Angiogenesis is one of the prerequisites for tumour growth and progression(Reference Dvorak1, Reference Nagy, Brown and Senger2). Without the angiogenic process, a tumour can only grow 1–2 mm in size. Angiogenesis is therefore a potential target in suppressing the spread and growth of cancer. Lycopene (a component of red fruits and vegetables) has been suggested as an anti-cancer dietary component by both epidemiological(Reference Ziegler3–Reference Rao and Agarwal5) as well as experimental studies(Reference Levy, Bosin and Feldman6–Reference Hwang and Lee9). Various modes of action for lycopene have been suggested for its anti-carcinogenic effects. These range from its antioxidant property(Reference Pennathur, Maitra and Byun10), reduction in DNA damage(Reference Rehman, Bourne and Halliwell7), inhibition of adhesion, invasion and migration(Reference Hwang and Lee9), cell cycle arrest(Reference Karas, Amir and Fishman8) and inhibition of tumour growth(Reference Levy, Bosin and Feldman6, Reference Yang, Yen and Uang11). Although each of these modes of action is important for tumour interception, angiogenesis inhibition is considered the most important target for blocking tumour growth. Recently, on subcutaneous implantation of human androgen-independent prostate carcinoma PC-3 cells into athymic nude mice, lycopene at concentrations of 4 and 16 mg/kg inhibited tumour growth while reducing plasma levels of vascular endothelial growth factor (VEGF)(Reference Yang, Yen and Uang11). Angiogenic factors such as VEGF, basic fibroblast growth factor and TNF-α are secreted by tumour cells in response to hypoxia and energy depletion, resulting in the recruitment of endothelial cells and their proliferation to mediate angiogenesis and hence tumour growth(Reference Bhat and Singh12).
In the present study, we have examined the effect of lycopene on angiogenesis in vitro. A number of angiogenesis models have been reported in the literature(Reference Auerbach, Lewis and Shinners13). These assays are based on the formation of tube-like structures, or pseudotubules, from endothelial cells upon their culture on an angiogenesis-supporting matrix. One such substrate is Matrigel™, which is the trade name for a solubilised basement membrane preparation marketed by BD Biosciences (San Jose, CA, USA). When endothelial cells grow on Matrigel™, they produce an intricate cell network, which is highly suggestive of the microvascular capillary system that provides tissues with nutrients and oxygen. Structural proteins such as laminin (60 %) and collagen type IV (30 %) make up the bulk of the Matrigel™ matrix and provide cultured cells with the adhesive peptide sequences they would naturally encounter in vivo. Also containing proteoglycans, heparin sulphate and entactin, the unique composition of Matrigel™ resembles the mammalian cellular basement membrane and thus provides a biologically active and physiologically relevant environment for studies of cell morphology, migration and angiogenesis. Angiogenesis can also be assessed by way of intricate co-culture of endothelial cells on a feeder layer of fibroblasts in high-priced ready-to-use angiogenesis kits or by using rat aortic rings. The latter, an ex vivo assay, involves thin aortic sections to be cultured on a supportive matrix such as Matrigel™ and the examination of outgrowth of endothelial and non-endothelial cells over a period of 7–14 d. Microvessel outgrowth from these primary explants can be quantified in terms of tubule length and number of junctions within the newly formed network. The rat aortic ring assay closely represents the in vivo environment of angiogenesis, as it involves both endothelial and surrounding non-endothelial cells(Reference Auerbach, Lewis and Shinners13). The present study examines the effect of lycopene on both the above assay systems.
Materials and methods
All reagents were obtained from Sigma-Aldrich (Gillingham, Dorset, UK) unless otherwise stated. Cell culture reagents were obtained from TCS Cellworks (Little Balmer, Bucks, UK). Matrigel™ basement membrane preparation was purchased from BD Biosciences. Standard cell culture plastics were obtained from Greiner Bio-one (Stonehouse, Gloucester, UK). Specialised culture plastics were obtained from Integrated BioDiagnostics (ibidi; Martinsried, Munich, Bavaria, Germany). The lycopene powder was a generous gift from DSM Nutritionals (Kaiseraugst, Switzerland).
