The chemoreception of fatty acids (FA) in the oral cavity, also known as fatty acid taste (FAT), is involved in the regulation of dietary fat intake(Reference Cartoni, Yasumatsu and Ohkuri1–Reference Zhang, Zhou and Ban3). Individuals with impaired FAT sensitivity are more likely to consume greater amounts of dietary fat due to compromised cephalic phase and post-ingestive satiety hormone responses following oral fat exposure(Reference Keast, Azzopardi and Newman4–Reference Wisén, Björvell and Cantor9). This is reflected throughout the alimentary canal, as individuals who are less sensitive to FAT in the oral cavity also have reduced hormonal response following FA stimulation in the gastrointestinal tract (GIT)(Reference Stewart, Seimon and Otto8). FAT sensitivity is attenuated by oral fat exposure and conversely can be increased by long-term reduced dietary fat intake(Reference Costanzo, Nowson and Orellana10–Reference Stewart and Keast12), which may be due to the regulation of some or all FAT receptors in the oral cavity.
Three types of lingual papillae – fungiform, foliate and circumvallate – house taste bud cells (TBC) which express FAT receptors. A recent study of our group localised several candidate FAT receptor genes – cluster of differentiation 36 (CD36), free fatty acid receptor 2 (FFAR2), FFAR4, G protein-coupled receptor 84 (GPR84) and a delayed rectifying K+ channel (K+ voltage-gated channel subfamily A member 2; KCNA2) – in TBC of human fungiform papillae(Reference Liu, Costanzo and Evans13). Each of these receptors has specificities with regard to the type of FA they bind(Reference Liu, Archer and Duesing14); however, the exact receptors that might be responsible for oral chemoreception of FA in humans remain unresolved. Previously, we showed that increased CD36 was associated with short-term fat intake, particularly saturated fat, and acute dislike of fatty foods(Reference Liu, Costanzo and Evans13). However, no association was found between any FAT receptors and FAT perception. Since FAT sensitivity can be modified by dietary fat intake(Reference Costanzo, Nowson and Orellana10–Reference Stewart and Keast12), it may be possible to use a dietary fat intervention to elucidate the receptor that might be responsible for long-term changes in FAT perception.
While no human studies have investigated changes in FAT receptors in response to changes in the dietary fat, animal studies have been conducted albeit with conflicting results. An 8-week high-fat (HF) (40 % energy from fat) diet induced significant down-regulation of CD36 in rat circumvallate TBC(Reference Zhang, Zhou and Ban3). However, this down-regulation was not observed in fasted mice fed an HF (34·2 %) diet for 4 weeks(Reference Chevrot, Bernard and Ancel15). Another study found that protein levels of CD36, rather than mRNA, decreased, while FFAR4 protein increased in raft membranes of human and mouse fungiform TBC following a 2-month HF (40 % energy from fat) diet(Reference Ozdener, Subramaniam and Sundaresan16). As for short-term, an acute HF (30 % energy from fat) oral exposure triggered an immediate decrease in CD36 protein in mice circumvallate TBC, which returned to preexposure levels following 11 h of fasting, with no obvious effect seen for FFAR4 protein(Reference Martin, Passilly-Degrace and Gaillard17). The discrepancies between the studies may be attributed to the fat content, the different area of the tongue used for analysis and/or fasting status. Diet-mediated regulation of FAT receptors has not been investigated in humans. Manipulating dietary fat intake, and in turn FAT taste perception, would elucidate the key receptors that are associated with long-term regulation of oral chemoreception of FA.
The present study aimed to determine the FAT receptors that are responsible for diet-mediated changes to FAT perception in humans. The aim was accomplished by assessing the association between changes to key FAT receptor genes expressed in fungiform papillae (as determined by Liu et al.(Reference Liu, Costanzo and Evans13)) and FAT perception following an 8-week dietary fat intervention. A secondary aim was to assess the association between changes in macronutrient intake and FAT receptor gene expressions.
Subjects and methods
The study presented here is an 8-week follow-up analysis of a previous study where the baseline data have been published(Reference Liu, Costanzo and Evans13).
Participants
A co-twin design was chosen as it controls for age, common environmental and partial genetic factors shared by co-twins in each experimental group. Twins Research Australia (TRA) invited via mail 1881 twin pairs (3762 individuals) from the Melbourne metropolitan area to participate in a larger study on the effect of diet and genes on FAT(Reference Costanzo, Nowson and Orellana10). Twins were eligible to participate in the study if they were aged between 18 and 69 years, were able to attend three laboratory sessions in Burwood, Victoria and were willing to alter their diet for a period of 8 weeks. Both monozygotic and dizygotic twin pairs were invited to participate. Subjects were excluded from recruitment if they had any dairy allergies and intolerances, if they had any illnesses preventing them from eating foods included in the study, or if they were pregnant or lactating. Due to the nature of the twin study design, if one individual from a twin pair was excluded or withdrawn from the study, their co-twin was also excluded. In sixty-six pairs, both twins expressed interest in participating and were then screened for eligibility. Forty-six twin pairs (ninety-two individuals) aged between 18 and 68 years were recruited into the larger study; however, only thirteen pairs (twenty-six individuals; ten monozygotic pairs, three dizygotic pairs) consented to the additional testing described in this paper (Fig. 1). Co-twins from each pair were randomised into either a low-fat (LF) or an HF diet, where one twin from each pair was allocated to the LF diet and the other twin allocated to the HF diet. Prior to recruitment, a block randomised sequence was generated with blocks of size two. TRA was responsible for recruitment and therefore characteristics of the participants were blinded to the researchers. Participants were allocated to the randomised sequence based on their TRA twin number; therefore, allocation of participants to diet group was concealed. Due to the nature of the intervention, blinding of participants was not feasible.
