In general, the worldwide population consumes much more Na as dietary salt (NaCl) than is necessary for physiological functions( Reference Elliott and Brown 1 ). Currently, average Na consumption is 3·95 g/d( Reference Powles, Fahimi and Micha 2 ), whereas the WHO recommends that adults, including pregnant and lactating women, should consume <5 g of salt/d (equivalent to 2 g Na)( Reference Elliott and Brown 1 ). Such values should be adjusted downwards for children, based on their energy requirements( Reference Elliott and Brown 1 ). The main public health concern related to excessive salt intake is its association with high blood pressure( 3 – Reference Ito, Takeda and Hamano 5 ), a major risk factor for CVD, which are the leading causes of death worldwide( Reference Tuomilehto, Jousilahti and Rastenyte 6 – Reference Tientcheu, Ayers and Das 9 ). Furthermore, raised blood pressure is also associated with renal failure, cerebrovascular diseases, impaired cognitive function, hippocampal atrophy, as well as an increased risk of stroke and dementia( Reference Kisser, Allen and Katzel 10 – Reference Korf, White and Scheltens 15 ). Cost-effective preventive policies to reduce salt intake result in reduction in blood pressure( Reference Elliott and Brown 1 ), which can decrease the risk of cerebrovascular problems( Reference Skoog and Gustafson 13 , Reference Blaustein, Zhang and Chen 16 – Reference Stocker, Monahan and Browning 18 ). On the other hand, the Framingham Heart Study did not consider salt intake to be an important influence on blood pressure, defining the environment modifying factors responsible for hypertension, such as smoking, physical activity, diet/alcohol and air pollution( Reference O’Donnell and Elosua 19 ). Clearly, more studies are necessary to clarify the impact of salt intake on brain health and disease.
Pregnancy is a decisive period for fetal metabolic programming( Reference Heijmans, Tobi and Stein 20 ); therefore, maternal salt intake should be controlled. Gestational hypertension increases the risk of pre-eclampsia, a clinical condition that increases fetal and maternal morbidity and mortality( Reference Amaral, Wallace and Owens 21 ). Elevated blood pressure during pregnancy can lead to adverse outcomes in the offspring, such as fetal growth restriction, high childhood blood pressure( Reference Staley, Bradley and Silverwood 22 , Reference Miliku, Bergen and Bakker 23 ), neurodevelopment delay( Reference Warshafsky, Pudwell and Walker 24 ), childhood obesity and an increased risk of CVD in adulthood( Reference Zhang, Wang and Leng 25 ). However, the underlying cellular and molecular mechanisms of these effects on metabolism are unknown. According to the Barker hypothesis, the prenatal and early postnatal environments, including maternal diet, exert long-term effects on the health status of offspring( Reference Hales and Barker 26 , Reference Morton, Cooke and Davidge 27 ) through the modification of gene expression by epigenetic mechanisms( Reference Hales and Barker 26 , Reference Mathias, Elmhiri and de Oliveira 28 , Reference Bale 29 ). In this context, metabolic programming in the uterus alters the risk of chronic disease development in adulthood( Reference Mathias, Elmhiri and de Oliveira 28 , Reference Canani, Costanzo and Leone 30 ).
A high-salt diet in rodents is used to model the phenotypic condition of hypertension( Reference Drenjančević-Perić, Jelaković and Lombard 31 – Reference Liu, Chen and Li 34 ), and it can give additional data concerning underlying cellular and molecular mechanisms, such as the involvement of oxidative stress in several tissues( Reference Mayyas, Alzoubi and Al-Taleb 35 – Reference Togliatto, Lombardo and Brizzi 37 ), as well as impaired synaptic plasticity( Reference Ge, Wang and Wu 38 ), although this remains elusive clinically. Although evidence from rodents fed a high-salt diet during pregnancy reinforces the long-term impact on the cardiovascular health of offspring( Reference Svitok, Molcan and Vesela 39 , Reference Maruyama, Kagota and Van Vliet 40 ), there is a lack of information about the effects of high maternal salt consumption on offspring brain development.
On the basis of worldwide dietary habits involving the consumption of a high-salt diet even during pregnancy and the wide range of its related complications for mother and offspring( Reference Mathias, Elmhiri and de Oliveira 28 , Reference Canani, Costanzo and Leone 30 ), we sought to investigate the effect of such a diet during pregnancy and lactation on the brains of offspring in terms of redox state and mitochondrial viability.
Methods
Reagents
The reagents were obtained from SIGMA® Chemical Co. and Life Technologies.
Animals
Wistar rats, 60-d-old female (forty animals) and 90-d-old male (twenty animals), were housed at Central Animal House of Departamento de Bioquímica, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil, under air-conditioned constant temperature (22±1°C) and humidity, on a 12 h light–12 h dark cycle, with free access to food (control diet or high-salt diet) and water. The diet was in accordance with expected macronutrients and micronutrients required by the population (National Research Council (US) Subcommittee on Laboratory Animal Nutrition).
