Maternal malnutrition during pregnancy and/or lactation exerts long-term effects on fetal development and postnatal growth, and may predispose offspring to metabolic diseases later in life(Reference Symonds, Stephenson and Gardner1). Maternal low-protein (LP) diet or dietary protein restriction has been widely adopted as a model to reveal the possible mechanisms. The majority of the studies were carried out in mammals, and there are evidences that maternal LP diet alters endocrine status of the mother and placenta serves as a gateway to selectively relay the signals from mother to developing embryos(Reference Myatt2). As a result, expression profiles for specific genes or gene networks in specific tissues of developing fetus are modified(Reference Hanson and Gluckman3), which, consequently, induces phenotypic changes later in life.
In mammals, glucocorticoid(Reference Drake, Walker and Seckl4), thyroid hormones(Reference Mostyn, Sebert and Litten5) and leptin(Reference Mostyn, Sebert and Litten5, Reference Miralles, Sánchez and Palou6) are the major hormones mediating the programming effect of maternal nutrition on offspring performances. Although, potentially, all the tissues are affected by maternal malnutrition, placenta and fetal brain are the most intensively studied and are believed to be highly susceptible to prenatal environment. In the placenta, three representative genes are reported to participate in the endocrine mediation of maternal influences on fetal development, namely 11β-hydroxysteroid dehydrogenase2, which inactivates cortisol to cortisone to protect the fetus from overexposure to glucocorticoid(Reference Stewart and Krozowski7), leptin receptor (LepR), which mediates the action of leptin(Reference Baratta8), and transthyretin (TTR), a thyroxine (T4)-binding protein to transport thyroid hormones into the embryo(Reference McKinnon, Li and Richard9). In the brain, corticotropin-releasing hormone (CRH) and thyrotropin-releasing hormone (TRH) are hypothalamic-releasing factors of the hypothalamic–pituitary–adrenal (HPA) and hypothalamic–pituitary–thyroid axes, respectively, which determines the ‘set point’ for growth and metabolism, as well as energy homeostasis. Corticosterone metabolic enzymes, glucocorticoid receptor (GR) and LepR expressed in the hypothalamus have been shown to fine-tune the HPA and hypothalamic–pituitary–thyroid functions(Reference Dutriez-Casteloot, Breton and Coupé10–Reference Arora12). It has been suggested that maternal nutritional or endocrine intervention may modify the pattern of gene expression in the fetal hypothalamus and thus cause a shift of the metabolic set point in mammals(Reference Adam, Findlay and Chanet13).
Birds offer some advantages over mammals in investigating the maternal influence or developmental programming because all the signals to be passed from mother to offspring are deposited in the egg. The hormone-mediated maternal effects have been investigated both descriptively and experimentally in birds, focusing on the effects of yolk steroids, especially testosterone, on postnatal growth and behaviour(Reference Groothuis and Schwabl14). By far, most of the work is related to behaviour ecology using wild birds as a model, and the maternal hormones in the egg yolk concern mainly sex steroids(Reference Groothuis, Muller and von Engelhardt15), glucocorticoids(Reference Hayward, Satterlee and Wingfield16) or thyroid hormones(Reference Wilson and McNabb17). Recently, we reported leptin deposition in the chicken egg and its correlations with maternal leptin concentrations in the plasma and liver, as well as embryonic and post-hatch growth of the offspring(Reference Hu, Ni and Ren18), adding a new member to the list of yolk hormones in avian species.
Nevertheless, it remains largely unknown how maternal hormones deposited in the egg yolk exert their actions on the embryo. Two basic questions need to be answered. First, are genes encoding response components such as metabolic enzymes, transporters or receptors expressed on the yolk-sac membrane and responsive to maternal nutrition? Second, what tissues and genes in the developing embryo are affected and how such modifications are associated with phenotypic changes of the offspring?
It is well known that nutritional interventions, such as fasting and protein deficiency, induce significant alterations in the endocrine status of chickens(Reference Scanes and Griminger19). However, there are scarcely any data linking maternal nutrition to yolk hormone deposition and progeny embryonic development in domestic fowls, and the pathways through which the maternal hormones in the yolk programme the progeny development and growth have not been examined.
