The black tiger shrimp (Penaeus monodon) is the second most cultured crustacean species worldwide(1). Due to the importance of protein for shrimp growth, its high cost in formulated feeds and the environmental implications of N losses, it is essential to gain a better understanding of N requirements and N utilisation in P. monodon. Available data on crude protein requirements (CP, % DM diet) of P. monodon show a large degree of variability, i.e. from 36–40 %(Reference Shiau and Chou2) up to 50 %(Reference Shiau3). Several factors, e.g. differences in protein source, dietary energy level, life stage, rearing conditions and, in particular, differences in feed intake (FI), can explain some of this variation(Reference Rollin, Mambrini and Abboudi4). The confounding effect of FI on dietary protein requirement estimates has also been illustrated with the pacific white shrimp, Litopenaeus vannamei (Reference Kureshy and Davis5, Reference Venero, Davis and Rouse6). The latter authors(Reference Venero, Davis and Rouse6) demonstrated that maximum weight gain could be obtained by a wide range of dietary CP levels (30–40 % DM diet) at different feed allowances (50, 75 and 100 % of typical daily intakes), underlining the importance of expressing protein requirements on absolute basis rather than as a percentage of the diet. The use of the factorial approach, which allows the distinction between maintenance and growth for estimating protein requirements, has been initially developed for terrestrial animals(Reference Wang and Fuller7, Reference Fuller, McWilliam and Wang8) and has also been applied for teleost fish(Reference Gatlin, Poe and Wilson9–Reference Abboudi, Mambrini and Ooghe11). For shrimp, data on protein requirements using the factorial approach have been documented for two marine species: L. vannamei (Reference Kureshy and Davis5) and the Kuruma prawn Marsupenaeus japonicus (Reference Teshima, Koshio and Ishikawa12), reported as having more carnivorous feeding habits than L. vannamei, and for the freshwater prawn Macrobrachium rosenbergii, with herbivorous–omnivorous feeding habits(Reference Teshima, Koshio and Ishikawa13). For P. monodon, so far, no studies applied the factorial approach in order to distinguish maintenance from growth protein requirements.
In studies on amino acid (AA) requirements, the dietary AA profile is mostly based on either shrimp whole body or tail muscle composition as the reference(Reference Millamena, Bautista-Teruel and Reyes14, Reference Alam, Teshima and Yaniharto15). With the increasing use of plant-based proteins in shrimp feed as an alternative to marine protein sources (fish, shrimp or squid meal), lysine and methionine will be the first two limiting indispensable AA (IAA)(Reference Gatlin, Barrows and Brown16). Data on the requirements of P. monodon for lysine and methionine are limited to the studies of Millamena et al. (Reference Millamena, Bautista-Teruel and Reyes14, Reference Millamena, Bautista-Teruel and Kanazawa17), who estimated requirements of post-larval P. monodon based on growth response using diets with fixed CP level (37 or 40 %) and different levels of lysine and methionine. The dose–response method has been criticised since the IAA balance differs in each of the test diets, in contrast to the ‘diet dilution’ technique that is based on a ‘summit’ diet with high-protein level being diluted with a protein-free or low-protein diet of similar IAA profile(Reference Gous18). Furthermore, a combination of both methods(Reference Wang and Fuller7, Reference Gous and Morris19), using diets in which the protein level is ‘diluted’ by non-protein (NP) nutrients while creating an IAA deficiency at each of the tested protein levels, has been used in studies with fish(Reference Fournier, Gouillou-Coustans and Métailler10, Reference Abboudi, Mambrini and Ooghe11, Reference Abboudi, Ooghe and Larondelle20). This provides simultaneous estimations of protein and IAA requirements in a single study, and enables to analyse the relationship between the level of IAA and that of total CP. In studies on fish, as in other animals, some controversy exists on potential interactions between levels of IAA and CP on for instance the utilisation efficiency of the first limiting IAA, possibly leading to wrong estimations of requirements(Reference Abboudi, Ooghe and Larondelle20, Reference Heger, Phung and Krizova21). Another point, not examined in shrimp, concerns the validity of applying the ideal protein pattern in high-protein diets, i.e. the question whether the different IAA are always required as a constant proportion of crude protein or not. This point has received a lot of attention in broilers(Reference Morris, Gous and Fisher22, Reference Sterling, Pesti and Bakalli23) and is of practical relevance for diets in which poor quality protein sources are included at higher than normal levels to provide a minimal level of IAA in the diet (% diet), resulting in imbalanced dietary AA profiles (% CP).
The aim of the present study was to determine, within a single feeding trial, the requirements for protein and for two IAA, lysine and methionine, for maintenance (N equilibrium) and maximal body protein deposition of juvenile P. monodon and to study the possible relationship between the limiting IAA and dietary CP supply. Different mathematical models were used to determine the requirements, giving consideration to the basic assumptions behind and to obtain original data on efficiency of N and AA utilisation in shrimp.