The study was approved by the local Ethics Commission of the Universidade Federal do Rio Grande do Sul (CEUA/UFRGS) under the reference no. 28100. All experiments were conducted according the National Animal Rights Regulation (Law 11.794/2008), the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH publication no. 80–23, revised in 1996), as well as the Animal Research: Reporting of In Vivo Experiments (ARRIVE) Guidelines for Reporting Animal Research. We further attest that all efforts were made to minimise the number of animals used and their suffering.
Experimental design
In all, forty adult female rats were randomly divided into two groups: control standard diet (0·675 % NaCl-containing diet) and high-salt diet (7·2 % NaCl-containing diet)( Reference Liu, Chen and Li 34 ). The nutritional experiment lasted 5 weeks, starting 1 week before mating, continuing throughout the gestational period and for 1 week of lactation. One male rat was allocated per two female rats over 48 h for mating, and pregnancy was diagnosed by the presence of a vaginal plug. On the 20th day of gestation, dams (pregnant rats) were caged individually for delivery and were observed twice a day (08.00 and 18.00 hours) in order to define the postnatal day (PND) 0 of each litter. Within 24 h after delivery, randomly selected pups were culled in each litter (20 litters/group of study) in order to maintain eight pups per dam to ensure equal nutrition. The offspring remained with the mother up to PND7. Male offspring were euthanised by decapitation without anaesthesia, and the cerebellum, hypothalamus, hippocampus and prefrontal and parietal cortex of each pup were dissected and stored at −80°C until biochemical analysis, except those samples used for flow cytometry, which were processed immediately. One pup from each litter was used for each technique (6 rats/group for biochemical assays; 8–12 rats/group for flow cytometry), in order to eliminate the litter effect.
High-salt-diet preparation
A high-salt diet containing 7·2 % NaCl was made by mixing commercial chow bran, water and commercial salt( Reference Liu, Chen and Li 34 ). To obtain the same texture, the control diet containing 0·675 % NaCl was prepared by mixing commercial chow bran and water. The chow was dried at low temperature and stored at 4–8°C until use. The body weight and chow intake of dams were recorded daily.
Sample preparation
For flow cytometry, 100 mg of fresh tissue was dissociated with a Pasteur pipette in PBS solution, pH 7·4, containing 1 mg% collagenase IV and 0·5 mg% DNAse. Dissociated tissue was filtered and then incubated with fluorescent probes.
For biochemical analysis, each brain structure was individually homogenised in ten volumes (1:10, w/v) of 20 mm sodium phosphate buffer, pH 7·4, containing 140 mm KCl. Homogenates were centrifuged at 1000 g for 10 min at 4°C to discard nuclei and cell debris. The pellet was discarded and the supernatant was used in biochemical assays.
Mitochondrial parameters and oxidant level measurement
According to the manufacturer’s instructions, the samples (100 μl) were incubated at 37°C with the following fluorescent probes: 4-amino-5-methylamino-2',7'-difluorofluorescein diacetate to measure nitric oxide levels, MitoSOX™ Red® (Invitrogen, Molecular Probes) to measure mitochondrial superoxide levels and MitoTracker® Green FM and MitoTracker® Red CM-H2Ros (Invitrogen, Molecular Probes) to measure mitochondrial mass and membrane potential, respectively. In addition, reactive species levels were measured using dichloro-dihydro-fluorescein diacetate (DCFH2) according to Crnkovic et al.( Reference Crnkovic, Riederer and Lechleitner 41 ). Cells were gated on the basis of the forward scatter (FSC) and side scatter (SSC) pattern of the sample cells and 10 000 events were acquired per sample in a FACSCalibur flow cytometer (BD Biosciences). Data were analysed using the software FlowJo®.
Antioxidant enzyme activities
Antioxidant enzyme activities were measured spectrophotometrically and the results are expressed as units/mg of protein.
Superoxide dismutase (SOD, EC 1.15.1.1) activity was measured by quantifying the inhibition of superoxide-dependent autoxidation of epinephrine, verifying the absorbance of the samples at 480 nm( Reference Boveris 42 ). SOD activity was expressed as the amount of enzyme that inhibits the oxidation of epinephrine by 50 %, which is equal to 1 unit.
Catalase (CAT, EC 1.11.1.6) activity was evaluated by measuring the change in absorbance at 240 nm, corresponding to hydrogen peroxide concentration ( Reference Aebi 43 ). One CAT unit is defined as 1 μmol of hydrogen peroxide consumed per minute.
Glutathione peroxidase (GPx, EC 1.11.1.9) activity was determined by a coupled GSH reaction, according to Wendel( Reference Wendel 44 ). The assay used tert-butyl hydroperoxide as substrate. NADPH disappearance was monitored spectrophometrically at 340 nm. One GPx unit is defined as 1 μmol of NADPH consumed per minute.
GSH level assay
GSH levels were measured by fluorimetry( Reference Browne and Armstrong 45 ). The samples were diluted in PBS 100 mm,pH 8·0, containing EDTA 5 mm, and incubated with o-phthaldialdehyde (1 mg/ml of methanol) for 15 min at room temperature. Fluorescence was measured using excitation and emission wavelengths of 350 and 420 nm, respectively. A standard curve of GSH (0·001–1 mm) was performed in parallel. Results are expressed as nmol/mg of protein.