Therefore, the present study was aimed to test the hypothesis that maternal LP diet may programme offspring growth by modifying maternal hormone deposition in the yolk and subsequently or simultaneously altering the pattern of gene expression in important tissues of developing chicken embryos. Metabolic hormones including corticosterone, thyroid hormone and leptin were measured. Yolk-sac membrane as a mother–offspring interface, fetal hypothalamus representing central regulatory system and pectoralis major muscle as an indication for fetal growth were sampled at embryonic day 14 (E14) to monitor the alterations in the pattern of gene expression. Genes presumably involved in mother–offspring signal transportation including 20-HSD, TTR and LepR in the yolk-sac membrane, genes participating in central metabolic regulation such as 20-HSD, GR, TRH, CRH and LepR in the hypothalamus, as well as genes regulating muscle growth such as insulin-like growth factors I and II (IGF-I and IGF-II) and IGF-I receptor (IGF-IR), were selected as targets of investigation.
Materials and methods
Animals and experimental design
A highly inbred Chinese indigenous broiler chicken strain, Langshan, was employed in the present study in order to ensure the homogeny of the genetic background, as most of the commercial broiler chicken lines are hybrids and may cause high individual variations. One hundred and twenty 44-week-old Langshan breeder hens were selected from the parent stock kept in the Institute of Poultry Science, Chinese Academy of Agricultural Sciences (Yangzhou, China) and randomly allocated to the control (Con) and LP diet groups. The Con group was fed a standard diet containing 15 % crude protein according to the nutritional standard established for the breed, while the LP group was fed diets with 10 % crude protein. Lysine and methionine contents were adjusted accordingly, while energy, vitamins and trace element contents were kept the same in the two groups. The diet composition is shown in Table 1. Hens were artificially inseminated. Eggs were collected in the 4th week after the initiation of the dietary treatment. The experiment lasted for a month. Body weight of hens was recorded at the beginning and the end of the experiment.
Table 1 Composition of the basal diet used in the present experiment
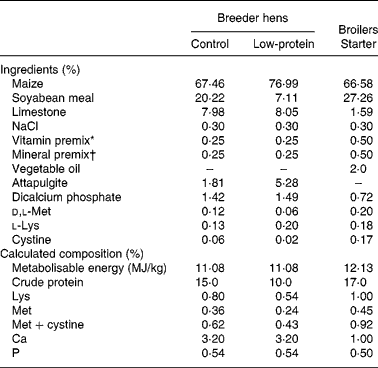
* Vitamin premix provided (per kg of diet): vitamin A, 84 000 IU; vitamin D3, 1500 IU; vitamin E, 11 mg; vitamin K3, 1·5 mg; thiamine, 1·1 mg; riboflavin, 6·6 mg; niacin, 66 mg; pantothenic acid, 16·5 mg; biotin, 0·11 mg; folic acid, 1·1 mg; vitamin B12, 13·2 g; ethoxyquin, 125 mg.
† Mineral premix provided (per kg of diet): manganese sulphate, 68 mg; zinc oxide, 55 mg; iron sulphate, 26 mg; copper sulphate, 4·4 mg; iodine, 1·0 mg; selenuim, 0·1 mg.
Eggs of similar size (ten for each group) were used to determine the yolk content of tri-iodothyronine (T3), T4 and leptin using RIA, and corticosterone using enzyme immunoassay. The yolk was separated from the albumen, homogenised and stored at − 20°C before the analysis.
One hundred and forty eggs from each group were incubated at 37·8°C with 60–70 % relative humidity following standard settings for automatic turning and ventilation. The eggs in Con and experiment groups were placed side by side and distributed equally on different shelves in the incubator to minimise the possible variations in the incubation condition. On E14, ten eggs from each group were taken from matched location for tissue sampling. The egg weight and embryo weight were recorded and the yolk sac was immediately removed and washed twice in saline. The pectoralis major muscle and the hypothalamus were dissected. All the tissue samples were snap-frozen in liquid N2 and then transferred to − 80°C until RNA extraction. The remaining eggs were incubated until hatch. At hatching, ten chicks from each group were killed for plasma and tissue sampling. The remaining chicks were reared under the same diet (Table 1) until 4 weeks of age. The body weight was recorded every week and the pectoralis major muscle was dissected and weighed at 1 and 28 d of age.