Materials and methods
Experimental diets
Ten semi-purified diets were formulated with four different levels of crude protein (CP) and two levels of lysine or methionine (adequate or 30 % deficient). The N source was casein and a blend of crystalline AA in a 55:45 ratio (casein:AA blend; Tables 1 and 2). NP, low-protein (LP), medium-protein (MP) and high-protein (HP) diets were formulated to supply 0, 1·6, 4·8 and 8·1 % N, respectively. Fish protein soluble concentrate (20 g/kg diet) and an attractant mix (glucosamine, taurine, betaine, glycine and alanine) (15 g/kg diet) were included in all diets in order to improve palatability, which provided an additional N source in all diets, including in the ‘protein-free NP diet’ (0·8 % N, Table 1). Gelatinised starch levels compensated for varying CP levels. The four diets, NP, LP, MP and HP, had a similar AA (indispensable and non-indispensable) profile (% CP; Table 3), which was based on the AA composition of shrimp whole body (‘ideal AA profile’, compiled from literature). The six other diets, lysine-deficient LP, methionine-deficient LP, lysine-deficient MP, methionine-deficient MP, lysine-deficient HP and methionine-deficient HP, were formulated to be 30 % deficient in either lysine (L diets) or methionine (M diets) (Table 3). The AA deficiencies (relative to the AA profile of the adequate diets) were obtained by replacing the tested AA from the AA blend by non-IAA in order to keep diets isonitrogenous at each level of total N supply. The ratio of cystine:methionine was kept constant at approximately 0·3 in all diets so that the levels of cystine were proportionally lower in the methionine-deficient diets (Table 2). Each AA blend (Table 2) was coated with agar(Reference Mambrini, Kaushik, Nunes, Portugal, Costa and Ribeiro24) dissolved in warm water (30°C; pH 5) before being mixed with the other ingredients. The experimental diets were manufactured by Institut National de la Recherche Agronomique at the experimental facility of Donzacq (France). Ingredients such as casein, cholesterol, lecithin, sodium alginate, cellulose, fish protein-soluble concentrate, gelatinised starch and attractants were first mixed together and homogenised before adding the coated AA blend and the fish oil. After thorough mixing, feed was pelleted (meat grinder, 3 mm), dried at 40°C and stored in sealed bags prior shipping to the experimental site in Madagascar, where it was stored at 4°C.
Table 1 Formulation and analysed composition of the ten experimental semi-purified diets fed to Penaeus monodon juveniles for 6 weeks

NP, non-protein; LP, low protein; LPM, methionine-deficient low-protein diets; LPL, lysine-deficient low-protein diets; MP, medium protein; MPM, methionine-deficientmedium-protein diets; MPL, lysine-deficient medium-protein diets; HP, high protein; HPM, methionine-deficient high-protein diets; HPL, lysine-deficient high-protein diets.
* Acros France; 95 % stabilised cholesterol; 98 % glycine; 98 % d-glucosamine; HCl; agar powder; pure casein (CAS 9000-71-9).
† Eurolysine and Acros (see Table 2 for details).
‡ Louis François (St Maur, France).
§ Sopropêche (Lorient, France).
∥ Roquette (Lestrem, France).
¶ Contained glucosamine, taurine, betaine, glycine and alanine as 5:3:3:2:2.
** Supplied the following (to provide g/kg mixture): magnesium oxide, 124; calcium carbonate, 215; KCl, 90; NaCl, 40; KI, 40 mg; copper sulphate, 3; cobalt sulphate, 20 mg; ferric sulphate, 20; manganese sulphate, 3; ZnSO4, 4; dibasic calcium phosphate, 500; NAF, 1.
†† Supplied the following (to provide g/kg mixture): retinyl acetate (A), 0·172, 1; thiamin (B1), 0·1; riboflavin (B2; 80 %), 0·5; nicotinic acid (B3), 1; calcium pantothenate (B5, 98 %), 2; pyridoxine (B6), 0·3; inositol (B7), 30; biotin (B8, 2 %), 1; folic acid (B9), 0·1; vitamin B12 (1 g/kg), 1; ascorbic acid (C, 35 %), 14·29; cholecalciferol (D3), 0·006; tocopheryl acetate (E), 3·7; menadione (K3, 50 %), 2; choline chloride (60 %), 167.
Table 2 Formulation of the amino acids blend added to the semi-purified casein-based diets (g/kg diet)

LP, low protein; LPM, methionine-deficient low-protein diet; LPL, lysine-deficient low-protein diet; MP, medium protein; MPM, methionine-deficient medium-protein diet; MPL, lysine-deficient medium-protein diet; HP, high protein; HPM, methionine-deficient high-protein diet; HPL, lysine-deficient high-protein diet.
* Acros, l-Arg 98 %, Gly 98 %.
† Eurolysine.
‡ Jerafrance.
Table 3 Analysed amino acid composition of the ten experimental diets (g/16 g nitrogen)

NP, non-protein; LP, low protein; LPM, methionine-deficient low-protein diet; LPL, lysine-deficient low-protein diet; MP, medium protein; MPM, methionine-deficient medium-protein diet; MPL, lysine-deficient medium-protein diet; HP, high protein; HPM, methionine-deficient high-protein diet; HPL, lysine-deficient high-protein diet.
Experimental animals
The feeding trial was undertaken at the hatchery facilities of Aqualma, Madagascar. Forty-three 150 litres fibreglass tanks (80 × 30·5; diameter × height) were used and stocked each with fifteen juvenile (85-d-old post-larvae) P. monodon (initial mean weight: 2·36 g (se 0·10)), reared in earthen ponds for 66 d before the experiment. At the start of the study, juveniles were individually weighed and allocated to the respective tanks. During the 10 d of adaptation, all groups were fed MP diet at an initial ration level of 2 % biomass per d. During the growth trial, which lasted 6 weeks, each of the ten experimental diets was distributed to four replicate groups allocated randomly. A commercial practical diet (CP: 46 %; crude fat: 7 %; Nutrima, La Réunion, Madagascar) was included as a control with three replicates to follow overall performance.