Protein concentration assay
Protein concentration was determined using folin phenol reagent, and bovine serum albumin was used as a standard, in accordance with Lowry et al.( Reference Lowry, Rosebrough and Farr 46 ). Data were expressed as mg protein/ml.
Statistical analysis
Data are expressed as means with their standard errors, and statistical analyses were performed using the GraphPad Prism 6.0 software. All data were tested for normality. Differences between nutritional groups were determined using a Student’s t test, and results were considered statistically significant when P<0·05. Daily body weight and food consumption were analysed using repeated-measures ANOVA.
Results
A hypersaline diet during pregnancy influences the weight gain of dams but does not affect consumption of chow or offspring birth weight
During pregnancy, adult female rats receiving control standard diet or high-salt diet were weighed daily. From the 8th up to 21st gestational day, high-salt-diet-receiving dams presented with a reduced body weight (F 1,16=16·20, P=0·001) (Fig. 1), as well as weight gain, during pregnancy (t(15)=2·305, P=0·036) (online Supplementary Fig. S1), compared with the control diet group. Food consumption was also measured daily; chow intake was calculated as consumed chow in g/100 g body weight. Food intake throughout pregnancy was similar in both groups (F 1,11=1·588, P=0·234) (online Supplementary Fig. S2). We further assessed pregnancy rate and pup body weight at birth, and these parameters were not affected by maternal diet (online Supplementary Fig. S3 and S4, respectively).
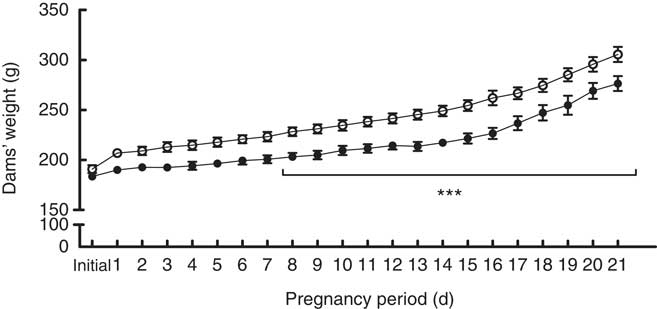
Fig. 1 Body weight measurements over the pregnancies of dams fed a control diet (![]() ) or high-salt diet (
) or high-salt diet (![]() ). Values are expressed in g. Values are means (n 6–7 dams/group) with their standard errors. *** Repeated-measures ANOVA showed a statistically significant difference between maternal control and high-salt diets (P<0·001).
). Values are expressed in g. Values are means (n 6–7 dams/group) with their standard errors. *** Repeated-measures ANOVA showed a statistically significant difference between maternal control and high-salt diets (P<0·001).
Maternal high-salt diet alters mitochondrial mass and membrane potential in offspring’s cerebellum
Mitochondrial mass and membrane potential were measured by flow cytometry as indicators of functional mitochondria( Reference Tal, Sasai and Lee 47 ) in the cerebellum, hypothalamus, hippocampus, prefrontal and parietal cortices from 7-d-old pups born to dams receiving control or high-salt diets (Fig. 2). As shown in Fig. 2, maternal high-salt diet promoted a statistically significant reduction in mitochondrial mass (t(18)=2·250, P=0·037) (Fig. 2(a)) and membrane potential (t(18)=2·477, P=0·023) (Fig. 2(b)) in the cerebellum of offspring, resulting in a 21 % reduction of both mitochondrial parameters. Mitochondrial mass and membrane potential in the hypothalamus (t(17)=0·776, P=0·448; t(17)=1·370, P=0·188, respectively), hippocampus (t(8)=1·962, P=0·085; t(8)=2·128, P=0·066, respectively), prefrontal cortex (t(10)=0·761, P=0·4641; t(10)=0·006, P=0·995, respectively) and parietal cortex (t(19)=0·122, P=0·904; t(18)=0·499, P=0·623, respectively) were not altered by maternal high-salt diet.
