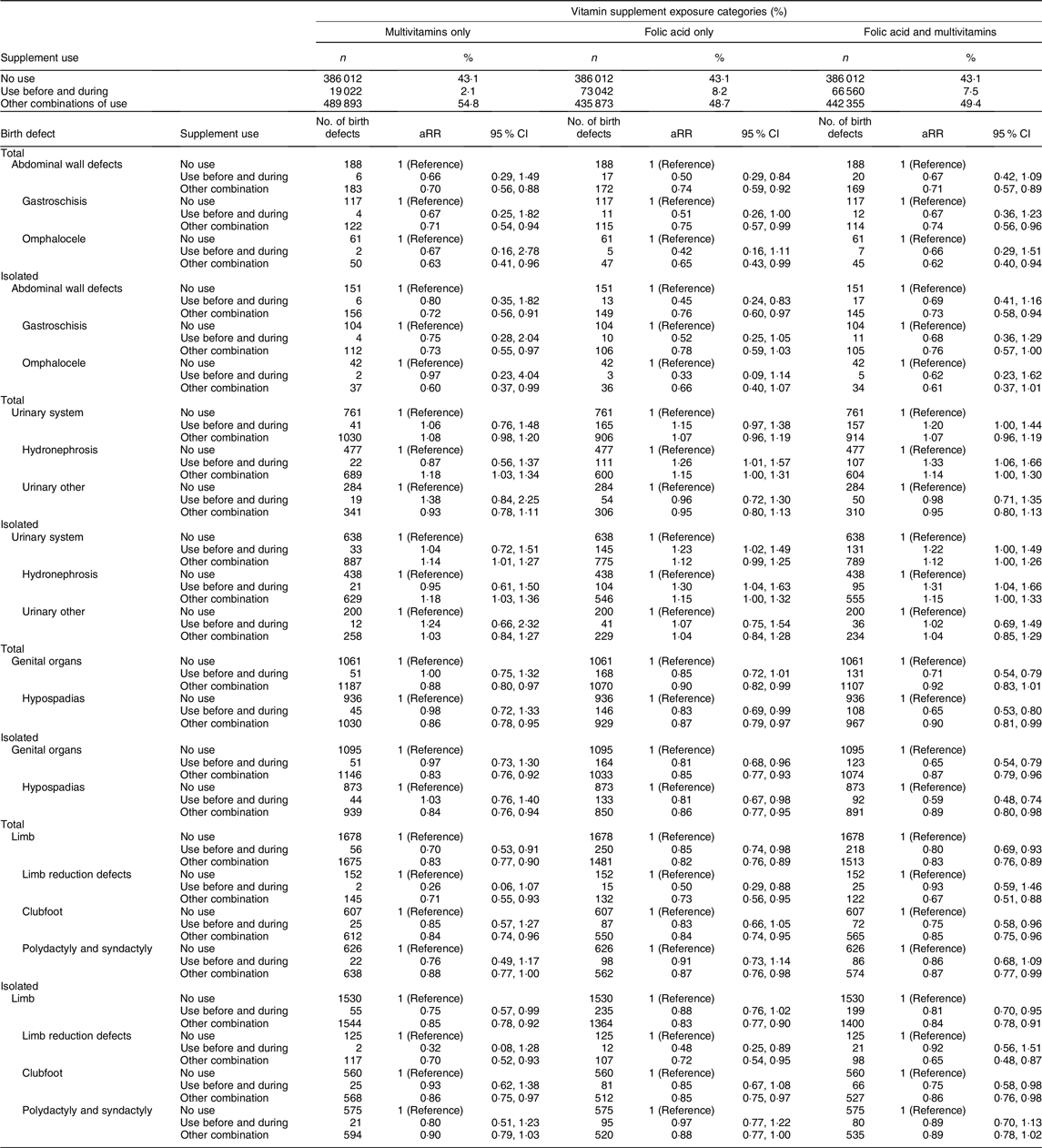
Table 4. Adjusted* relative risks† (aRR) for birth defects‡ by different vitamin supplement exposure§ categories among 894 927 live births and stillbirths, birth defect groups based on EUROCAT||definitions, Norway 1999–2013
(Numbers and percentages; adjusted relative risks and 95 % confidence intervals)
* Adjusted for the following: year of birth, maternal age, marital status, parity, maternal smoking habits at the beginning of pregnancy, pregestational diabetes and maternal epilepsy.