Sea water was filtered through a 50 μ sand filtration system before distribution into the tank. The minimum water exchange was 40 % of the tank volume and was done every morning before the first feeding. Temperature, pH and oxygen concentration of the water were measured twice daily (morning at 04.00 and afternoon at 17.00 hours). Salinity was recorded daily at 16.00 hours. Analyses for nitrate-N, nitrite-N and ammonia-N in the water in all the forty-three tanks were made every week using commercial kits (Nitraver®5 Nitrate, Nitriver®3 Nitrite and salicylate kit; Hach, Loveland, CO, USA). Average pH, oxygen concentration, temperature and salinity of the water were 8·0 (sd 0·1), 5·7 (sd 0·5) mg/l, 30·1 (sd 1·6)°C and 30·8 (sd 1·8) ppt, respectively. Total ammonia nitrogen, nitrate (N-NO3) and nitrite (N-NO2) were 0·17 (sd 0·26), 0·88 (sd 0·47) and 0·15 (sd 0·22) mg/l, respectively.
Special care was taken to assess actual feed consumption and to adapt feed distributions to the demands of each group. Feed distributions were done four times daily (08.00; 13.00; 18.00; 23.00 hours). At each distribution, feed was deposited into two small circular trays (20 cm diameter) placed inside the tank. Two hours after each feed distribution, the trays were checked and leftovers were counted, collected in a box and stored at − 20°C until the next collecting time. A code was established to determine the amount of feed to be distributed at the next meal:
0: all the feed was consumed, the next distribution increased by 30 % more than the previous one;
1: less than three pellets remained in the tray, the next distribution was of equal amount;
2: more than three pellets remained in the tray, the next distribution was decreased by 30 %.
All feed leftovers were collected from each tray and pooled per tank on a weekly basis. DM content of each uneaten feed sample was determined by drying to a constant weight at 90°C for 48 h. We could thus estimate actual FI for each tank on a weekly basis. N, lysine and methionine intakes were calculated from the total FI measured.
Biomass of each tank was measured every 2 weeks during the whole trial. Mortality was checked before and after each feeding period; dead animals were removed and weighed. Exuvia were removed from the tanks as soon as they were observed in order to avoid the animals to feed on them.
Performance calculations were as follows:

where BWI, BWF, initial and final BW (g) and average BW (kg) = ((BWI+BWF)/(1000 × 2); B F, B I, B D, initial, final and dead biomasses (g).
N Shrimp I and N Shrimp F, initial and final N content of shrimp (g/100 g fresh matter).
Proximate analysis of diets and shrimp whole body
At the beginning of the study, fifteen shrimp (12-h feed deprived) were selected for whole-body composition analyses. At the end of the trial, a pool of eight shrimp (12-h feed deprived) was analysed per treatment (two shrimp per tank). All samples were kept at − 20°C before analyses. Whole shrimp were ground and analysed for DM before being freeze dried. Gross energy content of feed samples was analysed using an adiabatic bomb calorimeter (IKA, Heitersheim, Germany). Feed samples as well as freeze-dried whole-body samples were analysed for DM (105°C for 24 h), ash (550°C for 12 h), lipid (Soxtherm, Gerhardt, Germany) and protein (N × 6·25, Kjeldahl Nitrogen analyser 2000, Fison Instruments, Milan, Italy). Based on comparative carcass analyses, gain and retention values were computed. The AA composition of the diets was analysed (AgroBio Laboratory, Rennes, France) after hydrolysis (6 m-HCl, 110°C, 23 h). After evaporation, samples were analysed in an automatic AA analyser (Biochrom-30, Biochrom Ltd, Cambridge, UK) using a sodium high resolution protein hydrolysate column (resin ultra pac 8). The AA were derivatised with ninhydrin and quantified at 570 and 440 nm for proline.
Data analysis
All data were analysed by a one-way ANOVA using dietary treatment as factor (n 11 diets) or by a two-way ANOVA using the level of protein and of IAA (lysine or methionine) as independent factors (n 6 diets), followed by the comparison of means using the Duncan's multiple range test in case of a significant effect (P < 0·05). ANOVA was performed using STATISTICA 5.0 software (StatSoft, Inc., Tulsa, OK, USA).
Four regression models were used to estimate the maintenance (X-value for zero gain) and growth requirements. The first models use a broken line (BLM) and a quadratic regression(Reference Robbins, Saxton and Southern25):
where Y, dependent variable as N gain (g/kg BW per d); X, independent variable as N, lysine or methionine intake (g/kg BW per d); U, slope of the first segment (nutrient utilisation efficiency); R, breakpoint X value (maximal growth requirement value); L, plateau value.
Maintenance requirements (Y = 0) were calculated as: X = (UR − L)/U with the BLM and as ![]() with the quadratic model.
with the quadratic model.
The third model used was the four parameters saturation nutrient kinetic model(Reference Mercer26):
where Y, dependent variable as N gain (g/kg BW per d); X, independent variable as N, lysine or methionine intake (g/kg BW per d); B, intercept on y-axis for X = 0; K 0·5, concentration for 1/2(R+B); R max, maximum Y response; n, apparent kinetic order.
Maintenance requirement (Y = 0) was calculated as: ![]() . Total requirement was estimated at 95 % of the maximum gain (R max)(Reference Rodehutscord, Jacobs and Pack27) following the above equation. Therefore, requirement (mg/kg BW per d) was calculated as
. Total requirement was estimated at 95 % of the maximum gain (R max)(Reference Rodehutscord, Jacobs and Pack27) following the above equation. Therefore, requirement (mg/kg BW per d) was calculated as
The fourth model was logistic model(Reference Gahl, Finke and Crenshaw28) and described as
where Y, dependent variable as N gain (g/kg BW per d); X, independent variable as N, lysine or methionine intake (g/kg BW per d); b, intercept on y-axis for X = 0; R max, maximum Y response; c, shaping parameter that locates the inflection point; k, scaling parameter.