Culture of endothelial cells
Pooled human umbilical vein endothelial cells (HUVEC) were obtained from TCS Cellworks and cultured in complete large vessel endothelial cell basal medium supplemented with 2 % complete large vessel endothelial cell growth supplement and antibiotics, as per the manufacturer's recommendations. Cells were passaged using a tailored passage kit, including a buffered rinsing solution, trypsin–EDTA and trypsin blocking solution. Incubation took place at 37°C in humidified air containing 5 % CO2. Care was taken not to grow HUVEC beyond 80 % confluence. All experiments involving HUVEC were carried out between passages three and six.
Preparation of lycopene
Lycopene was prepared fresh for each set of experiments. In short, a lycopene stock solution of 5 mm was prepared by dissolving the crystalline powder (DSM Nutritionals) in freshly purified tetrahydrofuran with 0·025 % butylated hydroxytoluene. Aliquots were stored at − 80°C under N2 gas. For experiments, the lycopene stock was diluted in the ratio 1:10 with fetal calf serum for enhanced stability and vortexed vigorously for 1 min. Cells were treated with lycopene at concentrations of 0·58, 1·15 (concentration previously shown to be achievable after the consumption of tomato products for 1 week)(Reference Lee, Thurnham and Chopra14) and 2·3 μmol/l. A tetrahydrofuran toxicity study on HUVEC revealed no effect on cellular viability at tetrahydrofuran concentrations < 0·25 %.
Rat aortic ring assay
The twenty-four centre wells of a forty-eight-well microtitre plate were coated with Matrigel™. A freshly dissected rat aorta was placed in PBS solution and cut into 1-mm rings using sterile scalpel blades in a sterile environment. The rings were then carefully placed into the Matrigel™-coated wells, one ring per well, and were maintained in Medium 200 containing a low serum growth supplement and 0·3 % of the anti-fungal agent, amphotericin. The plate was then incubated at 37°C in 5 % CO2 for 48 h before treatment. Following 48 h incubation, the rings were treated with lycopene at concentrations of 0·58, 1·15, 2·3 and 5 μmol/l in supplemented Medium 200 in duplicates. Control wells were treated with vehicle only. As outgrowth of cells from the aortic ring occurs over a period of 6–10 d and lycopene gradually degrades under cell culture conditions, the treatment medium was refreshed every day.
Analysis of the rat aortic ring assay
For imaging purposes, the rings were fixed in 10 % phosphate-buffered formalin (4 % formaldehyde, 0·4 % sodium dihydrogen orthophosphate and 0·65 % disodium hydrogen phosphate) and stained with 150 μl of a 0·02 % methylene blue solution for 24 h. The Matrigel™ was dried under a gentle stream of N2 gas. Images of each well were taken with a FUJI C750UZ digital camera (FUJIFILM Europe GmbH, Düsseldorf, Germany) and pseudotubule outgrowth was analysed using the angiogenesis analysis software AngioSys version 1.0 (TCS Cellworks), which measures tubule length, number of junctions, number of individual tubules and total tubule growth area.
Human umbilical vein endothelial cells monoculture assay
HUVEC were grown to 50 % confluence and treated with lycopene at concentrations of 0·58, 1·15 and 2·3 μmol/l for 24 h before seeding onto Matrigel™; or with TNF-α at concentrations of 5, 10 and 20 μg/l for 24 h before seeding; or with VEGF at concentrations of 1, 2, 4 and 8 ng/l upon seeding and for 24 h before seeding; or simultaneously with lycopene (0·58, 1·15 and 2·3 μmol/l) plus 10 μg/l TNF-α for 24 h; or with lycopene at concentrations of 0·58, 1·15 and 2·3 μmol/l for 24 h, followed by exposure to 8 ng/l VEGF upon seeding onto Matrigel™.