The experiment was undertaken following the guidelines of the Animal Ethics Committee in Nanjing Agricultural University.
Hormone assays
RIA
All hormones except corticosterone were measured using commercial RIA kits. Multi-species leptin RIA kit was purchased from Beijing North Institute of Biotechnology (Beijing, China) with an intra-assay CV of 5 %. Total T3 and T4 RIA kits were purchased from Shanghai Institute of Biological Products (Shanghai, China) with an intra-assay CV of 5 % for both kits. Egg yolk samples were extracted, as previously described(Reference Wilson and McNabb17), for thyroid hormone analysis. Yolk leptin was extracted according to De Pablo et al. (Reference De Pablo, Roth and Hernandez20). All samples were analysed in duplicate within one assay to avoid inter-assay variations. The commercial RIA kits were validated for measuring chicken samples(Reference Hu, Ni and Ren18, Reference Li, Yuan and Yang21).
Enzyme immunoassay
Yolk corticosterone was extracted before assay according to Williams et al. (Reference Williams, Ames and Kiparissis22) and was determined with a commercial enzyme immunoassay kit (Cayman Chemical Company, Ann Arbor, MI, USA; catalogue number: 500651-96). The detection range of the kit was 24–10 000 pg/well, and all determinations fell within this range. The inter- and intra-assay CV were 3·5 and 8·4 %, respectively. The cross-reactivity of the antibody with other steroids was < 0·5 %.
Total RNA extraction
A portion (about 30 mg) of the yolk-sac membrane and pectoralis major muscle were used to extract total RNA using TRIzol total RNA kit (Tiangen Biotech Co., Ltd, Beijing, China). The whole hypothalamus was ground in liquid N2 and a portion of about 50 mg was used for RNA extraction. The total RNA concentration was quantified by measuring the absorbance at 260 nm in a photometer (Eppendorf Biophotometer, Hamburg, Germany). The 260:280 ratios of all preparations were between 1·8 and 2·0. Aliquots of RNA samples were subjected to electrophoresis through a 1·4 % agarose–formaldehyde gel to verify their integrity.
Reverse transcription
Two micrograms of total RNA were reverse transcribed by incubation at 37°C for 1 h for the first-strand cDNA synthesis in a 25 μl mixture consisting of 1 × reverse transcription buffer, 10 mm-deoxynucleotide triphosphates, 10 U RNase inhibitor, 100 U moloney murine leukaemia virus reverse transcription and 2·5 μm random hexamer primers. The reaction was terminated by heating at 95°C for 5 min and quickly cooling on ice.
Real-time PCR
Real-time PCR were performed in Mx3000P (Stratagene, La Jolla, CA, USA). Different controls were set to monitor the possible contamination of genomic and environmental DNA both at the stage of reverse transcription and polymerase chain reaction. The pooled sample made by mixing equal quantity of total RNA from all samples was used for optimising the PCR condition and tailoring the standard curve. Melting curves were performed to confirm specific amplification for each gene. Two microlitres of the reverse transcription product, diluted 8- to 16-fold, were used in a final volume of 25 μl containing 12·5 μl synergy brands green real-time PCR master mix (code number: QPK-201; Toyobo, Japan) and 0·2–0·8 μmol/l primer pairs for IGF-I, IGF-II, IGF-IR, 20-HSD, TTR, GR, CRH, TRH and LepR, and chicken β-actin was used as a reference gene for normalisation purposes. The PCR products were sequenced for verifying specificities. The primer sequences and PCR conditions for each gene are shown in Table 2.
Table 2 Nucleotide sequences of specific primers and PCR conditions

IGF, insulin-like growth factor; IGF-IR, IGF-I receptor; TTR, transthyretin; CRH, corticotropin-releasing hormone; LepR, leptin receptor; GR, glucocorticoid receptor; 20-HSD, 20-hydroxysteroid dehydrogenase; TRH, thyrotropin-releasing hormone.