Maintenance requirement (Y = 0) was calculated as X = ( − 1/k) × Ln( − R max/(b+b × c − R max)).
Overall requirement (g/kg BW per d) estimated at 95 % R max(Reference Finke, DeFoliart and Benevenga29) was calculated as
Marginal efficiency of nutrient utilisation was calculated as the first derivative dY/dX, according to the following equation:
Protein requirements were determined using data from NP, LP, MP and HP treatments. Lysine requirements were determined using data from treatments NP, LP, lysine-deficient LP, MP, lysine-deficient MP, HP and lysine-deficient HP (excluding the methionine-deficient diets), and methionine requirements were determined using those from treatments NP, LP, methionine-deficient LP, MP, methionine-deficient MP, HP and methionine-deficient HP (excluding the lysine-deficient diets). Four replicate tanks for each treatment (n 4) were included in the analysis, except for HP (n 3) where one tank had to be excluded because of cannibalism. Graphical presentations and parameter estimates were made using GraphPad Prism 4.00 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Table 4 shows the performances of the shrimp analysed by a one-way (effect treatment, n 11) and two-way ANOVA (effect dietary protein and IAA level, n 6 diets). Results from the one-way ANOVA showed no significant effect of the dietary treatment on the survival of the shrimp (Table 4). FI (g/kg BW per d) in shrimp fed LP, MP and HP diets were higher than when fed NP diet or the practical diet (Table 4). The lowest final BW was found for shrimp fed the NP and LP diets (2·3–3·6 g), and the highest BW was found for those fed MP diets, the three HP diets and the practical diet (4·9–5·7 g), which were not significantly different. The deficient HP diets and the non-deficient MP diet led to similar growth rates, which were not different from growth rates obtained with the practical diet (Table 4). Among the experimental diets, LP and NP diets provided a significantly lower FE than MP or HP diets. The two-way ANOVA of the data in Table 4 showed a significant effect of the protein level on final BW, specific growth rate and FE (HP = MP>LP), but not on survival or FI. No significant effect of the IAA deficiency could be detected on any of the parameters (Table 4). Interestingly, for both IAA, the two-way ANOVA indicated a significant interaction between the levels of protein and IAA for growth performances (final BW and specific growth rate), which was more pronounced (lower P-value) for methionine than for lysine (Table 4). The interaction between the level of methionine and protein regarding growth, and also FE, can be explained by the fact that methionine deficiency reduced growth, and FE, at the MP level (methionine-deficient MP v. MP), but not at the higher or lower protein levels (Table 4).
Table 4 Survival, feed intake, growth and nutrient utilisation in juvenile Penaeus monodon fed the semi-purified diets for 6 weeks
(Mean values and standard deviations)

BW, body weight; SGR, specific growth rate; FE, feed efficiency; FI, feed intake; NP, non-protein; LP, low protein; LPM, methionine-deficient low-protein diet; LPL, lysine-deficient low-protein diet; MP, medium protein; MPM, methionine-deficient medium-protein diet; MPL, lysine-deficient medium-protein diet; HP, high protein; HPM, methionine-deficient high-protein diet; HPL, lysine-deficient high-protein diet.
a–f Mean values with different superscript letters were significantly different between groups (P < 0·05).
* n 4 per diet, except for HP and commercial diets for which n 3.
† P values given by the one-way ANOVA (n 11 diets).
‡ P values given by the two-way ANOVA (n 6 diets per analysis). NP and commercial diets were excluded from the test, as well the Met-deficient diets from the ANOVA on the effect of Lys and the Lys-deficient diets from the ANOVA on the effect of Met.
The analysis of whole-body composition of the shrimp (data not shown) revealed a low-lipid content (0·3–0·6 g/100 g shrimp) and an ash content of 3·4–4·0 g/100 g shrimp, without visible effect of the dietary treatment. There was a positive linear relationship (R 2 0·96) between the concentration of dietary and whole-body protein, which was 17·4, 19·1, 20·2 and 21·9 g/100 g shrimp for NP, LP, MP and HP diets, respectively. As intended, N intakes (data not shown) differed largely between the dietary treatments, being significantly higher with HP diet (5·7 g/kg BW per d) than with MP or LP diets (3·6 and 1·5 g/kg BW per d, respectively), and the lowest value being with the NP treatment (0·4 g/kg BW per d). Daily N gain (per unit BW) was significantly affected by N intake: methionine-deficient HP-fed animals had the highest N gain (0·67 g/kg BW per d), whereas NP-fed animals had a daily N loss of 0·07 g/kg BW per d. Daily N gains of shrimp fed HP and MP diets were not significantly different from the N gain obtained with the commercial diet (0·59–62 g/kg BW per d). Lysine or methionine deficiency did not affect the level of N intake or N gain (P>0·05). However, a significant interaction was found between dietary protein and methionine level on the daily N gain (P = 0·0026), related to the fact that the deficiency in methionine (Fig. 1(b)) affected N growth only at the MP level. Although the interaction between lysine and protein levels was not significant (P = 0·055), N gains presented in Fig. 1(a) show a similar tendency with the effect of a lysine deficiency being more pronounced at the medium than at lower or higher dietary protein levels.