After incubation with the test compounds, cells were harvested using 0·02 % EDTA, pelleted by centrifugation and duplicate cell counts were performed. A total of 5 × 103 cells per well were then seeded into the ibidi μ-slide angiogenesis and incubated for 12–24 h. Exposure to 20 μg/l suramine served as a negative control.
Analysis of angiogenesis
For imaging purposes, pseudotubules were fixed overnight in 10 % phosphate-buffered formalin and pictures of the wells were taken by phase contrast microscopy at overall magnifications of 18·75 × and 30 × . The overall length of the tubule networks and the numbers of junctions were determined using the angiogenesis analysis software AngioSys version 1.0 (TCS Cellworks). Good-quality images with a clear contrast of pseudotubules-to-background are required for successful image analysis with this program. Identical threshold settings were maintained for all images of the same experiment series to ensure accuracy of the results.
Statistical analysis
Experiments were repeated three times, with triplicate measurements done on each occasion. Results are therefore an average of nine measurements. The data sets were compared by paired t test using SPSS 16.0 for Windows (SPSS, Inc., Chicago, IL, USA), with the significance of difference set at a level of 0·05.
Results
The rat aortic ring assay closely represents the in vivo environment of angiogenesis, as it involves endothelial cells as well as the surrounding non-endothelial cells(Reference Auerbach, Lewis and Shinners13). Treatment of rat aortic rings with lycopene significantly reduced the pseudotubule network in terms of network length as well as the junction numbers (Figs. 1 and 2). Fig. 1 shows reduction in tubule length by 25 and 44 % with lycopene concentration of 1·15 (P = 0·04, paired t test) and 5 μmol/l (P = 0·001), respectively. The effect on junction numbers was even more pronounced, with a reduction in network branching of 44 % at 1·15 μmol/l lycopene and a reduction of 43·5 % at the highest lycopene concentration of 5 μmol/l. The cell outgrowth at 8 d after planting the rings is shown in Fig. 2. The vehicle control (tetrahydrofuran, Fig. 2(a)) shows a nicely structured pseudotubule network with clearly defined tubules and regular branching. All cells appear to be linked into the network, with no apparent cell sheet formation. With the introduction of lycopene into wells (Fig. 2(b)–(e)), the regular network was replaced by a less structured arrangement of cells and greatly increased formation of cell sheets. The lack of structure was most obvious in the well with the highest concentration of lycopene (5 μmol/l, Fig. 2(e)), in which seemingly random cell sheets and irregular junctions replaced the defined pseudotubule network observed in the control well (Fig. 2(a)).

Fig. 1 Tubule length (![]() ) and junction numbers (□) of the cell outgrowth of rat aortic rings treated with 1·15 and 5 μmol/l lycopene relative to the untreated control (0 μmol/l lycopene). Values are means of three experiments and standard deviations. Mean values were significantly different: * P < 0·05, ** P < 0·01 (comparison with control was done using a paired t test).
) and junction numbers (□) of the cell outgrowth of rat aortic rings treated with 1·15 and 5 μmol/l lycopene relative to the untreated control (0 μmol/l lycopene). Values are means of three experiments and standard deviations. Mean values were significantly different: * P < 0·05, ** P < 0·01 (comparison with control was done using a paired t test).

Fig. 2 Rat aortic rings treated with lycopene at concentrations of (a) 0, (b) 0·58, (c) 1·15, (d) 2·3 and (e) 5 μmol/l in tetrahydrofuran.
Effect of lycopene on human umbilical vein endothelial cells monoculture in Matrigel™
Fig. 3 shows that with increasing concentrations of lycopene, the overall tubule length of the network as well as junction numbers within this network were reduced. Although the lowest concentration of 0·58 μmol/l lycopene exerted no significant effect, 1·15 μμmol/l of lycopene significantly reduced overall network length by 11 % (P = 0·04, paired t test) and junction numbers by 17 % (P = 0·05) compared to the control. The highest concentration of lycopene at 2·3 μmol/l further reduced network formation and junction numbers, resulting in a total reduction of 24 % (P = 0·03) and 33 % (P = 0·007), respectively. This is also illustrated in Fig. 4, which shows representative images of the tubule network formed by untreated HUVEC (Fig. 4(a)), as well as after exposure of HUVEC to 2·3 μmol/l (Fig. 4(b)) and 20 μg/l suramine (Fig. 4(c) – negative control).