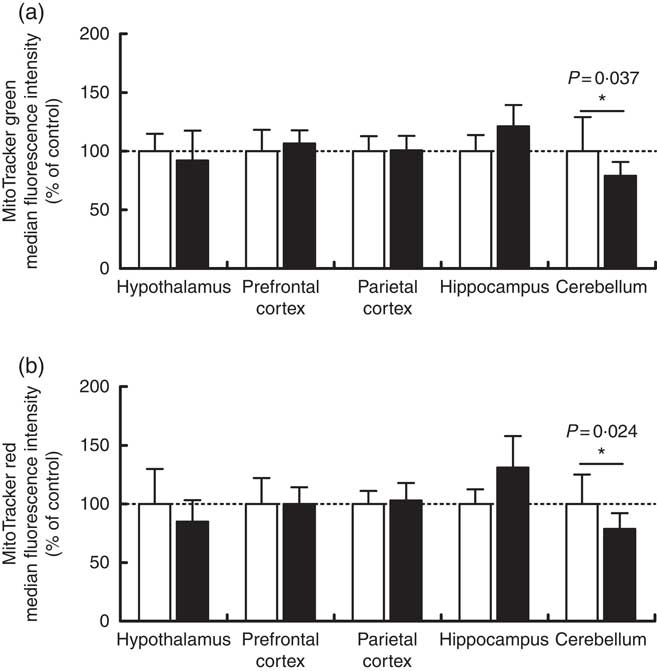
Fig. 2 Effect of a maternal high-salt diet on mitochondrial mass (a) and mitochondrial membrane potential (b) in the cerebellum, hypothalamus, hippocampus, and prefrontal and parietal cortices of 7-d-old pups born to dams receiving a control diet (![]() ) or high-salt diet (
) or high-salt diet (![]() ) during pregnancy and lactation. Values are means (n 8–12 pups/group) with their standard errors. * Student’s t test showed a statistically significant difference between maternal control and high-salt diets (P<0·05).
) during pregnancy and lactation. Values are means (n 8–12 pups/group) with their standard errors. * Student’s t test showed a statistically significant difference between maternal control and high-salt diets (P<0·05).
Maternal high-salt diet alters oxidative parameters differentially in encephalic regions
Overall reactive species, nitric oxide and mitochondrial superoxide levels were measured by flow cytometry in order to assess the oxidative status of the cerebellum, hypothalamus, hippocampus, prefrontal and parietal cortices from 7-d-old pups born to dams receiving control or high-salt diets (Fig. 3). DCFH2 is a target molecule of oxidant species, especially hydrogen peroxide, hydroxyl radical and peroxynitrite, giving rise to a fluorescent product if oxidised( Reference Kalyanaraman, Darley-Usmar and Davies 48 ). DCFH2 oxidation increased significantly in the cerebellum of pups born to dams fed a high-salt diet, by 15 % in comparison with control pups (t(8)=2·666, P=0·028) (Fig. 3(a)). Conversely, a statistically significant reduction (13·6 %) in hippocamal DCFH2 oxidation (t(19)=3·788, P=0·001) was observed in pups born to dams fed a high-salt diet compared with pups born to control-diet-fed dams. In the cases of the hypothalamus and prefrontal and parietal cortices, maternal high-salt diet did not alter DCFH2 oxidation (t(19)=0·324, P=0·749; t(19)=0·326, P=0·748; t(19)=1·503, P=0·149, respectively).
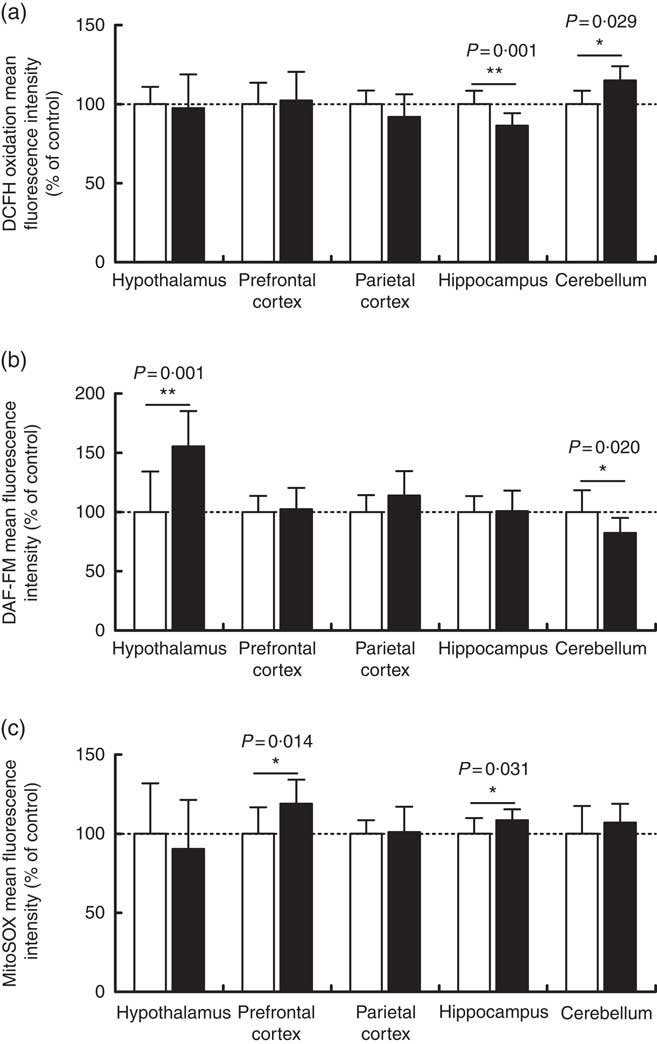
Fig. 3 Effect of a maternal high-salt diet on DCFH2 oxidation (a), nitric oxide levels (b) and mitochondrial superoxide (c) levels in the cerebellum, hypothalamus, hippocampus and prefrontal and parietal cortices of 7-day-old pups born to dams receiving a control diet (![]() ) or high-salt diet (
) or high-salt diet (![]() ) during pregnancy and lactation. Values are means (n 8–12 pups/group) with their standard errors. DAF-FM, 4-amino-5-methylamino-2',7'-difluorofluorescein diacetate. Student’s t test showed a statistically significant difference between maternal control and high-salt diets: * P<0·05, ** P<0·01.