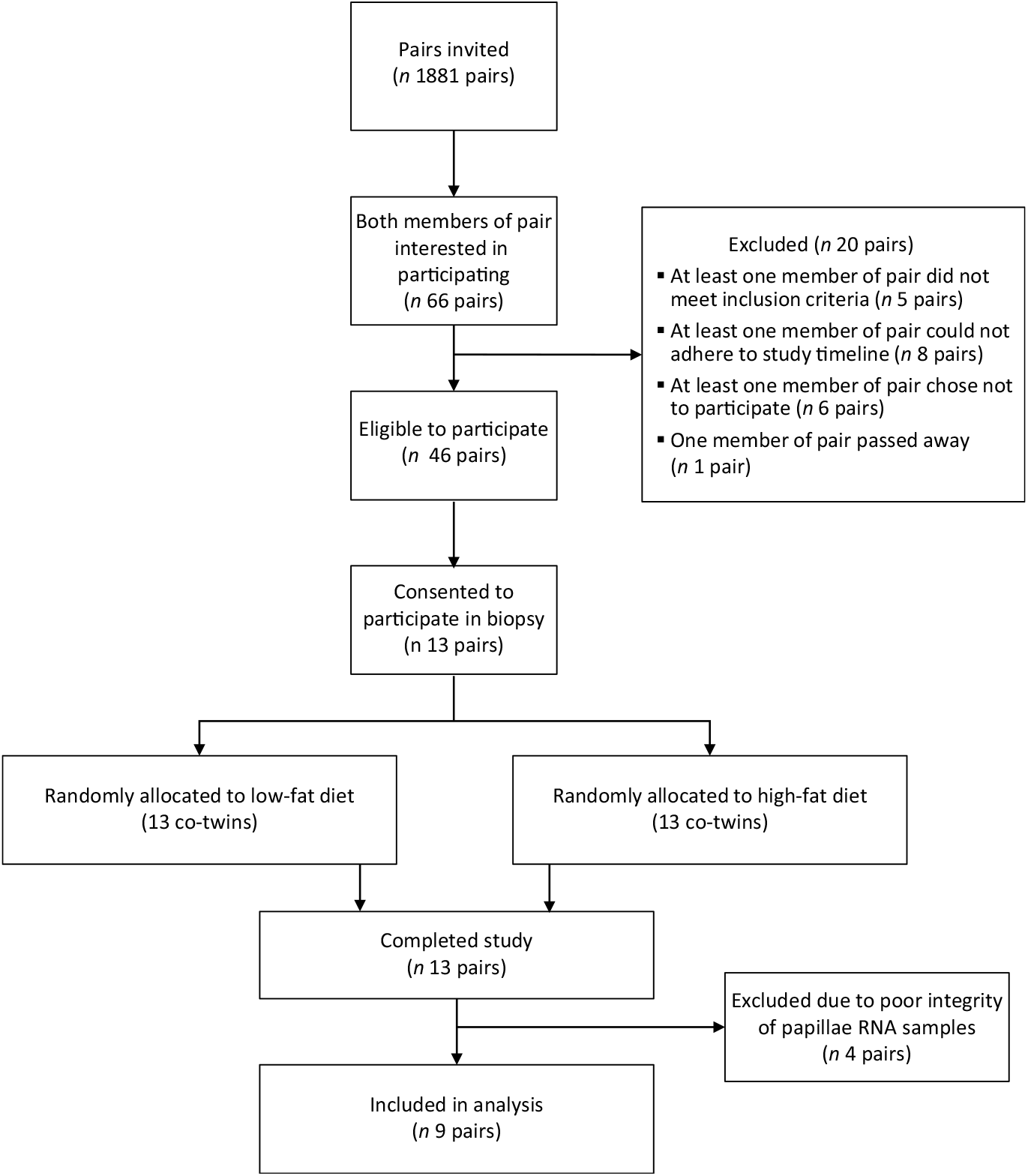
Fig. 1. Study CONSORT flow chart diagram.
Ethics
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Deakin University Human Research Ethics Committee (ID: 2013-163). Written informed consent was obtained from all subjects. The present study is registered as a clinical trial with the Australian New Zealand Clinical Trials Registry (ID: ACTRN12613000466741; https://www.anzctr.org.au/).