† Relative risks compared with infants of supplement non-users, relative risk calculated as the ratio of birth prevalence in the exposed group and the birth prevalence in the unexposed group (Reference group).
‡ Cases with one or more birth defect(s) within the same organ-specific group and no other organ affected were classified as isolated.
§ Supplement use: multivitamins before and during pregnancy, folic acid before and during pregnancy, or combined use of multivitamins and folic acid before and during pregnancy, other combination of supplement use: remaining groups of supplement use.
|| EUROCAT: European Surveillance of Congenital Anomalies (https://eu-rd-platform.jrc.ec.europa.eu/sites/default/files/appendices.pdf).
We observed an association between supplement use and genital defects (total aRR 0·81, 95 % CI 0·72, 0·91 and isolated aRR 0·76, 95 % CI 0·68, 0·86). Among genital defects, 88 % were hypospadias (total hypospadias aRR 0·77, 95 % CI 0·68, 0·88 and isolated hypospadias aRR 0·75, 95 % CI 0·65, 0·86). Multivitamin use (not concurrent with folic acid) was not associated with reduced risk of hypospadias (total hypospadias aRR 0·98, 95 % CI 0·72, 1·33 and isolated hypospadias aRR 1·03, 95 % CI 0·76, 1·40). However, protective associations for hypospadias were observed with folic acid only (total aRR 0·83, 95 % CI 0·69, 0·99 and isolated 0·81, 95 % CI 0·67, 0·98) and combined use of folic acid and multivitamins (total aRR 0·65, 95 % CI 0·53, 0·80 and isolated aRR 0·59, 95 % CI 0·48, 0·74; Table 4).
Supplement use was also associated with reduced risk of limb defects (total aRR 0·81, 95 % CI 0·74, 0·90), limb reduction defects (total aRR 0·64, 95 % CI 0·45, 0·92) and clubfoot (total aRR 0·79, 95 % CI 0·67, 0·93). Nearly identical results were found for isolated limb defects (Table 3). Use of multivitamins only, use of folic acid only and folic acid combined with multivitamins were associated with reduced risks for total and isolated limb defects (Table 4). Numbers were small among those with limb reduction defects that had mothers who only used multivitamins. Folic acid only was associated with reduced risks for limb reduction defects (total aRR 0·50, 95 % CI 0·29, 0·88 and isolated aRR 0·48, 95 % CI 0·25, 0·89). For clubfoot, we observed that folic acid combined with multivitamins was associated with reduced risks for clubfoot (total aRR 0·75, 95 % CI 0·58, 0·96 and isolated aRR 0·75, 95 % CI 0·58, 0·98; Table 4).
Finally, for urinary system defects, hydronephrosis amounted 65 % of the group. We found that use of folic acid was associated with an increased risk for hydronephrosis (total aRR 1·21, 95 % CI 1·03, 1·42 and isolated aRR 1·22, 95 % CI 1·03, 1·44). Use of folic acid combined with multivitamins showed the strongest association (total aRR 1·33, 95 % CI 1·06, 1·66), while the association was not present with multivitamins only (Table 4). Stratification by time period showed that supplement use was associated with an increased risk of hydronephrosis in the first time period, but not in the second (online Supplementary Table S2). Associations, albeit not statistically significant, were observed for NTD, respiratory system defects and digestive system defects. Analyses with supplement use during pregnancy only showed protective associations for some birth defects, including defects in the nervous system, abdominal wall, genital organs and limbs (Table 3).