Fig. 3 Effect of lycopene on angiogenesis of human umbilical vein endothelial cells. Cells were treated with increasing concentrations of lycopene for 24 h before seeding onto Matrigel™. The bar chart shows total tubule length (![]() ) and junction numbers (□) as a percentage of the control values and standard deviations. Mean values were significantly different from control: * P < 0·05, ** P < 0·01.
) and junction numbers (□) as a percentage of the control values and standard deviations. Mean values were significantly different from control: * P < 0·05, ** P < 0·01.

Fig. 4 (a) The pseudotubule network in an untreated control well. Effect of (b) 2·3 μmol/l lycopene and (c) 20 μg/l suramine on angiogenesis of human umbilical vein endothelial cells is shown.
Effect of TNF-α and lycopene on angiogenesis
The effect of TNF-α, a known pro-angiogenic factor, alone and in combination with lycopene was assessed. HUVEC were pre-treated with either TNF-α alone or in combination with varying concentrations of lycopene for 24 h before seeding onto Matrigel™ in the μ-slide angiogenesis. The initial TNF-α treatment course was used to establish a suitable concentration for HUVEC exposure in combination with lycopene. Cells were exposed to TNF-α at 5, 10 and 20 μg/l. Fig. 5(a) shows the effect of TNF-α on overall network length and junction numbers. TNF-α increased both the total tubule length and junction numbers at all concentrations. The latter increased by 14 % at 5 μg/l TNF-α and by 18 % at 10 μg/l (P = 0·002) of the inflammatory cytokine, whereas tubule length increased by 10 and 16 %, respectively. The 20 μg/l concentration of TNF-α did not result in a significant further increase of network formation compared to that at 10 μg/l. As a result, the 10 μg/l concentration of TNF-α was used in the subsequent experiments with lycopene.
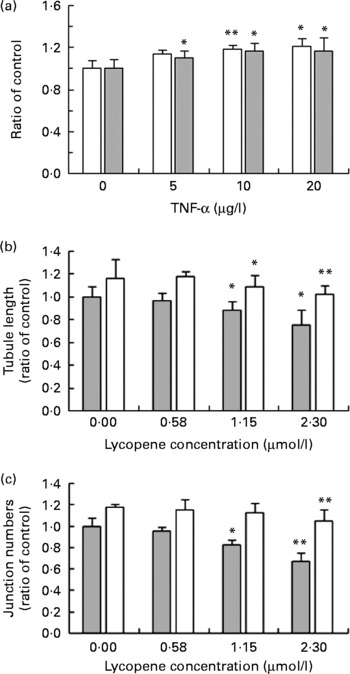
Fig. 5 The effect of TNF-α on angiogenesis of human umbilical vein endothelial cells. (a) Cells were treated with increasing concentrations of TNF-α for 24 h before seeding onto Matrigel™. Total tubule length (![]() ) and junction numbers (□) are shown as a percentage of the control values and standard deviations. The effect of lycopene on (b) total tubule length, (c) junction numbers, both in the presence (□) and absence (
) and junction numbers (□) are shown as a percentage of the control values and standard deviations. The effect of lycopene on (b) total tubule length, (c) junction numbers, both in the presence (□) and absence (![]() ) of TNF-α are shown. The bar chart shows the ratio of the control values and standard deviations. Mean values were significantly different from control: * P < 0·05, ** P < 0·01.
) of TNF-α are shown. The bar chart shows the ratio of the control values and standard deviations. Mean values were significantly different from control: * P < 0·05, ** P < 0·01.