Study outline
Participants attended two tasting sessions and two biopsy sessions at the Centre for Advanced Sensory Science at Deakin University, Burwood, Victoria. Recruitment and data collection occurred between July 2014 and May 2016. The first tasting session occurred on the day prior to beginning the dietary intervention, and the second tasting session occurred 8 weeks later on the last day of the intervention. Tasting sessions were conducted in a temperature- and sound-controlled environment with a 15-min break in the middle of their session to prevent fatigue. Participants were asked to avoid eating or drinking anything but water and to avoid brushing their teeth or using mouthwash up to an hour prior to each tasting session. Tasting sessions measured FAT threshold (FATT) for oleic acid (C18 : 1) and anthropometric measurements. A 24-h food recall was collected by a nutritionist during the first session. Between tasting sessions, participants recorded six 24-h diet records, two weekdays and one weekend during the first 4 weeks, and two weekdays and one weekend during the final 4 weeks of the trial.
Collection of fungiform papillae (biopsy) occurred the day after each tasting session. Participants fasted for at least 10 h prior to each fungiform papillae collection. Fungiform papillae tissue and serum blood samples were collected. After the biopsy session, participants were provided with an LF or HF breakfast snack depending on the diet that they were allocated.
Dietary intervention
The LF diet was defined as <20 % of energy from fats and the HF diet was defined as >35 % of energy from fats. These values were chosen as they fall outside the acceptable macronutrient distribution range for fat intake (20–35 %)(18). Participants on the HF diet were encouraged to choose foods higher in monounsaturated and polyunsaturated fats rather than saturated fats in order to maintain a healthy diet. A diet booklet for each diet was created with the aid of an accredited practicing dietitian, which described the parameters of each diet, a list of foods that should be and should not be eaten and some example recipes that adhere to the diet. Participants were given the HF or LF booklet, their assigned diet was explained and they were taught how to interpret a nutrition information panel in order to identify the food that were acceptable for their diet.
Participants were requested to start the assigned diet the day after the baseline measurement. As food were not provided in the present study, food choice was up to the participants. To maximise adherence to the diets, participants were contacted via phone fortnightly and questioned on their dietary habits. If the researcher felt that participants were not following the diet adequately, they were provided with suggestions and encouragement to aid in diet adherence. Participants were also asked a series of questions to ensure that they did not experience any negative effects from the diet. These questions included ‘Do you feel like you have less energy since starting the diet?’ ‘Do you feel like your weight has changed significantly since starting the diet?’ ‘Is the diet affecting your day-to-day activities?’ If the researcher felt that participants were suffering from major negative effects due to the diet (e.g. nausea, inability to work), they would be asked to stop the diet and were dropped out of the trial.
The first three completed diet diaries were inspected and reviewed at week 4 to assess compliance to the assigned diet.
Dietary assessment
A single three-pass 24-h dietary recall(19) of the day prior to the first tasting session was conducted by a trained nutritionist. For the ongoing diet records throughout the trial, participants were asked to avoid filling out diet records on a non-standard day (e.g. if they attended a wedding reception). They were taught to quantify foods in standard serving sizes (cups, teaspoons, tablespoons, etc.) using a food model booklet and asked to weigh their food and drinks wherever possible. Details such as brand, cooking method and foods additives (e.g. sugar added to coffee) were included in the diet records.
Food recall and records were analysed for carbohydrate, protein, fat and fibre intake (g and % of energy) using computer software FoodWorks (version 8; Xyris).
Anthropometry
Body weight was measured after removal of shoes, heavy clothing and any items in their pockets using electronic scales (OHAUS NV4101), and height was measured using a free-standing stadiometer (SECA). BMI was calculated as weight (kg)/height (m)2.
Fatty acid taste receptor expression
Fungiform papillae biopsy was conducted without anaesthetic by a registered doctor following the procedure described previously(Reference Spielman, Pepino and Feldman20,Reference Archer, Liu and Shaw21) . Fungiform papillae were chosen as they were the least invasive biopsy target and provide the best result compared with other oral sampling methods(Reference Haryono, Sprajcer and Keast22). For each participant, up to eight fungiform papillae were collected; four papillae chosen from the left side of the tongue and four from the right side of the tongue, which were chosen at random sites within the fungiform region of the tongue by the doctor. The eight papillae were pooled as an individual sample to reduce the impact of variation from different sites of collection. The gene expression of FAT receptors found within fungiform papillae tissue(Reference Liu, Costanzo and Evans13) including CD36, FFAR2, FFAR4, GPR84 and KCNA2 was analysed with real-time RT-PCR. RNA was extracted from the pooled fungiform papillae samples using TRIzol reagent (Life Technologies) following the manufacture’s protocol. The purified RNA pellet was dissolved in 20 μl RNase-free water treated with RNase-free DNase set (Qiagen) and quantified with NanoDrop ND-1000 spectrophotometer. The RNA integrity was measured with Bioanalyser 2100 (Agilent Technologies) and the cut-off value was set at 5. For the RT-PCR, 1 μg of total RNA was used to synthesise complementary DNA (cDNA) using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems). Each cDNA sample was diluted 1:5 at first. Standards were then prepared with a serial dilution of 1:5 from the top standard (an aliquot of all the 1:5 dilution cDNA samples). For the standard curve, one gene copy was assigned to the lowest concentrated standard, which had a Cp value. Gene concentration of other standards were set accordingly based on the dilution value. The notional concentration of each sample was calculated based on the standard curve (log concentration against Cp). The expression of the genes of interest were analysed with the Taqman gene expression assays (Life Technologies) (Table 1). For each gene analysis, a negative control of the sample that had not been reversely transcribed was included. Housekeeping genes glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and 60S acidic ribosomal protein P0 (RPLP0) were included for normalising the transcript numbers.