Fig. 1 Effect of dietary levels of protein and lysine (a) and protein and methionine (b) on daily individual nitrogen gain (mg/d) of juvenile Penaeus monodon fed the semi-purified diets for 6 weeks. LP, low protein; LPL, lysine-deficient LP; MP, medium protein; MPL, lysine-deficient MP; HP, high protein; HPL, lysine-deficient HP; LPM, methionine-deficient LP; MPM, methionine-deficient MP; HPM, methionine-deficient HP. Values are means (n 4 per treatment, except for HP where n 3), with standard deviations represented by vertical bars. a,b,c Mean values with unlike letters are significantly different (P < 0·05; one-way ANOVA). P values of the two-way ANOVA (protein × lysine) are as follows: protein, P < 0·0001; lysine, P = 0·936; protein × lysin, P = 0·055. P values of the two-way ANOVA (protein × methionine) are as follows: protein, P < 0·0001; methionine, P = 0·503; protein × methionine, P = 0·002.
Protein and indispensable amino acid requirement estimates
The four models used to estimate the requirements for N, lysine and methionine all gave a satisfactory regression coefficient (0·95 < R 2 < 0·99; Table 5). The four parameters saturation nutrient kinetic model gave the highest N requirement estimates and the BLM gave the lowest N requirement estimates (Tables 5 and 6). Based on the contrasting assumptions inherent to the model regarding nutrient utilisation efficiency (constant or diminishing returns)(Reference Gahl, Finke and Crenshaw28), we decided to further comment the results obtained only with the BLM (Fig. 2) and the logistic (Fig. 3) model.
Table 5 Parameters estimated by fitting the four regression models through the experimental data using nitrogen gain (g/kg body weight (BW) per d) as the response parameter and the different intake levels of nitrogen, lysine or methionine (g/kg BW per d) as input parameter in Penaeus monodon
(Mean values with their standard errors)

BLM, broken line model; Quad-1, quadratic model with one slope; SK-4, four parameters saturation kinetic model; df, degrees of freedom.
Table 6 Estimated requirements for nitrogen equilibrium (maintenance, M) and maximal nitrogen gain (nitrogen growth, G) for nitrogen, protein, lysine and methionine using the four regression models for juvenile Penaeus monodon
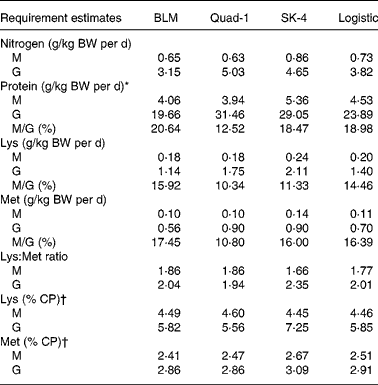
BLM, broken line model; Quad-1, quadratic model with one slope; SK-4, four parameters saturation kinetic model; BW, body weight.
* Nitrogen requirement × 6·25.
† Calculated ratio = 100 × (estimated indispensable amino acid requirement/estimated protein requirement).

Fig. 3 Non-linear regressions obtained with the logistic model of nitrogen gain v. nitrogen intake (a), lysine intake (b) and methionine intake (c) and their respective marginal efficiencies in juvenile Penaeus monodon. The six indispensable amino acid-deficient diets were excluded from the model (a). The three methionine- and lysine-deficient diets were excluded from the models (b) and (c), respectively. The parameters of the regression equations and the requirement estimates are summarised in Tables 5 and 6. Marginal instantaneous utilisation efficiency is defined as the incremental response in nitrogen gain per incremental unit of nitrogen intake (a), lysine intake (b) and methionine intake (c).
Nitrogen requirements for maintenance (N equilibrium) and maximal N gain were estimated to be 0·6–0·7 g N/kg BW per d and 3·1–3·8 g N/kg BW per d, respectively, corresponding to CP levels of 4·1–4·5 g and 19·7–23·9 g/kg BW per d (Table 6, Figs. 2(a) and 3(a)). Regarding the requirements for lysine, using N gain as response, both models led to similar estimates, being 0·18–0·20 g/kg BW per d for maintenance and 1·14–1·40 g/kg BW per d for maximal N gain (Table 6, Figs. 2(b) and 3(b)). Methionine requirement for maintenance or N balance was found to be 0·10–0·11 g/kg BW per d, whereas that found for maximum N gain ranged from 0·56 to 0·70 g/kg BW per d (Table 6, Figs. 2(c) and 3(c)). Based on the above data, we could estimate the need as percentage of protein requirement, which was 5·8 % for lysine 2·9 % for methionine (Table 6). Maintenance requirements for protein represented 19–21 % of the protein requirement for maximum growth, whereas the maintenance/growth ratio was 14·5–15·9 % for lysine and between 16·4 and 17·4 % for methionine (Table 6).
Marginal efficiencies of nitrogen and indispensable amino acid utilisation
The instantaneous marginal efficiency of N utilisation, calculated from the parameters obtained with the logistic model, showed a maximum efficiency of 38 %, occurring at an N intake level of 39 % of that needed for maximum N gain (Fig. 3(a)). The BLM estimate of constant N utilisation efficiency between maintenance and maximal growth was 24 % (Table 5). Lysine marginal efficiency, determined after plotting N gain against lysine intakes, peaked at 0·77 at a lysine intake level corresponding to 35·7 % of the predicted requirement for maximum N gain (Fig. 3(b)). Methionine marginal efficiency reached a maximum of 1·62 corresponding to 37·1 % of the requirement estimated for maximum N gain (Fig. 3(c)).