When cells were exposed to 10 μg/l TNF-α and lycopene simultaneously for 24 h, there was a significant reduction in network branching (junction numbers, number of tubules and tubule length) at both lycopene concentration of 1·15 (P = 0·05) and 2·3 μmol/l (P = 0·01, Fig. 5(b) and (c)). Lower concentrations of lycopene (0·58 μmol/l) did not appear to affect the assessed angiogenic parameters significantly.
Effect of vascular endothelial growth factor and lycopene on angiogenesis
HUVEC were exposed to increasing concentrations of VEGF at concentrations of 1, 2, 4 and 8 ng/l, either 24 h before or while seeding onto Matrigel™. An increase in overall tubule length, as well as tubule numbers and junction numbers, could be observed with VEGF exposure, as is illustrated in Fig. 6. Overall tubule length peaked at 4 ng/l VEGF with a 12 % increase, whereas VEGF at 8 ng/l resulted in an overall increase in tubule and junction numbers of approximately 20 % (Fig. 6(a)). There was no significant difference in the effect of VEGF on the angiogenic activity of HUVEC pre-treated with VEGF for 24 h compared to exposure to VEGF upon seeding onto Matrigel™ (results not shown).

Fig. 6 The effect of vascular endothelial growth factor (VEGF) on angiogenesis of human umbilical vein endothelial cells (HUVEC). (a) Cells were exposed to increasing concentrations of VEGF upon seeding onto Matrigel™. The bar chart shows the number of tubules (![]() ), junctions (□) and total tubule length (
), junctions (□) and total tubule length (![]() ) as a ratio of the control values and standard deviations. The effect of lycopene alone and lycopene+8 ng/l VEGF on angiogenesis of HUVEC is shown in (b) and (c). Cells were pre-treated with increasing concentrations of lycopene for 24 h and exposed to 8 ng/l VEGF upon seeding onto Matrigel™. The effect of lycopene on (b) total tubule length and (c) junction numbers, both in the presence (□) and absence (
) as a ratio of the control values and standard deviations. The effect of lycopene alone and lycopene+8 ng/l VEGF on angiogenesis of HUVEC is shown in (b) and (c). Cells were pre-treated with increasing concentrations of lycopene for 24 h and exposed to 8 ng/l VEGF upon seeding onto Matrigel™. The effect of lycopene on (b) total tubule length and (c) junction numbers, both in the presence (□) and absence (![]() ) of VEGF. Data points are presented as a ratio of the control values and standard deviations. Mean values were significantly different from control: * P < 0·05, ** P < 0·01.
) of VEGF. Data points are presented as a ratio of the control values and standard deviations. Mean values were significantly different from control: * P < 0·05, ** P < 0·01.
Pre-incubation of HUVEC with lycopene for 24 h prior to exposure of cells to 8 ng/l VEGF resulted in a reduction of both tubule length and junction numbers. Exposure of HUVEC to the lowest lycopene concentration of 0·58 μmol/l abrogated the stimulatory effect of VEGF on both the junction numbers and overall network length, but results were statistically significant for tubule length only (P = 0·05). With increasing lycopene concentrations, both parameters decreased further (Fig. 6(b) and (c)), resulting in a maximum reduction of tubule length by 15 % at 2·3 μmol/l (P = 0·008) and 7 % at 1·15 μmol/l (P = 0·03) lycopene relative to the untreated control. The reduction in junction numbers was more pronounced at 2·3 μmol/l lycopene (29 % reduction, P = 0·03) than at 1·15 μmol/l (24 % reduction, P = 0·05). Lycopene appeared to have a greater inhibitory effect on the network branching, that is, junction numbers, mediated by exposure to VEGF than on the overall tubule length.