Table 1. Taqman gene expression assays used for the real-time RT-PCR analysis
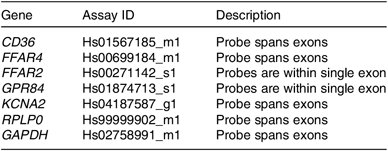
CD36, cluster of differentiation 36; FFAR4, free fatty acid receptor 4; FFAR2, free fatty acid receptor 2; GPR84, G protein-coupled receptor 84; KCNA2, delayed rectifying K+ channel (K+ voltage-gated channel subfamily A member 2); RPLP0, 60S acidic ribosomal protein P0; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Fatty acid taste threshold
Fat-free milk (Devondale) solutions containing C18 : 1 (Sigma Aldrich) at varying concentrations (0·02, 0·06, 1·00, 1·40, 2·00, 2·80, 3·80, 5·00, 6·40, 8·00, 9·80, 12·00 and 20·00 mM) were prepared using established methods(Reference Haryono, Sprajcer and Keast22). Control samples were prepared in the same way but without added C18 : 1.
FATT for C18 : 1 were determined using an ascending series three-alternate forced choice methodology(Reference Costanzo, Nowson and Orellana10). To prevent confounding non-taste sensory inputs, participants wore nose clips and all tests were conducted under red light. FATT was transformed to an ordinal variable ranging from 0 to 12, with a higher score implying lower FAT sensitivity(Reference Costanzo, Orellana and Nowson2).
Statistical analysis
Analyses were conducted using computer software SPSS (v24.0; IBM). Null hypotheses were rejected at P < 0·05. The present study is a secondary analysis of participants from a larger study(Reference Costanzo, Nowson and Orellana10), who provided consent to additional testing; therefore, sample size of the present study was determined by feasibility of recruitment.
The effect of the diet on FAT receptor gene expressions was assessed using linear mixed models including diet (LF and HF), time (baseline and week 8) and the time–diet interaction as fixed effects; with twin pair as a random effect and co-twin as the subject with repeated measures to account for the correlation between co-twins. A post hoc Sidak’s test was used to compare means between each fixed effect and to correct for multiple comparisons. Time–diet interaction, post hoc Sidak’s test and CI are reported. The above model was also used to assess differences between diet groups at baseline and to determine compliance to the prescribed diets.
To explore the strength of the association (![]() ) between change in FATT and change in FAT receptor gene expression over the 8-week period, the same model as above was conducted with Δ FATT (Δ, week 8 − baseline) as the outcome and Δ FAT gene expression as a fixed effect and twin pair as a random effect. Pearson’s correlations (r) for each association are also reported for descriptive purposes. The same analysis was repeated for the association between Δ macronutrient intake (total fat, saturated fat, monounsaturated fat, polyunsaturated fat, carbohydrate, protein and dietary fibre) (g) and Δ FAT gene expression.
) between change in FATT and change in FAT receptor gene expression over the 8-week period, the same model as above was conducted with Δ FATT (Δ, week 8 − baseline) as the outcome and Δ FAT gene expression as a fixed effect and twin pair as a random effect. Pearson’s correlations (r) for each association are also reported for descriptive purposes. The same analysis was repeated for the association between Δ macronutrient intake (total fat, saturated fat, monounsaturated fat, polyunsaturated fat, carbohydrate, protein and dietary fibre) (g) and Δ FAT gene expression.
Results
Thirteen twin pairs were recruited in the present study. However, four twin pairs were not included in the RT-PCR analysis of gene expression due to low concentration or poor integrity of the collected RNA samples according to the Nanodrop and Bioanalyser analyses. As a result, we present here nine pairs (seven monozygotic and two dizygotic; eight female pairs and one male pair) with valid samples that underwent RT-PCR analysis (Fig. 1). No harmful or unintended effects were observed in either group. None of the participants reported any significant changes to their weight, energy levels or ability to perform day-to-day tasks throughout the dietary intervention. Baseline characteristics of the participants are described in Table 2. No significant differences in baseline characteristics were observed between diet groups.
Table 2. Baseline characteristics of participants
(Mean values and standard deviations)
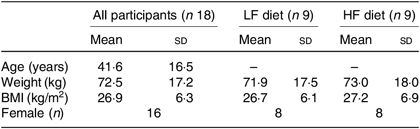
LF, low fat; HF, high fat.