Discussion
The survival and whole-body mass increases of the shrimp fed the semi-purified MP and HP diets are comparable to those reported in other feeding trials with P. monodon of similar size ranges(Reference Glencross, Smith and Tonks30, Reference Glencross and Smith31). Only few studies with shrimp species applied a factorial approach to determine the part of N losses to be attributed to maintenance or to estimate N utilisation between maintenance and maximal growth response(Reference Kureshy and Davis5, Reference Teshima, Koshio and Ishikawa12, Reference Teshima, Koshio and Ishikawa13). As for IAA requirements, there is, to our knowledge, no such data available for crustaceans. Some of the methodological dissimilarities between the present and latter studies with shrimp susceptible to affect requirement estimates(Reference Fuller and Garthwaite32, Reference Bodin, Govaerts and Abboudi33) concern the feed supply, response criterion and mathematical model. In the present study, the different diets were fed on an ad libitum basis with careful monitoring of intakes instead of supplying a single diet at fixed ratios as in the study with L. vannamei (Reference Kureshy and Davis5). In line with Teshima et al. (Reference Teshima, Koshio and Ishikawa12, Reference Teshima, Koshio and Ishikawa13), protein accretion (daily N gain/unit BW) was used as response rather than total weight(Reference Kureshy and Davis5), considered less pertinent as response criterion than N accretion(Reference Gahl, Finke and Crenshaw28, Reference Rodehutscord, Becker and Pack34). Regarding the model, we decided to focus on a linear (broken line) and non-linear (logistic) regression because of the contrasting assumptions regarding the marginal utilisation efficiency, the former assuming constant efficiency and the latter being recommended in vertebrates for the determination of diminishing returns when approaching maximal intake(Reference Gahl, Crenshaw and Benevenga35).
Protein requirements for maintenance and growth
The maintenance requirement for protein, defined as the amount of protein ingested by the shrimp to maintain its N equilibrium (N synthesis equals N breakdown), was estimated from both models at the X-intercept level. The protein requirements for maintenance for P. monodon estimated in the present study (2·4 g initial BW) ranged between 4·1 and 4·5 g CP/kg BW per d, showing only minor variations according to the model used. These estimates are superior to data from juvenile L. vannamei (1·8–3·8 g CP/kg BW per d) using zero BW gain as a response parameter(Reference Kureshy and Davis5). The low protein maintenance requirement of 0·2 g CP/kg BW per d reported for M. rosenbergii (initial BW: 0·15 g)(Reference Teshima, Koshio and Ishikawa13) and of 1·1 g CP/kg BW per d for M. japonicus (initial BW: 1·69 g)(Reference Teshima, Koshio and Ishikawa12) reflects, in fact, the daily obligatory N losses at zero feed intake, which was assumed as reflecting maintenance requirements. If calculated in the same manner, daily N losses in the present study were 0·16 g/kg BW per d, equivalent to 0·98 g CP/kg BW per d, a value close to that reported for M. japonicus (Reference Teshima, Koshio and Ishikawa12).
Protein requirement for maximum N gain in P. monodon juveniles ranged between 19·7 and 23·9 g CP/kg BW per d, in line with the protein requirement for growth (20·5–23·5 g CP/kg BW per d) reported for subadult L. vannamei (Reference Kureshy and Davis5). The protein requirements of 7·1 g CP/kg BW per d found for M. rosenbergii (Reference Teshima, Koshio and Ishikawa13) and of 10 g CP/kg BW per d for M. japonicus (Reference Teshima, Koshio and Ishikawa12) are lower than in the present study, possibly due to the relatively poor growth in their studies as compared to ours. It is also worth noting that in the present study growth of the shrimp fed the MP and HP semi-purified diets was similar to that obtained with a commercial practical diet.
The ratio between maintenance and growth requirement, which reflects the proportion of a nutrient used by an animal to maintain N balance as compared to that needed for maximal N gain, was about 20 %. This is much higher than the maintenance/growth ratio reported for L. vannamei juveniles (3·9–8·8 %) and subadults (6·4–10·2 %)(Reference Kureshy and Davis5), suggesting a relatively higher protein requirement for maintenance of N balance in juvenile P. monodon. However, the present maintenance/gain ratio is within the same range as found in juvenile teleost fish, i.e. 12·3 % for Atlantic salmon (Salmo salar) fry(Reference Abboudi, Mambrini and Ooghe11), 15·1 % in channel catfish (Ictalurus punctatus) fingerlings(Reference Gatlin, Poe and Wilson9), 16·7 % in juvenile Nile tilapia (Oreochromis niloticus)(Reference Kaushik, Doudet and Médale36) or 21 % for juvenile two-banded seabream (Diplodus vulgaris)(Reference Ozorio, Valente and Correia37).
Efficiency of protein utilisation for maximal nitrogen gain
The study of marginal N utilisation efficiency, which reflects the efficiency of N utilisation between maintenance and maximum growth, depends on the biological assumption of constant efficiency or not, and thus the mathematical model. We found that marginal efficiency of N utilisation in P. monodon reached a maximum of 38 at 39 % of the maximal growth requirement, after which N utilisation efficiency decreased to 5·6 % at maximal N gain intake levels. Diminishing returns are important to consider from an economical perspective for choosing the requirement, with estimates for optimal utilisation efficiency occurring at lower N intakes than for optimal growth(Reference Gahl, Crenshaw and Benevenga35). The average value of the marginal (instantaneous) efficiencies obtained with the logistic model was 24·9 %, which agrees well with the constant efficiency of 24 % obtained with broken line model (U estimate). The comparison with other data from shrimp is difficult since no data on the regression coefficients were given (simple linear regression)(Reference Teshima, Koshio and Ishikawa12, Reference Teshima, Koshio and Ishikawa13) or since weight gain rather than N gain/kg BW was used as response criterion without precision of efficiency values(Reference Kureshy and Davis5). However, the present N utilisation efficiency (24 %) is inferior to values similarly obtained by broken line regression(Reference Bodin, Govaerts and Abboudi33)or simple linear regression(Reference Fournier, Gouillou-Coustans and Métailler10) in other species, such as teleost fish showing N utilisation efficiencies as high as 37·9 % for gilthead seabream (Sparus aurata)(Reference Fournier, Gouillou-Coustans and Métailler10) or 39·6 % for European seabass (Dicentrarchus labrax)(Reference Fournier, Gouillou-Coustans and Métailler10) and 34–44 % for rainbow trout (Oncorhynchus mykiss)(Reference Fournier, Gouillou-Coustans and Métailler10, Reference Bodin, Govaerts and Abboudi33).