A total of 31 927 infants were from multiple pregnancies, and among these, 1063 (3·3 %) had birth defects, while 17 319 (2·0 %) of the singletons had birth defects. Excluding multiple births from the analysis did not affect the results much (online Supplementary Table S3). However, infants in multiple births had increased risk of total birth defects compared with singletons, RR 1·63 (95 % CI 1·55, 1·76). Yet, mothers of infants in multiple births used supplements before and during pregnancy slightly more than did mothers of singletons (25·6 and 21·4 %, respectively). The percentage of IVF among mothers with multiple births was 21·6 % compared with 2·0 % among mothers with singletons. Among all infants with birth defects (n 18 382), 3·6 % of them were from IVF (n 666). Infants from IVF pregnancies had an increased risk of total birth defects compared with naturally conceived infants (RR 1·33, 95 % CI 1·23, 1·44).
Discussion
The present study included eleven major non-chromosomal birth defect groups in the entire birth population of Norway during 1999–2013. The study found that folic acid and/or multivitamin supplement use before and during pregnancy was associated with a slight reduction in risk of infant total birth defects among live- and stillborn babies (aRR 0·94, 95 % CI 0·91, 0·98). The reduced risk was observed for birth defects in the abdominal wall, genital organs and limbs. Protective associations were also suggested for NTD, respiratory system defects and digestive system defects although CI included the null value of 1.
Mothers who smoked, who were younger, single or had higher parity were less likely to use folic acid supplements before and during pregnancy (Table 2). These factors could be related to a lack of pregnancy planning(Reference Nilsen, Mastroiacovo and Gunnes25). The rates of unplanned pregnancies in Norway are unknown, but in a large Norwegian cohort, 20 % reported that they had not planned the pregnancy(Reference Suren, Roth and Bresnahan26). Folate depletion increases if inter-pregnancy intervals are short(Reference Smits and Essed27), and we found that higher parity was associated with less use of supplements. Because of increased requirements of folate throughout pregnancy, depletion could result in an increased risk of birth defects in the subsequent neonate(Reference Kwon, Lazo-Escalante and Villaran28). Also, research has indicated that folate levels are lower in pregnant smokers than in non-smokers(Reference Tuenter, Bautista Nino and Vitezova29), and fetuses of pregnant smokers could possibly benefit from higher doses of folic acid. Still, we observed that smoking mothers used less folic acid supplements than non-smokers did (Table 2). This may be related to an educational gradient in smoking habits(Reference Grotvedt, Kvalvik and Groholt30). Nearly half of women with pregestational diabetes were defined as supplement non-users. This is probably partly due to missing data from the hospitals but could also be related to unplanned pregnancies. Research has indicated that folic acid use may be even more important in women with pregestational diabetes to prevent birth defects(Reference Parker, Yazdy and Tinker31,Reference Correa, Gilboa and Botto32) .
Comparison with previous studies
We have previously examined the relationship between folic acid or multivitamin supplement use and infant NTD(Reference Gildestad, Øyen and Klungsøyr33), oral clefts(Reference Gildestad, Bjørge and Vollset34) and heart defects(Reference Leirgul, Gildestad and Nilsen35) from the same source population (MBRN). There were several differences between the previous three studies and the current one. For example, the current paper separates total and isolated NTD. More than 20 % of infants with NTD had associated anomalies. We observed associations between supplement use and some of the anomalies that co-occurred with NTD (limbs, urinary, digestive defects, abdominal wall defects). Yet, the association measures were nearly identical for total and isolated NTD. A 2015 Cochrane review assessed the effects of folic acid supplementation on birth defects(Reference De-Regil, Pena-Rosas and Fernandez-Gaxiola19) and concluded that folic acid, alone or in combination with vitamins and minerals, prevents NTD, with high-quality evidence according to Grades of Recommendation Assessment, Development and Evaluation (GRADE) criteria(Reference Guyatt, Oxman and Vist36). However, there was insufficient evidence for other birth defects, motivating efforts to quantify the associations between use of folic acid and birth defects other than NTD(Reference Guyatt, Oxman and Kunz37). Supplement use was inversely associated with several of the birth defects that co-occurred with oral clefts. A 2018 systematic review from observational studies concluded that folic acid supplementation reduced the risk of non-syndromic oral clefts(Reference Jahanbin, Shadkam and Miri38), whereas in the 2015 Cochrane report, there were insufficient data from randomised controlled trials to evaluate the effects on oral clefts(Reference De-Regil, Pena-Rosas and Fernandez-Gaxiola19).