Dietary compliance
Intake of energy from fat throughout the trial was within the aim of the study, with the LF group consuming 14·8 (95 % CI 10·0, 19·7) % energy from fat and the HF group consuming 39·9 (95 % CI 35·0, 44·7) % energy from fat (online Supplementary Table S1). Intake of energy from fat at baseline was already considered high in the HF diet group at 36·4 (95 % CI 31·6, 41·3) %, so there was no significant change in fat intake across the trial in this group. A significant decrease in the intake of energy was observed from fat in the LF diet group (−20·3 (95 % CI −26·2, −14·4) %, P < 0·001). No significant change in total energy intake was observed in either diet group, though a between-group difference was observed at week 8 (2·3 (95 % CI 0·5, 4·2) MJ, P = 0·021).
Effect of dietary fat intake on fatty acid taste threshold
No difference in FATT was observed between the LF and HF diet groups at baseline (Table 3). Over the 8 weeks, FATT decreased by 76 % in the LF diet group (P < 0·001) and increased by 23 % in the HF diet group (P = 0·049). At week 8, FATT in the HF diet group was 3·8 times higher than the LF diet group (P < 0·001), and a significant time–diet interaction was observed for FATT (P < 0·001) (Table 3).
Table 3. Means and between-group differences in fatty acid taste threshold (FATT) and fatty acid taste receptor gene expression levels (relative gene copy number) in fungiform papillae taste bud cells over the 8-week trial‡
(Mean values and 95 % confidence intervals)
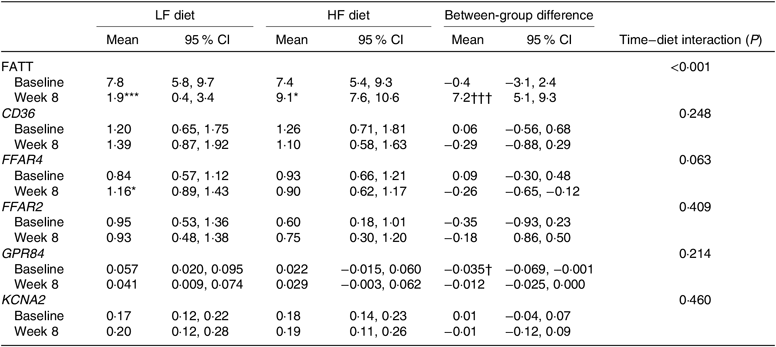
LF, low fat; HF, high fat; CD36, cluster of differentiation 36; FFAR4, free fatty acid receptor 4; FFAR2, free fatty acid receptor 2; GPR84, G protein-coupled receptor 84; KCNA2, delayed rectifying K+ channel (K+ voltage-gated channel subfamily A member 2).
Significantly different from baseline: * P < 0·05, *** P < 0·001.
Significant between-group difference: † P < 0·05, ††† P < 0·001.
‡ Between-group difference calculated as HF diet − LF diet. Estimated means, CI and P values obtained under a mixed model including twin pair as a random effect. Post hoc Sidak’s test, CI and time–diet interaction are reported.
Effect of dietary fat intake on fatty acid taste receptor gene expressions
There was evidence for a time–diet interaction for FFAR4 expression (P = 0·063), as expression increased by 38 % in the LF diet group (P = 0·023) (Table 3). No significant time–diet interactions were observed for CD36, GPR84, FFAR2 and KCNA2 expression, though GPR84 expression was 61 % greater in the LF diet group compared with the HF diet group at baseline.
Associations between fatty acid taste threshold and fatty acid taste receptor gene expressions
The relationship between FAT sensitivity and FAT receptor expression was assessed by comparing change (Δ) in FATT with change in receptor expression from baseline to week 8 (Table 4) (Fig. 2). A significant negative association was observed between Δ FATT and Δ FFAR4, indicating that as FFAR4 expression increased, FATT also decreased concurrently (i.e. sensitivity to FAT increased). Conversely, the positive association between Δ FATT and Δ GPR84 indicates that as GPR84 expression increased, FATT also increased (i.e. sensitivity to FAT decreased). There were no significant associations between Δ FATT and Δ CD36, Δ FFAR2 or Δ KCNA2.
Table 4. Associations between Δ fatty acid taste threshold (FATT) and Δ fatty acid taste receptor gene expressions*
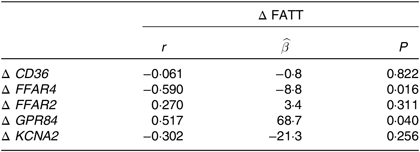
Δ, Week 8 − baseline; CD36, cluster of differentiation 36; FFAR4, free fatty acid receptor 4; FFAR2, free fatty acid receptor 2; GPR84, G protein-coupled receptor 84; KCNA2, delayed rectifying K+ channel (K+ voltage-gated channel subfamily A member 2).
* ![]() and P values obtained under a mixed model including twin pair as a random effect and time as a repeated effect; r obtained using Pearson’s correlation for descriptive purposes only.
and P values obtained under a mixed model including twin pair as a random effect and time as a repeated effect; r obtained using Pearson’s correlation for descriptive purposes only.