) during pregnancy and lactation. Values are means (n 8–12 pups/group) with their standard errors. DAF-FM, 4-amino-5-methylamino-2',7'-difluorofluorescein diacetate. Student’s t test showed a statistically significant difference between maternal control and high-salt diets: * P<0·05, ** P<0·01.
To investigate the contribution of other oxidant species, we further measured nitric oxide (Fig. 3(b)) and mitochondrial superoxide (Fig. 3(c)) levels in encephalic regions. Offspring cerebellar (t(18)=2·549, P=0·020) and hypothalamic (t(18)=3·884, P=0·001) nitric oxide levels were significantly altered by maternal diet. Nitric oxide was reduced by 17·6 % in the cerebellum and increased by 55 % in the hypothalamus of offspring born to high-salt-diet-fed dams in comparison with control-diet-fed dams. Although parietal cortex nitric oxide levels tended to be increased (14 %) (t(19)=1·748, P=0·096) in the offspring of dams fed a high-salt diet, this was not statistically different from the control diet group. Similarly, nitric oxide levels in the hippocampus (t(19)=0·105, P=0·917) and prefrontal cortex (t(19)=0·326, P=0·748) were not altered by a maternal high-salt diet. By analysing offspring cerebellum (t(18)=1·092, P=0·289), hypothalamus (t(19)=0·689, P=0·499) and parietal cortex (t(19)=0·1777, P=0·861), mitochondrial superoxide levels were found to be similar to those in the control group. Conversely, maternal high-salt diet caused mitochondrial-specific superoxide levels to increase in the hippocampus (t(19)=2·325, P=0·031) and prefrontal cortex (t(19)=2·720, P=0·013) of offspring. Such results were statistically significant and account for an increase of 8·5 % in hippocampus and 19 % in the prefrontal cortex.
Maternal high-salt diet modulates antioxidant enzymatic activity in the brains of offspring
The activities of the antioxidant enzymes SOD, CAT and GPx were measured as part of the assessment of the effects of a maternal high-salt diet on the antioxidant system of offspring in the encephalic regions. SOD activity (Fig. 4(a)) increased significantly in the cerebellum of pups born to the maternal high-salt diet group compared with the maternal control diet group (t(10)=3·106, P=0·011), by 92 %. In contrast, SOD activity was similar in the hypothalamus (t(10)=1·096, P=0·299), hippocampus (t(10)=0·767, P=0·461), prefrontal (t(10)=1·894, P=0·087) and parietal cortices (t(10)=1·445, P=0·179) of both diet groups’ offspring.
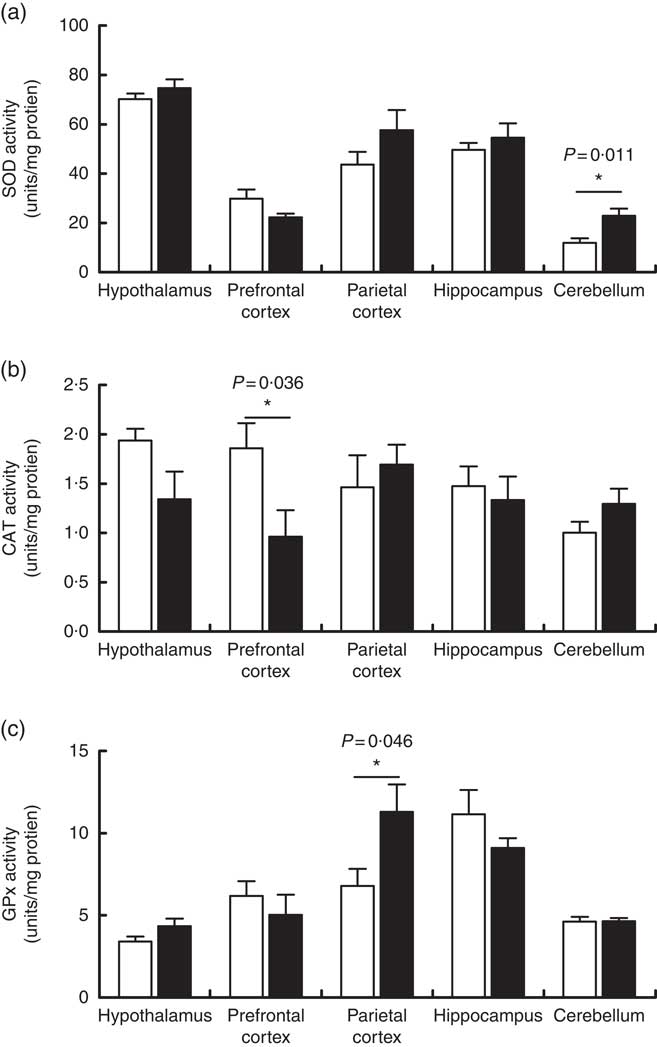
Fig. 4 Effect of a maternal high-salt diet on antioxidant enzyme activities: superoxide dismutase (SOD) (a), catalase (CAT) (b) and glutathione peroxidase (GPx) (c) in the cerebellum, hypothalamus, hippocampus and prefrontal and parietal cortices of 7-d-old pups born to dams receiving a control diet (![]() ) or high-salt diet (
) or high-salt diet (![]() ) during pregnancy and lactation. Values are means (n 6 pups/group) with their standard errors. *Student’s t test showed a statistically significant difference between maternal control and high-salt diets (P<0·05).