The association between folic acid and multivitamin supplement use and congenital heart defects has been reported in similar studies from Norway and Denmark(Reference Øyen, Olsen and Basit20,Reference Leirgul, Gildestad and Nilsen35) . In the study by Leirgul et al. (Reference Leirgul, Gildestad and Nilsen35), individuals with heart defects were identified from four data sources, including MBRN, whereas the present study used data from the MBRN updated with additional 4 years. Yet, the finding in the present study (aRR 1·05, 95 % CI 0·98, 1·12) was similar to the previous paper (aRR 1·10, 95 % CI 1·03, 1·16). Further, in two national birth cohorts in Norway and Denmark, with prospective data from nearly 200 000 births and 2247 individuals with heart defects, folic acid supplement use was not associated with heart defects (aRR 0·99, 95 % CI 0·80, 1·22)(Reference Øyen, Olsen and Basit20). These findings were also in accordance with the Cochrane review from 2015(Reference De-Regil, Pena-Rosas and Fernandez-Gaxiola19), albeit with low-quality evidence according to GRADE.
In our study, use of folic acid or multivitamins was associated with a reduced risk of abdominal wall defects, including both gastroschisis and omphalocele. This corresponds well with the literature(Reference Botto, Mulinare and Erickson39–Reference Paranjothy, Broughton and Evans41), although there are exceptions(Reference Godwin, Sibbald and Bedard42,Reference Feldkamp, Carmichael and Shaw43) .
Use of folic acid supplements before and during pregnancy was associated with reduced risk of infant hypospadias in our study, but we did not observe associations with multivitamins (Table 4). A Hungarian case–control study suggested a dose-dependent relationship between folic acid supplement use and risk for infant hypospadias(Reference Mavrogenis, Urban and Czeizel44). The folic acid doses were considerably higher than in our study, and however, our findings suggested similar associations. In a systematic review by Goh et al., no associations between multivitamin supplements and hypospadias were found from the selected cohort studies and randomised controlled trials(Reference Goh, Bollano and Einarson45).
We observed that the use of multivitamins and/or folic acid was associated with reduced risk of limb defects, with the largest risk reduction for the limb reduction subgroup (Table 3). Several studies have shown similar results, with risk reductions of about one-third(Reference Shaw, O’Malley and Wasserman46,Reference Wolf, Hegaard and Huusom47) . However, the quality of the evidence has not been convincing. We found a non-significant, but somewhat lower point estimate for limb reduction defects among infants of mothers using multivitamins only than among infants of folic acid only users (Table 4). The association nearly disappeared for use of folic acid combined with multivitamins. There was limited power to detect associations due to the small number of infants in the multivitamin group. In contrast to limb reduction defects, we found that the use of folic acid combined with multivitamins was associated with a reduced risk of infant clubfoot, while multivitamin use was not. One study has found that elevated plasma homocysteine levels were associated with increased risk of clubfoot(Reference Vollset, Refsum and Irgens48), which suggest that folate insufficiency contributes to the risk.
Use of folic acid supplements was associated with an increased risk of urinary system defects, mainly driven by the large proportion of infants with hydronephrosis in this category. This corresponds well with the literature(Reference Godwin, Sibbald and Bedard42,Reference Groen In ’t Woud, Renkema and Schreuder49,Reference Canfield, Collins and Botto50) . However, the association was not present for infants where mothers used multivitamins only, which does not correspond with previous findings(Reference Botto, Olney and Erickson17,Reference Wolf, Hegaard and Huusom47) . According to EUROCAT, there are discrepancies in diagnostic criteria for hydronephrosis cases(Reference Morris, Springett and Greenlees51), and registration of hydronephrosis is recommended only if the renal pelvis is 10 mm or more after birth, while the MBRN does not specify this distinction. Further, the ascertainment of hydronephrosis in the MBRN may be lower than that of birth defects that are visible after birth.