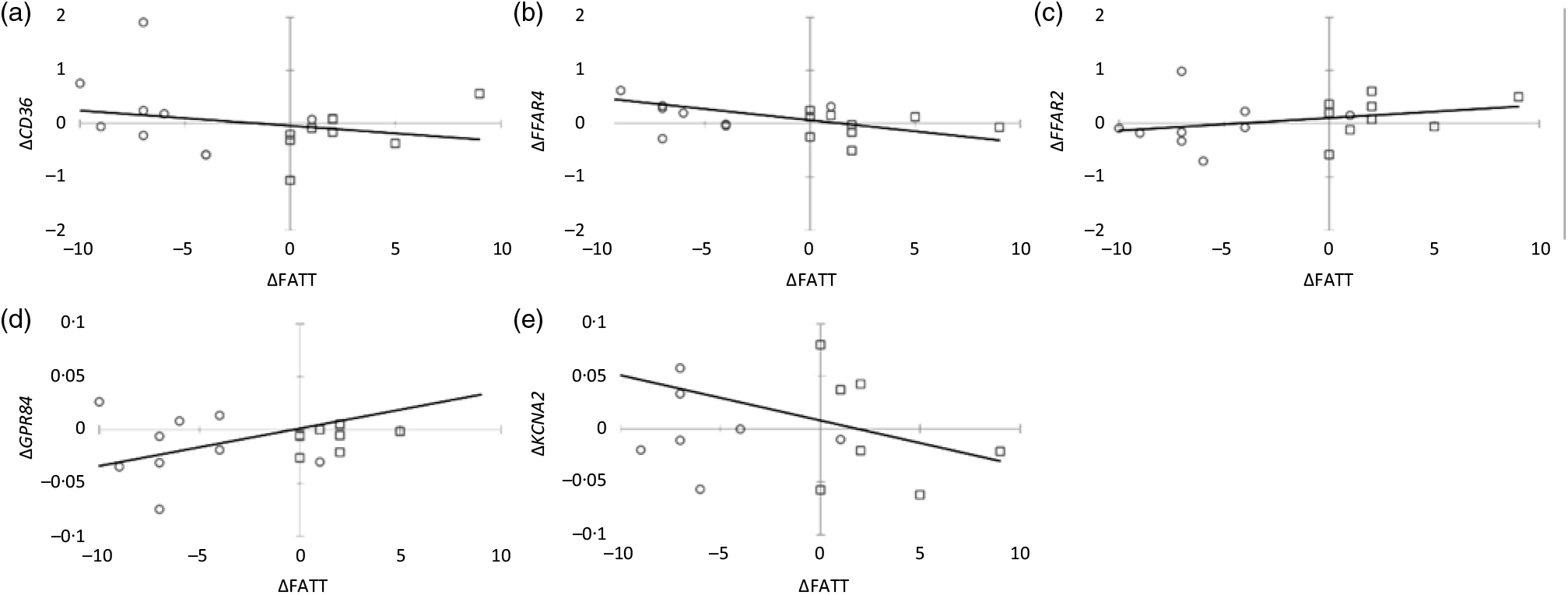
Fig. 2. Scatterplots of Δ fatty acid taste threshold (FATT) and Δ fatty acid taste receptor gene expressions. Circle markers (○) indicate participants on the low-fat diet (n 9) and square markers (□) indicate participants in the high-fat group (n 9). (a) Δ FATT v. Δ cluster of differentiation 36 (CD36); (b) Δ FATT v. Δ free fatty acid receptor 4 (FFAR4); (c) Δ FATT v. Δ free fatty acid receptor 2 (FFAR2); (d) Δ FATT v. Δ G protein-coupled receptor 84 (GPR84); (e) Δ FATT v. Δ K+ voltage-gated channel subfamily A member 2 (KCNA2).
Associations between dietary macronutrient intake and fatty acid taste receptor gene expressions
Significant negative associations were observed between
Δ FFAR4 and Δ fat intake (g) (![]() = −159·4; r −0·744; P < 0·001), Δ saturated fat intake (g) (
= −159·4; r −0·744; P < 0·001), Δ saturated fat intake (g) (![]() = −79·4; r −0·759; P < 0·001), Δ monounsaturated fat intake (g) (
= −79·4; r −0·759; P < 0·001), Δ monounsaturated fat intake (g) (![]() = −53·4; r −0·711; P = 0·001) and Δ polyunsaturated fat intake (g) (
= −53·4; r −0·711; P = 0·001) and Δ polyunsaturated fat intake (g) (![]() = −14·8; r −0·533; P = 0·023). There was a statistical trend for a negative association between Δ KCNA2 and Δ polyunsaturated fat intake (g) (
= −14·8; r −0·533; P = 0·023). There was a statistical trend for a negative association between Δ KCNA2 and Δ polyunsaturated fat intake (g) (![]() = −72·7; r −0·459; P = 0·056). Finally, there was a significant positive association between Δ FFAR2 and Δ dietary fibre intake (g) (
= −72·7; r −0·459; P = 0·056). Finally, there was a significant positive association between Δ FFAR2 and Δ dietary fibre intake (g) (![]() = 22·5; r 0·560; P = 0·016). No significant associations were observed for Δ CD36 or Δ GPR84, though a significant association was observed between baseline GPR84 expression and intake of energy from dietary fibre (%) (
= 22·5; r 0·560; P = 0·016). No significant associations were observed for Δ CD36 or Δ GPR84, though a significant association was observed between baseline GPR84 expression and intake of energy from dietary fibre (%) (![]() = 8·8; r 0·399; P = 0·023).