Statistical analysis
The method of 2− ΔΔCT was used to analyse the real-time PCR data expressed as the fold change relative to the Con group(Reference Livak and Schmittgen23). All data were presented as mean values with their standard errors. Statistical analyses were carried out with SPSS 11.0 for Windows (SPSS Inc., Chicago, IL, USA). The differences were tested with ANOVA using t test for independent samples. A P-value < 0·05 was considered significant.
Results
Performance of hens and offspring
Low dietary protein level did not affect the body weight of hens, but significantly (P < 0·05) decreased egg weight and the laying rate (Table 3). LP diet did not affect the fertility rate (LP 97·4 % v. Con 97·7 %), but decreased hatchability of fertile eggs from 82·9 to 74·5 %. Since no replicates were set up for each group during incubation, statistical analysis was not possible for fertility or hatchability. Embryo weight at E14 did not show difference between the two groups, but both body weight and pectoralis major muscle weight at hatching were significantly decreased in the LP group compared with the Con (P < 0·001 and P = 0·024, respectively). The LP offspring demonstrated significantly higher post-hatch growth rate, which resulted in significantly heavier body weight and pectoralis major muscle weight (P = 0·047 and 0·001, respectively) at 4 weeks post-hatching (Table 4).
Table 3 Effect of low-protein (LP) diet on breeder body weight (n 15), egg weight (n 120) and egg-laying rate (n 30)
(Mean values with their standard errors)

Mean values were significantly different from that of the control group: *P < 0·05, **P < 0·01.
Table 4 Effect of maternal low-protein (LP) diet on body weight and pectoralis muscle weight
(Mean values with their standard errors)
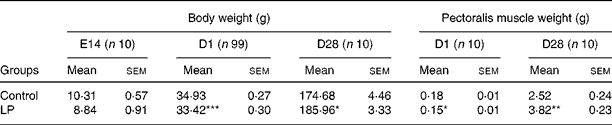
E14, embryonic day 14; D1, day 1; D28, day 28.
Mean values were significantly different from that of the control group: *P < 0·05, **P < 0·01, ***P < 0·001.
Plasma and yolk hormone concentration
The yolk leptin content was significantly lower in eggs laid by LP hens (P = 0·026), while no differences were detected for yolk contents of corticosterone, T3 or T4 (P = 0·314, 0·72 and 0·066, respectively). The LP offspring had significantly higher serum T3 concentration at hatching (P = 0·006), yet no difference was found in plasma T4 (P = 0·228; Table 5).
Table 5 Effect of maternal low-protein (LP) diet on yolk hormone contents and serum hormone concentrations in the progeny at hatching
(Mean values with their standard errors)

D1, day 1; T3, tri-iodothyronine; T4, thyroxine.
Mean values were significantly different from that of the control group (*P < 0·05, **P < 0·01, n 10).
Gene expression in yolk-sac membrane, hypothalamus and pectoralis major muscle
As shown in Fig. 1, 20-HSD mRNA expression was significantly down-regulated in the yolk-sac membrane of the LP embryo at E14 (P = 0·043), whereas no significant alterations were observed for the TTR and LepR mRNA expression (P = 0·943 and 0·376, respectively).

Fig. 1 Effect of maternal low-protein (LP) diet on mRNA expression of (a) 20-hydroxysteroid dehydrogenase, (b) leptin receptor and (c) transthyretin in the yolk-sac membrane of embryonic day 14 embryos. The difference between the two groups was significant (*P < 0·05, n 10). Con, control.
Hypothalamic expression of 20-HSD, GR, TRH and LepR increased significantly in LP embryos compared with their control counterparts (P = 0·04, 0·016, 0·024 and 0·024, respectively), while no difference was observed for the CRH mRNA expression (P = 0·737; Fig. 2).

Fig. 2 Effect of maternal low-protein (LP) diet on mRNA expression of (a) corticotropin-releasing hormone, (b) thyrotropin-releasing hormone, (c) glucocorticoid receptor, (d) leptin receptor and (e) 20-hydroxysteroid dehydrogenase in the hypothalamus of embryonic day 14 embryos. The difference between the two groups was significant (*P < 0·05, n 10). Con, control.