Discussion
Tumours can remain dormant for years, during which time their size is maintained by a balance between apoptosis and proliferation of cells. This, however, maintains the tumour size at the limit for simple diffusion of nutrients and gases like CO2 and O2. To increase its growth, tumour cells secrete angiogenic factors, which lead to the recruitment of endothelial cells and their proliferation for neovascularisation. Anti-angiogenic intervention is therefore considered important in cancer interception because of the crucial role that neovascularisation plays in the pathology of cancer. In the present investigation, lycopene at a final concentration ≥ 1·15 μmol/l significantly inhibited the angiogenic process in all the test systems, that is, rat aortic ring assay as well as TNF-α- and VEGF-stimulated angiogenesis in HUVEC. To extrapolate these results to an in vivo situation, it is important to consider whether the endothelium is likely to be exposed to such concentrations in vivo. Both inter-individual differences in response and bioavailability from different tomato products will overall influence the changes in plasma lycopene after supplementation(Reference Porrini and Riso15, Reference Maiani, Caston and Catasta16). Plasma levels of up to 1·2 μmol/l have been obtained with the use of supplements at a dose of 15 mg lycopene/d (equivalent to 600 g of raw tomatoes or 100 g of tomato paste) for 3 months(Reference Olmedilla, Granado and Southon17). A similar increase in plasma lycopene was observed after the consumption of 200 g tomato soup plus 230 g canned tomatoes providing approximately 46 mg lycopene/d for 1 week(Reference Lee, Thurnham and Chopra14). An increase in plasma lycopene to approximately 1·2 μmol/l has also been reported after supplementation with tomato sauce-based pasta for 3 weeks providing approximately 30 mg lycopene/d(Reference Porrini, Riso and Testolin18). However, several other studies with supplements or foods containing a lycopene content of 6–25 mg and a duration period ranging from 1 to 8 weeks have shown a maximum increase of plasma lycopene to 0·80 μmol/l(Reference Richelle, Bortlik and Liardt19–Reference Bowen, Chen and Stacewicz-Sapuntzakis22). It is therefore important to note that, although levels of up to 1·2 μmol/l can be achieved in vivo, these levels are likely to be achieved when individuals are either supplemented for a longer period of time or with high concentrations of a more bioavailable form of lycopene. In our previous study, when participants were allowed to choose from different red fruits and vegetables providing approximately 45 mg lycopene/d, mean plasma lycopene concentrations were raised to 0·85 μmol/l only, and the change was lower in smokers compared to non-smokers(Reference Chopra, O'Neill and Keogh23). Lycopene bioavailability is known to vary between tomato products, and bioavailability is higher from processed tomatoes compared to raw tomatoes(Reference Richelle, Bortlik and Liardt19, Reference Gartner, Stahl and Sies24, Reference Stahl and Sies25). Both processing and presence of fat have been show to increase the bioavailability of lycopene(Reference Stahl and Sies25). However, prolonged heating can also reduce the carotenoid content, especially if peeled tomatoes are used(Reference Graziani, Pernice and Lanzuise26). Furthermore, the lycopene content of tomato products can also vary(Reference O'Neill, Carroll and Corridan27). Observational studies have reported a reduction in risk of cancer incidence, especially that of prostate cancer, with the consumption of approximately 200 g tomato products per day(Reference Etminan, Takkuche and Cammano-Isoma28). An increase in plasma lycopene to 0·55 μmol/l has been reported with the consumption of 300 g raw tomatoes for 7 d and up to 0·80 μmol/l with consumption of 60 g tomato puree(Reference Richelle, Bortlik and Liardt19). In a prospective study, patients with aggressive prostate cancer were reported to show a plasma lycopene concentration of 356 ng/ml (0·66 μmol/l) compared to controls with plasma values of 388 ng/ml (0·72 μmol/l)(Reference Gann, Ma and Giovannucci29). In our present study, at a test concentration of 0·58 μmol/l, very little effect was observed on angiogenesis. Also, we did not examine the effect within a concentration range of 0·58–1·15 μmol/l. However, it is possible that a small increment in the concentration of lycopene >0·6 μmol/l might have shown a significant effect and perhaps highlighted the minimum lycopene concentration that is likely to intercept the angiogenic process. Baseline plasma concentrations of 0·50–0·60 μmol/l (equivalent to the lowest concentration used in the present study) has been reported by several studies(Reference Lee, Thurnham and Chopra14, Reference Porrini, Riso and Testolin18, Reference Richelle, Bortlik and Liardt19, Reference Paetau, Khachik and Brown30); however, few studies have also reported plasma levels of lycopene as low as 0·34 μmol/l with their habitual diet(Reference Porrini, Riso and Brusamolino20, Reference Kim, Paik and Kim21). Likewise, low lycopene levels reported for cancer patients and controls also vary between studies(Reference Gann, Ma and Giovannucci29, Reference van Eenwyk, Davis and Bowen31–Reference Lu, Hung and Heber33). The amount of lycopene that may be required to affect angiogenesis in vivo is therefore likely to vary between individuals. It is therefore difficult to speculate on the amount of tomato products that can be recommended for a possible angiogenic interception in vivo. The results of our study therefore can only highlight that at a baseline concentration that has been reported by several studies, that is, 0·58 μmol/l, lycopene has a minimal effect on angiogenesis; however, >1 μmol/l lycopene can significantly inhibit angiogenesis in vitro.