A more common parameter in nutritional studies on shrimp is total N retention, i.e. the ratio of total N gain to cumulated N intakes, without identification of the part of N losses due to maintenance. N retentions varied between 11 (HP diets) and 17 % (lysine-deficient MP). Low N retentions, comprised between 10 and 15 % of N intakes, were also reported for adult Litopenaeus stylirostris under laboratory conditions(Reference Gauquelin, Cuzon and Gaxiola38). The N retention obtained with the commercial treatment (24·7 %) agrees with N retentions in intensive shrimp farms in which approximately 20 % of total dietary N input was recovered in the harvested P. monodon (Reference Briggs and Funge-Smith39, Reference Jackson, Preston and Thompson40). Higher N retentions of up to 31 % for P. monodon (Reference Thakur and Lin41) or up to 46 % of N input for post-larval L. vannamei (Reference Perez-Velazquez, Gonzalez-Felix and Gomez-Jimenez42) have been attributed to the natural productivity (development of bacteria and phytoplankton populations) taking place in a static system (without water renewal), which constitutes a source of N intake and, hence, might overestimate N retention. In the present study, the water renewal (>40 % of each tank/d) is believed to have maintained the recycling of N wastes through natural productivity close to zero. Although special care was taken for monitoring intakes, efficiencies of N utilisation with the experimental diets might be slightly underestimated due to some unseen feed losses or to leaching related to the slow feeding behaviour of the shrimp, as underlined before by others(Reference Teshima, Koshio and Ishikawa12). Also the loss of exuvia during the growth of the animal, not taken into consideration in the present work, accounts for a part of N losses, which leads to underestimation of actual N retentions.
Lysine and methionine requirements for nitrogen maintenance and maximal nitrogen gain
Data obtained here on lysine and methionine requirements for maintenance are the first ever estimates of IAA requirements for N balance in crustacean shrimp. The proportion of ingested AA spent to cover the N maintenance requirement as compared to the requirement for maximal N gain was 14·5–15·9 % for lysine and 16·4–17·4 % for methionine. The similarity in maintenance contributions to total requirement for both IAA differs from the lower maintenance contribution for lysine than methionine reported in rainbow trout (4 % for lysine v. 10 % for methionine)(Reference Rodehutscord, Becker and Pack34) or in growing pigs (6 % for lysine v. 12 % for sulphur AA)(Reference Heger, Van Phung and Krizova43). In this respect, it would be of interest to evaluate the contributions at zero AA gain, given the possible underestimation of requirement based on N gain, related to shifts in the mobilisation of the type of body protein at intake levels near maintenance(Reference Gahl, Crenshaw and Benevenga35, Reference Edwards, Fernandez and Baker44).
Expressing the current lysine and methionine requirements for maximal N gain (1·1–1·4 and 0·6–0·7 g/kg BW per d, respectively) as a proportion of protein requirement enables comparisons with the two other studies available in literature on lysine and methionine requirements for growth of the same species. Expressed this way, the lysine and methionine requirements for maximal N gain (5·8 and 2·9 % of the protein requirement) are very close to the lysine requirement of 5·2 % and the methionine requirement of 2·4 % reported for post-larval P. monodon (approximately 20 mg initial BW) using the dose–response technique(Reference Millamena, Bautista-Teruel and Reyes14, Reference Millamena, Bautista-Teruel and Kanazawa17). The above requirements, however, exceed those found for L. vannamei, being 4·5–5·2 % for lysine(Reference Fox, Lawrence and Li-Chan45) and only 1·26 % for methionine(Reference Fox, Davis, Wilson, Suarez, Marie, Salazar, Lopez, Cavazos, Cruz and Ortega46). Expressing maintenance requirements the same way (g/16 g N), the proportion of protein requirement for maintenance covered by both IAA was only slightly less than that seen for maximal growth, being 4·5 % for lysine (using both models) and 2·4–2·5 % for methionine. Lower contribution of IAA to maintenance than to growth (g/16 g N)(Reference Fuller, McWilliam and Wang8, Reference Fournier, Gouillou-Coustans and Métailler10, Reference Mercer26, Reference Chung and Baker47) can be partly explained by the sparing of IAA due to the preferential oxidation of dispensable AA at N equilibrium, as suggested very early in the rats displaying lower requirements for IAA than for dispensable AA near maintenance(Reference Dreyer48) and in chickens for which dispensable AA were suggested to maintain and replete protein reserves(Reference Shapiro and Fisher49). In salmon fry, dispensable AA enabled a reduction in N losses, suggesting their implications in protein metabolism under maintenance conditions(Reference Abboudi, Mambrini and Larondelle50).