) during pregnancy and lactation. Values are means (n 6 pups/group) with their standard errors. *Student’s t test showed a statistically significant difference between maternal control and high-salt diets (P<0·05).
As shown in Fig. 4(b), we observed that a maternal high-salt diet induced a significant reduction (48 %) in CAT activity in the prefrontal cortex of pups (t(10)=2·423, P=0·036) in comparison with the control diet group. There was no significant difference in the CAT activity in the cerebellum (t(10)=1·551, P=0·152), hypothalamus (t(10)=1·952, P=0·079), hippocampus (t(10)=0·457, P=0·657) or parietal cortex (t(10)=0·597, P=0·563). Conversely, maternal high-salt diet induces a significant increase (66 %) in GPx activity in the parietal cortex of pups (t(10)=2·278, P=0·046) but does not affect GPx activity in the cerebellum (t(10)=0·065, P=0·949), hypothalamus (t(10)=1·713, P=0·117), hippocampus (t(10)=1·294, P=0·225) or prefrontal cortex (t(10)=0·746, P=0·472) (Fig. 4(c)).
Maternal high-salt diet did not affect GSH content in offspring’s brain
GSH content was measured in the cerebellum, hypothalamus, hippocampus, prefrontal and parietal cortices of 7-d-old pups born to dams receiving a control or high-salt diet (Fig. 5). Hippocampal GSH content was reduced by 33·6 % in maternal high-salt diet pups; although a tendency was observed, the difference was not statistically significant (t(10)=2·075, P=0·065). Similarly, maternal high-salt diet did not affect GSH content in the cerebellum (t(10)=1·070, P=0·121), hypothalamus (t(10)=0·292, P=0·776), prefrontal cortex (t(10)=1·728, P=0·115) or parietal cortex (t(10)=0·337, P=0·743) of offspring.
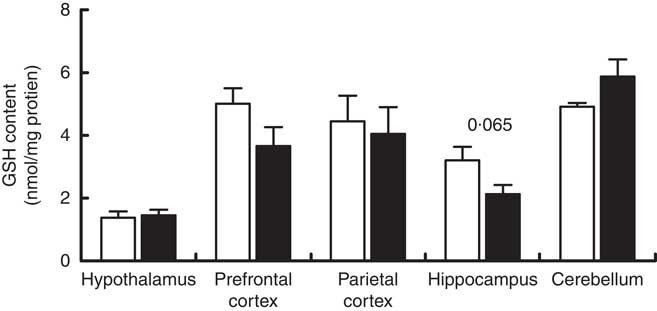
Fig. 5 Maternal high-salt diet has no effect on GSH content in the cerebellum, hypothalamus, hippocampus, and prefrontal and parietal cortices of 7-d-old pups. ![]() , Control diet;
, Control diet; ![]() , high-salt diet. Values are means (n 6 pups/group) with their standard errors. Student’s t test showed no statistically significant difference between maternal control and high-salt diets.
, high-salt diet. Values are means (n 6 pups/group) with their standard errors. Student’s t test showed no statistically significant difference between maternal control and high-salt diets.
Discussion
Salt intake at concentrations above physiological requirements is a common food habit worldwide, and, if combined with an unhealthy lifestyle, can represent a public health problem( 49 ). The main effect of excessive salt intake is the elevation of blood pressure, which in addition to other factors, such as hyperlipidaemia, hyperglycaemia and high BMI, contributes to an increased risk of hypertension and vascular diseases( Reference Vishram 50 ). In the present study, we hypothesised that maternal high-salt diet during pregnancy and lactation might programme the brain metabolism of offspring differentially from a standard diet, with regard specifically to cellular mitochondrial function and redox status. We demonstrate a redox status modulation elicited by maternal high-salt diet in the cerebellum, hypothalamus, hippocampus and prefrontal and parietal cortices of 7-d-old pups. Furthermore, maternal high-salt diet impairs cerebellar mitochondrial function. Few studies have investigated the effect of a high-salt diet on neurocellular metabolism( Reference Liu, Chen and Li 34 , Reference Ge, Wang and Wu 38 , Reference Cao, Li and Wang 51 – Reference Su, Liu and Cui 54 ) and, to the best of our knowledge, we are the first to investigate its effects on the developing brain.