In the pectoralis major muscle, expression of IGF-I and IGF-IR mRNA was significantly increased (P = 0·011 and 0·019, respectively) in LP embryos, while that of IGF-II mRNA was not affected by maternal dietary protein restriction (P = 0·069; Fig. 3).

Fig. 3 Effect of maternal low-protein (LP) diet on mRNA expression of (a) insulin-like growth factor (IGF)-I, (b) IGF-II and (c) IGF-I receptor in the pectoralis muscle of embryonic day 14 embryos. The difference between the two groups was significant (*P < 0·05, n 10). Con, control.
Discussion
Stringent feed restriction has been a routine practice in the broiler breeder management to reduce body size in order to improve egg production. Earlier studies demonstrated that reducing crude protein intake of broiler breeders from 16 to 10 %, while maintaining intake of critical amino acids, reduced egg weight and hatch weight, but no lasting effect was observed on offspring weight at 48 d, suggesting a catch-up growth after hatching(Reference Lopez and Leeson24). Unfortunately, this phenomenon did not attract enough attention beyond its economic significance at the time and the underlying mechanism was not explored. The catch-up growth or compensatory growth in the progeny of hens fed LP diet was also observed in the present study, although the contents of limiting amino acids were adjusted according to the crude protein values. However, the deficiencies of limiting amino acids, especially methionine and lysine, may be responsible for the decreased laying rate observed in the present study in LP hens, which is in accordance with the early finding(Reference Bowmaker and Gous25).
Maternal nutrition may programme placental function, offspring embryogenesis and postnatal growth in mammals(Reference Symonds, Stephenson and Gardner1). In birds, sex steroid hormones(Reference Groothuis and Schwabl14, Reference Groothuis, Muller and von Engelhardt15), corticosterone(Reference Hayward, Satterlee and Wingfield16), thyroid hormones(Reference Wilson and McNabb17) and, recently, leptin(Reference Hu, Ni and Ren18) have been reported to be present in egg yolk and presumably involved in mediating the maternal effect. While most of the experiments applied hormone supplementation to achieve alterations in maternal endocrine status and thus yolk hormone contents, few studies focused on alterations of hormone content in egg yolk induced by maternal nutrition. We determined the yolk content of corticosterone, T3 and T4, as well as leptin in the present study and all the values were similar to what was previously reported(Reference Hayward, Satterlee and Wingfield16, Reference Hu, Ni and Ren18, Reference Sechman and Bobek26). No differences were detected for yolk corticosterone, T3 or T4, but leptin content was found to be significantly reduced in the yolk of eggs laid by hens fed LP diet. Since no significant alterations were found in egg composition and egg quality traits (data not shown), and eggs of similar size were used for measuring yolk hormones, it is unlikely that the reduced yolk leptin content in LP eggs was a consequence of altered egg characteristics.
Chicken leptin has been a sensitive and widely disputed issue(Reference Sharp, Dunn and Waddington27). Discrepancies in detecting leptin gene expression in chicken and failure to map the published chicken leptin sequence to chicken genome led to a conclusion that the existence of chicken leptin gene and its encoded protein has not been clarified. However, leptin or leptin-like immunoreactivity has been detected in chicken plasma(Reference Dridi, Williams and Bruggeman28), gastroenteric tract(Reference Neglia, Arcamone and Gargiulo29), as well as liver and egg(Reference Hu, Ni and Ren18) by using RIA, immunocytochemistry and/or Western blot analysis. Furthermore, the existence of a functional chicken LepR(Reference Adachi, Takemoto and Bungo30) and the biological responses of different systems in the chicken to exogenous recombinant leptin(Reference Dridi, Taouis and Gertler31–Reference Sirotkin and Grossmann34) strongly suggest the presence of a leptin-like signalling system in the chicken. The detected leptin-like immunoreactivity in the present and other studies may represent the endogenous ligand for LepR, which is yet to be identified.