= 8·8; r 0·399; P = 0·023).
Discussion
The present study assessed changes in fasting expression of FAT receptor genes following 8 weeks of LF or HF dietary intake, for the first time in humans. It is well established that FAT sensitivity is modulated by dietary fat intake(Reference Costanzo, Nowson and Orellana10–Reference Stewart and Keast12). It was hypothesised that expression of FAT receptors would be modulated due to dietary fat intake, similar to rodent models(Reference Zhang, Zhou and Ban3,Reference Martin, Passilly-Degrace and Gaillard17) . However, only FFAR4 was affected by the dietary intervention, with expression of FFAR4 increasing by 38 % in the LF diet group from baseline to week 8. While the magnitude of reduction in FFAR4 expression in the HF group (3 %) was much lower than the increase seen in the LF group, this is likely because in the HF group, fat (g) increased by only 34 %; whereas in the LF group, there was a 72 % reduction in fat consumed. Further, change in FFAR4 expression was associated with change in FATT, or in essence, as FFAR4 increased, FAT sensitivity also increased concurrently. This is supported by the association between FFAR4 and intake of fat, saturated fat, monounsaturated fat and polyunsaturated fat, indicating that dietary fat with any level of FA saturation down-regulates FFAR4. These results indicate that FFAR4 expressed in fungiform papillae may be responsible for long-term changes in FAT perception. To that point, increasing expression of FFAR4 might lead to an increased secretion of intestinal peptides following chemoreception of FA in the oral cavity or GIT(Reference Stewart, Seimon and Otto8), reducing subsequent desire to eat and therefore energy intake. Also, since systemic FFAR4 is involved in the facilitation of energy homoeostasis(Reference Ichimura, Hirasawa and Poulain-Godefroy23), brown fat activation(Reference Quesada-Lwpez, Cereijo and Turatsinze24) and inflammation(Reference Talukdar, Bae and Imamura25), the ability to up-regulate FFAR4 via dietary intervention may have the potential to manage obesity and metabolic disease.
Interestingly, there was no effect of diet on CD36 in fungiform papillae in the present study, which contradicts the finding in our previous research(Reference Liu, Costanzo and Evans13). In the present study, there was a small trend in the same direction as FFAR4, in that CD36 increased by 16 % in the LF diet and decreased by 13 % in the LF diet after 8 weeks; however, no significant time–diet interaction was observed. This is likely because the baseline dietary data were based on a single 24-h recall, whereas the intervention dietary data were based on six diet records over the course of 8 weeks. If participants consumed an HF meal on the night before testing, there might have been some residual effect on gene expression. Short-term oral exposure to dietary fat in mice decreased CD36 levels by 2-fold within 1 h of re-feeding(Reference Martin, Passilly-Degrace and Gaillard17). However, 11 h of fasting returned CD36 levels to preprandial levels. Therefore, it is possible that TBC up- and down-regulate CD36 relatively quickly following acute oral exposure to fat compared with FFAR4. We speculate that the role of CD36 may be to mediate short-term response to dietary fat and is down-regulated throughout an eating event, then up-regulated to premeal expression following a prolonged fast; whereas FFAR4 is involved in regulating long-term response to dietary fat between meals with greater gradual change in expression over longer periods of time. Analysis of CD36 in human papillae immediately before and after consumption of dietary fat is necessary to confirm this. Some studies have also shown association between CD36 and hedonic preference for fatty foods in humans(Reference Liu, Costanzo and Evans13) and rodents(Reference Camandola and Mattson26–Reference Braymer, Zachary and Schreiber29), which strengthens the argument that the role of CD36 is more specific to mediating fat intake within a given meal. There is also evidence of interaction between FFAR4 and CD36 in TBC(Reference Abdoul-Azize, Selvakumar and Sadou30), though the significance of this interaction remains unclear.
While the dietary intervention was not designed to control for polyunsaturated fat intake as participants were able to choose to consume any food sources of fat, intake of polyunsaturated fat increased on the LF diet and decreased on the HF diet. Despite this, time–diet interaction for KCNA2, which is the receptor gene specific to PUFA chemoreception, was not significant. An 18 % increase in KCNA2 was observed in the LF diet group, which was in the hypothesised direction, and no change in KCNA2 in the HF diet group was likely due to the relatively minor increase in fat intake over 8 weeks. There was a statistical trend for a negative association between Δ KCNA2 and Δ polyunsaturated fat intake (P = 0·056), which indicates some effect of polyunsaturated fat intake on KCNA2. Therefore, it could be suggested that KCNA2 might be involved in the regulation of PUFA intake, where an individual who is not meeting the PUFA intake requirement might express greater KCNA2 levels on TBC to promote greater subsequent PUFA intake. However, a dietary intervention that is well-controlled for PUFA intake is necessary to confirm this.