Initially, we assessed the effect of high-salt intake on maternal body weight, weight gain, food consumption, as well as pup birth weight. In accordance with other studies( Reference Seravalli, de Oliveira and Zago 55 – Reference Gray, Al-Dujaili and Sparrow 58 ), we observed no effect of high salt on food intake. Notwithstanding, dams fed a high-salt chow displayed a reduction in weight gain of 14 % during pregnancy, and also had a body weight lower than that of dams fed control chow. Data from literature about the effects of a high-salt diet body weight and food intake are controversial. Gray et al.( Reference Gray, Al-Dujaili and Sparrow 58 ) and Reynolds et al.( Reference Reynolds, Vickers and Harrison 57 ) have demonstrated that a 4 % NaCl diet did not evoke increased food consumption or an alteration of dam body weight. Similarly, Ding et al.( Reference Ding, Lv and Mao 56 ) and Piecha et al.( Reference Piecha, Koleganova and Ritz 59 ) demonstrated that an 8 % NaCl diet did not affect dam body weight. However, contrary to the former, which did not observe any effect of a high-salt diet in food intake, the latter showed that an 8 % NaCl diet increased food intake( Reference Ding, Lv and Mao 56 , Reference Piecha, Koleganova and Ritz 59 ). Discrepancies are observed not only in dams but also in males( Reference Liu, Chen and Li 34 , Reference Ge, Wang and Wu 38 , Reference Su, Liu and Cui 54 , Reference Da Silva, de Souza and da Silva-Santos 60 ) and young rodents( Reference Pitynski-Miller, Ross and Schmill 61 ). In concordance with our data, Pitynski-Miller et al.( Reference Pitynski-Miller, Ross and Schmill 61 ) demonstrated that an 8·3 % NaCl diet reduced body weight but did not alter food intake in female weaning Sprague–Dawley rats. On the other hand, Ge et al.( Reference Ge, Wang and Wu 38 ) observed a reduction in body weight despite increased food intake in male mice fed 8 % NaCl for 7 weeks. On the basis of these data, such differences do not seem to be due to salt load or rodent strain. In agreement with the literature( Reference Porter, King and Honeycutt 62 , Reference Reynolds, Vickers and Harrison 63 ), the birth weight of pups born to high-salt-fed dams was similar to that of the control group, despite lower gestational weight gain.
Previous research has demonstrated that mitochondrial dysfunction and redox status imbalance play an important role in the pathogenesis of hypertension( Reference Montezano and Touyz 64 – Reference Rubattu, Pagliaro and Pierelli 68 ), which is thought to be associated with the development of cerebrovascular diseases in clinics( Reference Launer, Hughes and Yu 12 – Reference Korf, White and Scheltens 15 , Reference Iadecola, Yaffe and Biller 69 ). The brain is highly susceptible to oxidant species and a high-salt diet is able to induce inflammation( Reference Qi, Yu and Shi 70 ) and oxidative stress( Reference Su, Liu and Cui 54 ). High maternal salt intake seems to affect the structure and function of different tissues, including the heart and kidney( Reference Maruyama, Kagota and Van Vliet 40 , Reference Ding, Lv and Mao 56 , Reference Li, Lv and Wu 71 , Reference Mao, Liu and Bo 72 ), and to a much lesser extent the hypothalamus( Reference Porter, King and Honeycutt 62 ), during fetal development, as well as in adult offspring. We showed that a maternal high-salt diet differentially affected biochemical parameters in all brain areas studied. Mitochondrial mass and membrane potential indicators were decreased in the cerebellum, indicating a reduction in the number of functional mitochondria. Data published by our research group demonstrated that maternal exercise causes mitochondrial biogenesis in offspring( Reference Marcelino, Longoni and Kudo 73 ), and others showed an association between mitochondrial biogenesis and neurogenesis( Reference Cheng, Hou and Mattson 74 – Reference Wilkins, Harris and Carl 76 ). In contrast, this study suggests that a maternal high-salt diet can promote mitochondrial dysfunction, at least in the cerebellum. Despite superoxide levels remaining unchanged, we verified an increase in SOD activity, which, allied with non-altered CAT and GPx, might be connected to increased hydrogen peroxide levels, one of the oxidants measured by DCFH oxidation. This oxidant molecule can be reduced to a hydroxyl radical by iron in the Fenton reaction( Reference Michiels, Raes and Toussaint 77 , Reference Halliwell 78 ). It is important to note that our cells do not have enzymatic defences to eliminate hydroxyl radicals( Reference Ribeiro, Queiroz and Pelúzo 79 ). Enhanced levels of hydrogen peroxide and/or hydroxyl radicals favour oxidative stress and can result in oxidative damage to carbohydrates, proteins, lipids and nucleic acids( Reference Halliwell 78 ). In addition, hydrogen peroxide is able to overcome the membrane and affect multiple cellular compartments( Reference Barreiros and David 80 ). Further, the offspring of high-salt-diet-fed dams presented reduced levels of nitric oxide in the cerebellum. Nitric oxide plays several important roles in our body, including vasodilation, maintenance of the endothelial barrier of blood vessels and apoptosis regulation, besides being a neurotransmitter( Reference Rosselli, Keller and Dubey 81 ). The cerebellum is responsible for learning and motor control, as well as being involved with cognitive functions( Reference Chalimoniuk, Jagsz and Sadowska-Krepa 82 ). Concerning the role of nitric oxide on vasodilatation, the reduced levels seen in this study could be crucial for an increased risk of hypertension and cerebrovascular disease development in adulthood( Reference Katusic and Austin 83 ). Data from the literature have demonstrated that oxidative damage can impair the physiological function of the cerebellum and, owing to its connection with other brain structures such as the cerebral cortex, can spread the damage to adjacent areas( Reference Roostaei, Nazeri and Sahraian 84 ).