Study strengths and limitations
Major strengths of our study include the prospective design as well as the large study population of almost 900 000 infants, with detailed information on maternal periconceptional supplement use, more than 18 000 infants with birth defects, and information on several potential confounders. With a study of this magnitude, the effect size across all birth defects may be highly statistically significant even if associated with small deviations of the relative risk from 1. For example, it is possible to detect a 10 % risk reduction of a rare event, which may seem small at the level of the individual if the focus is on the clinical importance of the prevention strategy. One should bear in mind, however, that a small relative risk may have an important impact from a public health perspective.
Our study had some limitations. First, misclassification of exposure may have biased the association measures. The exposure information was in most cases gathered early in pregnancy, which reduces the risk of maternal recall bias, because mothers do not know at this point the birth defect status of their babies. Errors in reporting by the hospitals concerning supplement type and timing may have occurred. The group of non-users was a mixture of mothers who did not use supplements and mothers where the hospitals did not report the use (missing). This non-differential misclassification of the exposure is considered to be present and could have biased the association measures towards the null. According to a recent study from the MBRN that explored the epidemiology of limb reduction defects, including their relation with maternal use of folic acid/multivitamin supplements, approximately 12 % of all births (excluding TOPFA) had missing information on folic acid and multivitamin supplement use during 1999–2016(Reference Klungsøyr, Nordtveit and Kaastad52). When multiple imputation was applied to handle missing data on folic acid and multivitamin supplement use, the estimate was close to our finding for limb reduction defects (aRR 0·7, 95 % CI 0·6, 0·9)(Reference Klungsøyr, Nordtveit and Kaastad52).
There is a risk of misclassification of birth defects in the MBRN, with varying ascertainment for the different defects. Validation studies have been performed for certain birth defects (oral clefts(Reference Kubon, Sivertsen and Vindenes53), gastroschisis(Reference Kazaura, Lie and Irgens54) and Down syndrome(Reference Melve, Lie and Skjaerven55)) showing satisfactory results. However, for oral clefts, the ascertainment depended on the visibility and severity of the cleft, indicating that some birth defects may be under-reported to the MBRN. For example, it is likely that the diagnosis of hydronephrosis reported to the MBRN depends on a prenatal diagnosis by ultrasound, and many women only have the routine ultrasound around 18 gestational weeks.
Second, the lack of information on supplement use in TOPFA could have introduced selection bias. If women who terminated a pregnancy for a birth defect were less likely to use folic acid, the associations would be underestimated. However, if women who used folic acid were more likely to opt for a termination of pregnancy due to birth defects, it would bias the risk ratio towards the null. This situation could be plausible if increased folic acid supplement use was associated with better prenatal care, which in turn could be associated with a prenatal diagnosis of birth defects. However, in Norway, prenatal screening with ultrasound is free of charge and offered to all pregnant women at 18 gestational weeks or earlier for women with a high-risk pregnancy. Therefore, such differential bias is not likely. Except for nervous system defects (51·4 % TOPFA) and omphalocele (37·2 %), most pregnancies affected by the described birth defects were continued to delivery. Selection bias is thus mostly a concern for the association between supplement use and NTD and omphalocele. Women who use IVF are more likely to have a multiple gestations and are, by definition, pregnancy planners and therefore much more likely to use folic acid(Reference Vollset, Gjessing and Tandberg56). Birth defects occur more frequently in infants from IVF(Reference Hansen, Kurinczuk and Milne57) and multiple(Reference Glinianaia, Rankin and Wright23) pregnancies as compared with naturally conceived infants and singletons. However, birth defects from IVF pregnancies only accounted for 3·6 % of total birth defects, and the associations were nearly unchanged in analyses restricted to singletons. Thus, selection bias due to IVF and multiple gestations could be ruled out.