β-Carotene and lycopene are the two major carotenoids in tomatoes as well as in human plasma(Reference Porrini, Riso and Testolin18, Reference Richelle, Bortlik and Liardt19, Reference Chopra, O'Neill and Keogh23, Reference O'Neill, Carroll and Corridan27). In one study, oral β-carotene supplementation of mice at 0·25 % concentration for 2 weeks is reported to inhibit angiogenesis evoked by HeLa and SKv-t tumour cell lines, but increased the angiogenesis induced by lymphocytes(Reference Szmourlo, Marcozak and Rudnicka34). Another study examined a concentration range of 0·3–3 μmol/l of β-carotene and reported an increase in basic fibroblast growth factor-induced angiogenesis in a mouse Matrigel™ model at a concentration of 3 μmol/l of the carotenoid(Reference Dembinska-Kiec, Polus and Kiec-Wilk35). In contrast, there are reports of a dose-dependent inhibition of tumour-specific angiogenesis in HUVEC and rat aortic rings by β-carotene at concentration range of 1–10 μg/l (1·8–18 μmol/l) in vitro (Reference Guruvayoorappan and Kuttan36) as well as in an in vivo mouse model(Reference Huang, Liao and Hu37). A recent study reported a dose-related inhibition of angiogenesis by lycopene in HUVEC at a concentration range of 1–10 μmol/l(Reference Sahin, Sahin and Gumuslu38). In agreement with their results, our experiments also show an inhibitory effect at concentrations >1 μmol/l and a small, though mostly insignificant, effect at concentrations as low as 0·58 μmol/l. In the study by Sahin et al. (Reference Sahin, Sahin and Gumuslu38), HUVEC were grown in a medium supplemented with VEGF and fibroblast growth factor, and both these are considered important angiogenic growth factors. Angiogenesis inhibitors can either act by inhibition of the formation of these growth factors or through blocking of their action(Reference Bhat and Singh12). In the previous studies, in vivo supplementation of lycopene was shown to reduce the plasma levels of VEGF(Reference Yang, Yen and Uang11) and inhibit the VEGF expression by nude mice injected with tumour cells(Reference Huang, Liao and Hu37). VEGF levels are reported to be raised in cancer patients(Reference Fuhrmann-Benzakein, Ma and Rubbia-Brandt39). The present study has demonstrated a slight but significant reduction in VEGF-mediated angiogenesis of HUVEC after 24 h pre-treatment of cells with lycopene, both with regard to overall pseudotubule network length and junction numbers. These effects were seen at concentrations as low as 1·15 μmol/l, but were most pronounced at the higher concentration of 2·3 μmol/l of lycopene.