The high-salt-diet pro-oxidative effect was previously demonstrated by Koga et al.( Reference Koga, Hirooka and Araki 85 ), reporting an association between high salt intake and the production of oxidant species in the rostral ventrolateral medulla in an animal model of hypertension. Furthermore, the authors observed increased expression levels of angiotensin II receptor and NAD(P)H oxidase activity. Results indicated that the increased production of oxidants may be associated with the activation of NAD(P)H oxidase by angiotensin II( Reference Koga, Hirooka and Araki 85 ). Another study has shown that the administration of a hypersaline diet (8 % NaCl) during 3 weeks was able to increase angiotensin II levels in heart and kidney of Dahl salt-sensitive rats( Reference Pacurari, Kafoury and Tchounwou 86 ). Angiotensin II participates in the renin–angiotensin system (RAS) and is produced in response to decreased blood pressure, stimulating the vasoconstrictor response. Angiotensin II does not pass the blood–brain barrier under physiological conditions and the brain has a local RAS that works independently of the peripheral RAS. However, the CNS receives the peripheral signal from angiotensin II by receptors located in circumventricular areas( Reference Wright and Harding 87 , Reference Irigoyen, Consolim-Colombo and Krieger 88 ). Furthermore, the permeability of the blood–brain barrier seems to be altered in hypertensive rats, suggesting that angiotensin II could overpass it( Reference Biancardi, Son and Ahmadi 89 – Reference Zhang, Fang and Wan 91 ). It is already known that hypertensive individuals present with RAS hyperactivity( Reference Drenjančević-Perić, Jelaković and Lombard 31 ). In contrast to the cerebellum results, we detected increased levels of nitric oxide in the hypothalamus from offspring delivered to high-salt-treated dams. Insofar as angiotensin-converting enzyme levels are supposedly increased in the hypothalamus suggesting a dysfunction in the RAS( Reference Su, Liu and Cui 54 ), nitric oxide levels may represent an adaptation to imbalance in the RAS, in order to normalise the function of the vascular endothelium. However, more studies are required to confirm this hypothesis.
Finally, we reported increased levels of mitochondrial superoxide in the hippocampus and prefrontal cortex, allied with reduced CAT activity in the latter as a result of maternal high-salt diet during pregnancy and lactation. In this context, Liu et al.( Reference Liu, Chen and Li 34 ) demonstrated that a high-salt diet induced high levels of superoxide production, reduced GSH concentration and decreased CAT and SOD activities in the hippocampus of adult mice, allied to cognitive decline. On an overview, these data suggest that superoxide anions probably produced by the mitochondria favour hippocampal oxidative stress in response to a maternal high-salt diet during fetal and newborn development. Further studies are needed to reveal whether a high-salt diet during pregnancy and lactation has long-lasting effects on the offspring’s brain cellular function, as well as on cognitive function.
Conclusions
The intra-uterine environment and the lactation period are known to be influenced by the external environment, including maternal lifestyle, resulting in the adaptation of fetal and newborn metabolism. Herein, we have demonstrated for the first time that a high-salt diet during pregnancy and lactation promotes a pro-oxidant state, modulating oxidant species levels and the enzymatic antioxidant system, in addition to impairing mitochondrial function in the brains of offspring, suggesting that maternal diet is able to programme offspring neurometabolism. Clinical extrapolation of our experimental results raises the need for population awareness concerning salt intake, particularly during pregnancy and lactation, owing to possible adverse effects on the metabolism and development of offspring.
Acknowledgements
The authors thank Central Animal House of Departamento de Bioquímica, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil, for their help in performing this study.
This study was funded by CNPq (Universal 2014), FAPERGS, PROPESQ-UFRGS.
D. P. S. designed the research, conducted experiments, analysed the results, formatted the graphs and wrote the paper. C. P. K. conducted experiments, analysed the results, formatted the graphs and wrote the paper. A. B. S., P. M. A., N. C. M. and P. R. G. C. conducted the experiments. M. E. K. H. helped to design the research. C. M. searched for funding, designed research, analysed the results, and assisted with manuscript preparation.
The authors declare that there are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518000235