Maternal LP isoenergetic diet significantly decreased maternal plasma leptin levels in rats(Reference Fernandez-Twinn, Ozanne and Ekizoglou35), yet no data are available describing the effect of protein restriction on leptin concentration in the chicken. Nevertheless, as in mammals, plasma leptin level in the chicken was also considered as an indicator for consistent food supply and more stable energy status(Reference de Beer, McMurtry and Brocht36). Unfortunately, the plasma leptin concentration of hens was not measured in the present study, because any invasive procedure, other than modest dietary treatment, was not approved to apply to the highly protected parent stock of the pure-bred indigenous chicken. Therefore, the effect of LP diet on plasma leptin concentration in breeder hens and the correlation between leptin levels in plasma of hens and egg yolk are still to be determined.
Both maternal protein restriction(Reference Bautista, Boeck and Larrea37, Reference Zambrano, Bautista and Deás38) and prenatal leptin exposure(Reference McMillen, Edwards and Duffield39) have been shown to programme offspring performance in mammals. In the present study, the reduced yolk leptin content was found to be associated with faster post-hatch growth, which agrees with what we reported recently that higher yolk leptin content corresponded to attenuated post-hatch growth in offspring of dietary cysteamine-treated hens(Reference Hu, Ni and Ren18). It is well known that chicken hatch weight is closely correlated with egg weight and, indeed, a strong positive correlation between egg weight and hatch weight (R 2 0·9275, P < 0·01; data not shown) was observed in the present study. It is possible that the decreased hatch weight in the LP group resulted from the lower egg weight, but the significantly higher growth rate demonstrated in the LP offspring implicates a more complex mechanism.
Chicken embryo develops independent of the mother and all the nutritive substances and regulatory signals including hormones must be contained in the egg. The yolk sac envelops the yolk and produces enzymes and transporters that help to mobilise and transport the nutrients from the yolk to the developing embryo(Reference Holdsworth and Wilson40, Reference Powell, Deans and Speake41). Similarly, for maternal hormones in the yolk to exert actions on the embryo, receptors, binding proteins or transporters for specific hormones must be present on the yolk-sac membrane. However, information is scarce regarding the expression and function of proteins responsible for maternal signal transportation or transduction in the yolk-sac membrane.
LepR mRNA was detected in the turkey yolk-sac membrane(Reference Richards and Poch42), but its function is largely unknown. Since leptin is found to exist in the chicken egg yolk, we assumed that yolk leptin may bind to its receptor on the yolk-sac membrane to achieve either its transfer across the membrane or its signal transduction to regulate the yolk-sac function per se. However, maternal LP diet did not affect the LepR mRNA expression in the chicken yolk-sac membrane, although the yolk leptin content was significantly reduced.
In mammals, maternal undernutrition and low birth weight was associated with the reduction of placental 11β-hydroxysteroid dehydrogenase2 activity(Reference Lesage, Sebaai and Leonhardt43). In birds, 20-HSD is the most abundantly and more ubiquitously expressed enzyme, which transforms glucocorticoids to inactive 20-dihydrocorticosterone(Reference Kucka, Vagnerová and Klusonová44). We detected, for the first time, 20-HSD mRNA expression in the chicken yolk-sac membrane and a significantly decreased expression was shown in the LP group. It remains unknown how maternal protein restriction may affect the yolk-sac expression of 20-HSD. There is evidence indicating a role of leptin in regulating hepatic 11β-hydroxysteroid dehydrogenase2 expression(Reference Gluckman, Lillycrop and Vickers45). However, whether and how yolk leptin is involved in the regulation of yolk-sac 20-HSD mRNA expression is worthy of further investigation.
Serum T4-binding protein, TTR (formerly prealbumin), is made by hepatocytes and choroid plexus epithelium in adults and by yolk-sac cells in embryogenesis(Reference Soprano, Soprano and Goodman46). TTR is one of the important T4 transporters in birds and was suggested to transport thyroid hormones into the embryo(Reference Southwell, Duan and Tu47). However, neither yolk thyroid hormones nor TTR mRNA expression in the yolk sac were affected by maternal LP diet in the present study.