The ideal protein concept has been applied in IAA requirement studies for both terrestrial and aquatic species(Reference Mambrini and Kaushik51–Reference Furuya, Pezzato and Barros54), including crustaceans(Reference Millamena, Bautista-Teruel and Reyes14, Reference Alam, Teshima and Yaniharto15). In the present study, the AA profile of the balanced diets (LP, MP and HP) was based on a range of published AA profiles of P. monodon whole body. The created deficiency in lysine was probably not sufficient to impact growth performances of the shrimps, although a tendency for lower N gain was observed at the MP level. In contrast, methionine deficiency affected significantly N gains, however, only at the MP level, but not with the LP or HP diets. This interaction between methionine and dietary protein indicates that requirements for IAA, when expressed as % CP, should be evaluated together with requirements for protein, as in the present and some other mentioned studies(Reference Wang and Fuller7, Reference Fournier, Gouillou-Coustans and Métailler10, Reference Bodin, Govaerts and Abboudi33, Reference Nang, Parkouda and de Saeger55).
Interestingly, growth of the shrimp was not depressed by supplying excess N. Moreover, at intake levels exceeding N requirements, imbalances in the dietary IAA profile did not negatively affect FI or growth, as shown by the similarity in performances between shrimp fed the methionine-deficient HP or the balanced HP diet. This observation hence suggests that the ideal protein concept of ‘a perfect and constant ratio among individual IAA and dietary N’(Reference Boisen, Hvelplund and Weisbjerg53) should be applied only up to the N intake level providing maximal N gain, in line with broiler studies showing that lysine requirements are to be expressed as % CP at intake levels below but not above protein requirement(Reference Urdaneta-Rincon, de Lange and Pena-Ortega56). Also in kittens, increasing dietary CP while keeping methionine levels (% diet) constant did not reduce growth or feed intake(Reference Strieker, Morris, Kass and Rogers57), in contrast to earlier findings, referred to as AA imbalances, in rat and other animals(Reference Harper, Benevenga and Wohlhueter58). These aspects are important to consider when poor-quality proteins are included at higher than normal levels to provide a minimal level of IAA in the diet.
Efficiency of lysine and methionine utilisation for nitrogen gain
For vertebrates, a large debate continues to exist as to whether marginal IAA utilisation for growth (N or AA gain) is constant(Reference Rollin, Mambrini and Abboudi4, Reference Abboudi, Mambrini and Ooghe11, Reference Heger, Phung and Krizova21, Reference Heger, Van Phung and Krizova43, Reference Edwards, Fernandez and Baker44, Reference Hauler, Carter and Edwards59–Reference Heger and Frydrych62) or not(Reference Rodehutscord, Jacobs and Pack27, Reference Gahl, Finke and Crenshaw28, Reference Bodin, Govaerts and Abboudi33–Reference Robbins, Saxton and Southern35, Reference Fatufe and Rodehutscord63, Reference Peres and Oliva-Teles64). Because of this uncertainty and the fact that there is currently no such information for crustaceans, we compared efficiency values for both IAA obtained with the broken line and logistic regression, which fitted the data equally well (R 2>0·95). Based on the logistic model, the instantaneous efficiency (N gain/g AA intake) reached a maximum of 0·77 for lysine and of 1·62 for methionine, whereas diminishing returns in N gain began, respectively, at an intake level of 37·1 and 35·7 % of that required for maximal N gain. This observation is consistent with data from growing rats in which diminishing return responses in N gain started for each of the ten IAA at less than 40 % of maximum gain(Reference Gahl, Finke and Crenshaw28). In the same line, highest efficiencies were observed in rats fed diets providing 30–60 % of the requirement of the limiting IAA(Reference Heger and Frydrych62). Assuming a linear relationship between AA intake and N gain and thus a constant efficiency value, the marginal efficiency (N gain/g AA intake) of AA utilisation in the present study was 0·65 for lysine and 1·35 for methionine (U slopes BLM). The latter value agrees well with the efficiency of lysine utilisation for protein gain generally accepted in pigs (0·55–0·65)(Reference Heger, Van Phung and Krizova43). Regarding the efficiency of methionine utilisation, studies in terrestrial animals suggest that the growth response to changes in methionine intake depends on the presence of other dietary substrates (cystine, choline and betaine), which may spare the use of dietary methionine for metabolic processes (such as transmethylation to S-adenosylmethionine and transulphuration to cystine) other than for lean body growth(Reference Heger, Krizova and Sustale65). In this respect, in the presence of excess cystine, methionine retention in growing pigs was found to be a linear function of methionine intake (ranging from 45 to 90 % of the requirement)(Reference Chung and Baker47). When both cystine and methionine are limiting, e.g. as in the present study by keeping cystine/methionine (0·3/1) ratios constant, the increased demand for non-protein synthesis may result in decreased methionine utilisation(Reference Heger, Krizova and Sustale65). For P. monodon or other shrimp species, the relative contribution of cystine to the total sulphur AA requirement and the effect of cystine on methionine utilisation still remain to be elucidated.
Acknowledgements
The authors acknowledge the team of the Aqualma facility, with a special thanks to Christian Ramamonjisoa, Abel Randrianandrazana and Andry Rakotojaona for their technical assistance. From the Institut National de la Recherche Agronomique team, special thanks are due to Christiane Vachot, Fred Terrier and Peyo Aguirre for their help during diet manufacturing and to Marie Jo Borthaire for assistance with the laboratory analyses. P.-P. B. and V. R. contributed to the organisation of the experiments in Madagascar. S. J. K. and I. G. designed the study. L. R. did the data analysis. L. R., S. J. K. and I. G. contributed to the drafting of the paper. There are no contractual agreements for the presented data, which might cause conflicts of interest. The authors acknowledge UNIMA and institutional funds from Institut National de la Recherche Agronomique for funding the present study and Association Nationale de la Recherche Technique (France) for the scholarship to L. R. (CIFRE PhD Research Grant).