Another interesting outcome of the present study was that there was no effect of the diet on FFAR2, despite there being strong evidence for an association between FFAR2 and fat intake, particularly saturated fat, in the baseline data of the present study(Reference Liu, Costanzo and Evans13). The previous finding was unexpected as FFAR2 is mainly responsible for SCFA chemoreception(Reference Liu, Archer and Duesing14). Instead FFAR2 might not be regulated by fat intake but rather by total energy, as high saturated fat intake (g and %) is highly correlated with increased energy and reduced dietary fibre intake in this sample (unreported). Further to this, changes in FFAR2 was associated with changes to dietary fibre intake which is likely due to incidental changes to fibre intake as an artifact of the diets. This result is noteworthy, as dietary fibre intake leads to production of SCFA by biota in the GIT(Reference Kles and Chang31). Since regulation of receptors is presumably analogous throughout the alimentary canal(Reference Stewart, Seimon and Otto8), it is possible that increased exposure to SCFA in the GIT may cause increased FFAR2 in TBC. Similarly, although no time–diet interaction was found for GPR84 expression, an association was found between GPR84 and energy from dietary fibre (%). As GPR84 is mainly involved in the chemoreception of SCFA, this too may be due to increased exposure to SCFA in the GIT.
There was a strong time–diet interaction for FATT (P < 0·001), suggesting a physiological change to taste mechanisms was causing the change in FATT. While this is likely due to changes in FAT receptor gene expressions, namely FFAR4, there are many complexities to the taste system that should be discussed. First, small changes in gene expression may result in large physiological effects due to potent effects of a protein, differences in the amount of protein products and the half-life of the mRNA. To that point, we only measured gene expression in fungiform papillae as it was the least invasive tissue(Reference Archer, Liu and Shaw21), whereas there may have been larger changes in gene expression in foliate and circumvallate papillae(Reference Gilbertson, Liu and Kim32). Second, coordination and interaction of receptors may confound the results. For example, up-regulation of either FFAR4 or CD36 independently may have an insignificant effect on FATT; but when these up-regulated together, it resulted in a larger attenuation of FATT due to intracellular signal transduction(Reference Abdoul-Azize, Selvakumar and Sadou30). Finally, there may be salivary factors that were not measured in the present study that are additionally causal to the FATT change(Reference Mounayar, Septier and Chabanet33,Reference Poette, Mekoué and Neyraud34) .
This randomised controlled trial has some limitations that should be noted when interpreting these results. First, the analysis of FAT receptor gene expressions was only conducted in fungiform papillae, as collection of foliate and circumvallate papillae in living humans is fairly invasive. The relative sensitivities and expressions of these genes within and between human taste papillae are not clear(Reference Mattes35,Reference Costanzo36) , so the results from the present study should be interpreted only as indicative of the entire oral cavity. Second, the sample size was small and there was only one male twin pair in the sample, so there may be limited power to detect small changes in gene expression. Third, while we explored associations between nutrient intake and FAT receptor gene expressions, the present study was designed as an intervention to dietary fat intake. Changes in other nutrient intake were incidental, and therefore the observed association need to be confirmed in trials that are designed around those nutrients specifically. Lastly, due to the small sample of twin pairs, quantitative genetic effects could not be evaluated in the present study.
In summary, FFAR4 is the only FAT receptor on fungiform papillae associated with FAT perception in the fasted state, and both are mediated by diet concurrently. The role of FFAR4 appears to regulate long-term diet-mediated changes in FAT perception. Increasing the expression of FFAR4 in papillae, via diet or otherwise, could aid in reducing taste-mediated passive overconsumption of dietary fat. In addition, there is some evidence to suggest that CD36 is involved in regulating short-term within-meal responses to oral FA exposure, and KCNA2 may also have some role in regulating PUFA intake, though the future research should be conducted to confirm these.
Acknowledgements
The authors would like to acknowledge Dr Andrew Garnham for performing the papillae biopsies, Dr Dieuwerke Bolhuis for her assistance with the conception of the study, Kathryn Colla for her assistance with data collection and Saria McDonald and Katherine McDermott for their assistance with data entry.
Funding was provided by a National Health and Medical Research Council (NHMRC) grant (no. 104780) and the Centre for Advanced Sensory Science at Deakin University. This research was facilitated through access to TRA, a national resource supported by a Centre of Research Excellence Grant (ID: 1079102) from the NHMRC. The NHMRC and TRA had no role in the design, analysis or writing of this article.
C. N., K. D., N. A. and R. K. designed the research; A. C. and D. L. conducted the research; A. C., D. L. and S. B. analysed the data; A. C. and D. L. wrote the manuscript; and R. K. had primary responsibility for the final content.
There are no conflicts of interest.
Supplementary material
To view supplementary material for this article, please visit http://doi.org/10.1017/S0007114519002368


 = −159·4;
= −159·4; 