The hypothalamus is one of the most susceptible targets for fetal programming induced by maternal nutrition(Reference Symonds, Stephenson and Gardner1). The chicken embryo is structurally complete by E14(Reference Moran48), and most of the neuroendocrine regulatory networks in the hypothalamus establish and start to function about this time(Reference De Groef, Grommen and Darras49). We speculated that the profiles of hypothalamic expression of genes involved in metabolic and energy homeostasis regulation may be modified by maternal protein restriction at this stage of chicken embryonic development. The HPA axis has been suggested to be one of the major targets for metabolic programming and is known to be involved not only in stress response, but also in energy homeostasis in mammals(Reference Vieau, Sebaai and Leonhardt50, Reference McMillen and Robinson51). A short period (48 h) of maternal nutrient restriction inhibited CRH mRNA expression in the fetal hypothalamus of guinea pigs(Reference Go, Lingas and Wheeler52), yet, in the present study, maternal LP diet did not affect hypothalamic CRH mRNA expression in E14 chicken embryos. A number of factors, including species, method of restriction, timing of sampling, etc., may contribute to such discrepancy.
It is known that peripheral corticosterone feeds back at the level of the hypothalamus to regulate CRH mRNA expression and thereby HPA function in the chicken(Reference Vandenborne, De Groef and Geelissen53). Therefore, GR and corticosterone metabolic enzyme (20-HSD) in the hypothalamus may potentially be the targets for HPA programming. Indeed, mRNA expressions of GR and 20-HSD were both significantly up-regulated in the hypothalamus of E14 LP embryos, indicating a profound impact of maternal nutrition on the HPA axis in the chicken progeny.
TRH in the hypothalamus plays a major role in regulating the hypothalamic–pituitary–thyroid axis(Reference Nillni and Sevarino54), the most important neuroendocrine system in growth regulation in the chicken(Reference De Groef, Vandenborne and Van As55). We observed a significant up-regulation of TRH mRNA in the hypothalamus of the E14 embryo of the LP group, which may account, to some extent, for higher growth rate after hatching. However, it is unknown whether increased hypothalamic TRH mRNA expression at E14 is responsible for higher serum T3 at hatching, because no significant change was observed for serum T4 level. Other pathways, including the somatotropic axis, may be involved in the regulation of accelerated post-hatch growth in the LP progeny. The significant up-regulation of LepR mRNA expression in the hypothalamus may hint possible changes in the regulation of energy metabolism and homeostasis.
Skeletal muscle, especially breast muscle, is the major product in the broiler industry. In the present study, the LP offspring demonstrated significantly heavier body weight and pectoralis major muscle weight 4 weeks post-hatching. We hypothesised that maternal protein restriction may also programme myogenesis during chicken embryonic development. Among all the factors involved in regulating myogenesis, IGF-I and II are key regulators for vertebrate muscle development and growth through endocrine and/or paracrine/autocrine pathways(Reference Duclos56). In birds, locally produced IGF are more important for embryonic myogenesis since hepatic IGF-I mRNA expression and circulating IGF-I level were very low until the late stage of embryonic development(Reference Lu, McMurtry and Coon57). Unlike mammalian species, both IGF-I and II seem to share a common receptor, IGF-IR, in birds(Reference Duclos58). Expression of IGF-I and IGF-IR mRNA was significantly up-regulated in the pectoralis major muscle of the LP offspring, while that of IGF-II showed a trend of increase, which may contribute, at least partly, to the accelerated muscle growth during early post-hatch development.
Taken together, the present results demonstrated that maternal protein deficiency exerts a profound influence on embryonic development and post-hatch growth of the progeny, which is associated with modifications in leptin deposition in the yolk, as well as alterations in the pattern of gene expression in yolk-sac membrane, hypothalamus and muscle of developing chicken embryos. These results provide evidences for the fetal programming effect of maternal nutrition in the chicken, and offer hints for further investigations into the signal transmission pathways involved in mediating such effects.
Acknowledgements
The present work was supported by National Basic Research Program of China, 2004CB117505 (R. Z.) and Sino-German Bilateral Cooperation in Agriculture, No. 28 (R. Z. and R. G.). K. R., J. X. and X. Y. were responsible for the quantification of gene expression and contributed to the drafting of the manuscript; L. C. carried out the animal study, took samples and measured hormone levels; R. G. and R. Z. designed the experiment, supervised the whole process and finalised the manuscript. The authors have no conflicts of interest.