Third, we were able to adjust for a priori selected confounders known to be associated with birth defects and supplement use. Adjustment did not change the estimates much. However, stratification by time period showed that from 2006 onwards, associations were stronger for some defects. A possible explanation for this finding could be that more women complied with the recommendations in the latest time period. The reported prevalence of supplement use before and during pregnancy in the two time periods was 13·8 % (1999–2005) and 28 % (2006–2013). Analyses conditioned on complete smoking information produced similar results as when assigning missing to a separate category. Adjusting for year of birth slightly weakened the association measure (aRR total birth defects without adjusting for year of birth: 0·90, 95 % CI 0·87, 0·94), possibly due to differences in the prevalence of supplement use, and natural variations in outcome prevalence over the study period. The observed associations in our study could be due to unmeasured confounding. We had no information on maternal BMI(Reference Persson, Cnattingius and Villamor58) or baseline diet(Reference Carmichael, Yang and Feldkamp59).
The exact mechanism of why folic acid supplementation prevents NTD or other birth defects is not known, but associations have been established with erythrocyte folate concentration(Reference Bailey and Hausman60). Women below the cut-off for what is considered optimal erythrocyte are thought to have elevated risk of having babies with NTD(Reference Botto and Mastroiacovo61). Folate functions as a coenzyme in one-carbon metabolism during the methylation cycle and is crucial for DNA synthesis, and during pregnancy, there are higher rates of one-carbon transfer reactions(Reference Gernand, Schulze and Stewart4). The randomised controlled trials establishing a preventive role for NTD were specific to folic acid. Dietary folate is less bioavailable than folic acid, and consumption of dietary folates is considered to result in a significantly smaller increase in erythrocyte folate concentration than folic acid(Reference Cuskelly, McNulty and Scott62,Reference McNulty and Pentieva63) . Since food fortification with folic acid is not mandatory in Norway, the prevention strategy with a supplementation programme requires a high proportion of planned pregnancies and high compliance with the programme. Many Norwegian women are not aware of the importance of supplementation before pregnancy and have diets that do not meet the recommendations. According to MBRN online statistics (http://statistikkbank.fhi.no/mfr/), maternal use of folic acid supplements before pregnancy was reported for around a third of infants in 2018. The proportion of women who use folic acid supplements before conception is even lower in some subgroups of the population(Reference Nilsen, Daltveit and Iversen64). Since the prevalence of folic acid supplement use remains low, efforts to increase supplement compliance are needed.
Our findings suggest that some birth defects may be prevented by maternal use of folic acid or multivitamins, but that the extent of the risk reduction differs for the different types of birth defects and type of exposure (multivitamin or folic acid). Better definitions of the exposures regarding specific supplement types, timing and dosage would be beneficial in understanding the different roles of specific micronutrients in the aetiology of birth defects. Thus, in unplanned pregnancies, even though the time window to prevent NTD is closed, initialising supplementation during pregnancy could be beneficial to reduce defects that occur later in the first trimester. Since our study suggests that folic acid or multivitamin supplementation could have protective associations for other birth defects besides NTD, the prevention potential may be larger than anticipated. With an annual birth rate of nearly 60 000 and a birth defect prevalence of about 2 %, folic acid supplements used by the 40 000 women reported as non-users could prevent birth defects in nearly fifty infants annually in Norway, assuming the protective associations of 6 % risk reduction in our study were causal.
In conclusion, use of folic acid and/or multivitamin supplements before and during pregnancy was associated with a small reduction in the risk of total birth defects among live births and stillbirths. More specifically, the risk reduction was observed for abdominal wall defects, genital defects and limb defects. Folic acid supplement use was associated with increased risk for hydronephrosis. Protective associations were suggested for supplement use and NTD, respiratory system defects and digestive system defects although CI included the null value of 1.
Acknowledgements
The study was funded by the Western Norway Regional Health Authority (Helse Vest), project no. 911647 (to T. G.) and no. 911629 (to N. Ø.).
T. G., T. B., Ø. A. H., K. K., S. E. V. and N. Ø. all substantially contributed to the conception and interpretation of data. T. G. drafted the article and analysed data in collaboration with biostatistician Ø. A. H. All authors revised it critically for important intellectual content and gave their approval of the final version.
None of the authors reported a conflict of interest related to the study.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520001178