The pro-inflammatory cytokine, TNF-α, has also been shown to act as an autocrine growth factor for tumour angiogenesis(Reference Minuzzo, Moserle and Indraccolo40) and to affect the angiogenesis process directly(Reference Minuzzo, Moserle and Indraccolo40, Reference Sainson, Johnston and Chu41) as well as through its effects on VEGF formation(Reference Sainson, Johnston and Chu41, Reference Malaguarnera, Imbesi and Di Rosa42). The present study has demonstrated angiogenesis interception by lycopene in both TNF-α-mediated (Fig. 5(b) and (c)) as well as VEGF-induced angiogenic potential of HUVEC (Fig. 6(b) and (c)). With circulating levels of TNF-α being raised in prostate cancer(Reference Nakashima, Tachibana and Ueno43, Reference Michalaki, Syrigos and Charles44), lycopene might be beneficial in reducing the pro-angiogenic action of TNF-α in these patients. Epidemiological prospective data and dietary case–control studies suggest that a high intake of tomatoes and tomato-based products, with a resulting high plasma concentration of circulating lycopene, is associated with a highly significant reduction in the risk of cancer, in particular prostate cancer(Reference Giovannucci45). This has been found to be especially true for the more aggressive forms of prostate cancer. Also, lower serum and prostatic tissue levels of lycopene were demonstrated in men with prostate cancer when compared to healthy age-matched controls(Reference Gann, Ma and Giovannucci29, Reference Rao, Fleshner and Agarwal46). Likewise, lower plasma lycopene levels have been reported in patients with cervical cancer(Reference van Eenwyk, Davis and Bowen31, Reference Batieha, Armenian and Norkus32) and colorectal adenomas(Reference Erhardt, Meisner and Bode47). The anti-angiogenic activity of lycopene is therefore a likely candidate not just for prostate but also for other cancers owing to the crucial role that angiogenesis plays in growth and sustainment of tumours. It has been suggested that agents that exhibit both anti-angiogenic as well as anti-metastatic activity are likely to evoke a greater effect on tumour response/therapy than treatment with a single agent of these classes(Reference Teicher48). The results of the present study combined with previously published anti-metastatic effects of lycopene(Reference Hantz, Young and Martin49–Reference Palozza, Simone and Catalano51) not only support the findings of observational studies that show an inverse correlation between tomato consumption and risk of cancer but also offer a therapeutic hope for cancer inhibition.
Angiogenesis is a fundamental step in the transition of tumours from a quiescent to a malignant state, and is a requirement for both tumour progression and metastasis(Reference Cavallaro and Christofori52). The tumour vasculature has been identified as a strong prognostic marker for tumour grading(Reference Heidenreich, Aus and Bolla53). Endothelial cells represent a suitable target for anti-angiogenic treatment because they are non-transformed host cells and unlikely to acquire resistance to treatment compounds. Being part of the circulatory system, they also facilitate administration of a given therapeutic agent. Natural products are an attractive option for tumour interception, especially if they can be shown to intercept the tumorigenic processes at biologically achievable concentrations. The present investigation has demonstrated an inhibitory effect of lycopene on the angiogenic response in HUVEC and rat aortic rings at concentrations possible to be achievable in vivo after tomato product consumption. As lycopene was shown to exhibit its anti-angiogenic effects at achievable concentrations in vivo, it indeed can be regarded as a promising anti-angiogenic compound and can also explain why this compound has been highlighted as an important anti-cancer dietary component. Further in vivo studies are however warranted to confirm the in vitro effects of lycopene seen in the present study.
Acknowledgements
The authors would like to thank DSM Nutrition Products Limited for their generous gift of the crystalline lycopene used in the present study. The study was partly funded by HJ Heinz Limited (Hayes, Middlesex, UK). S. E. was supported by funding from the Institute of Biomedical and Biomolecular Science (IBBS, Portsmouth, Hampshire, UK). M. C. directed the present research and was responsible for preparing and submitting the manuscript. A. C. was the co-supervisor on the present project and helped S. E. with the rat aortic rings assay. S. E. conducted the experiments and wrote the methods and results section. All three have contributed towards the preparation of this paper. The authors declare no conflict of interest.








