Epidemiological studies have frequently shown higher vitamin C serum/plasma concentrations in women than in men( Reference Birlouez-Aragon, Delcourt and Tessier 1 – Reference Ravindran, Vashist and Gupta 10 ). These studies generally did not focus on the underlying reasons for the observed sex differences, were mostly conducted in young and middle-aged subjects and were often performed without consideration for confounding variables.
Vitamin C intake and other lifestyle factors, such as smoking behaviour, have been shown to influence vitamin C serum/plasma concentrations( Reference Galan, Viteri and Bertrais 4 , Reference Faure, Preziosi and Roussel 5 , Reference Schleicher, Carroll and Ford 9 – Reference Dietrich, Block and Norkus 12 ). Additionally, oxidative stress results in an increased consumption of antioxidants, including vitamin C( Reference Stephens, Khanolkar and Bain 13 ). Therefore, sex differences in those parameters could account for the observed sex differences in vitamin C status. In addition to those parameters, sex differences in the pharmacokinetics of vitamin C may exist, seeing that women reach the plasma ascorbate concentration plateau at lower vitamin C intake levels than men( 14 ). Furthermore, at equal vitamin C intake levels, women exhibit higher serum/plasma concentrations than men( Reference Vioque, Weinbrenner and Asensio 6 , Reference Cahill, Corey and El-Sohemy 8 , Reference Schleicher, Carroll and Ford 9 ).
In 1991, Blanchard( Reference Blanchard 15 ) postulated that the sex-related differences in the pharmacokinetics of vitamin C could be the result of sex differences in body composition. In addition, Sinha et al. ( Reference Sinha, Block and Taylor 16 ) showed that body weight functions as a negative predictor of vitamin C status in middle-aged men. Accordingly, an individual with high amounts of fat-free mass (FFM) can be assumed to have a larger distribution volume of hydrophilic vitamin C than a person with lower FFM.
The objectives of the present study were (1) to investigate whether sex differences in vitamin C plasma concentrations are also present in elderly subjects and (2) whether these differences are due to sex-specific lifestyles, total antioxidant status (TAOS) and/or body composition. We concentrated on the hypothesis that sex differences in vitamin C plasma concentrations might be explained by a higher distribution volume in men than in women, given that men show a higher FFM than women.
Subjects and methods
Study population
The subjects were participants in an ongoing cohort study in which the nutrition and health status of independently living senior citizens in Giessen, Germany, has been observed since 1994 at annual intervals and since 1998 at biannual intervals (GISELA study). The main objectives of the GISELA study are to investigate age-related changes in body composition, anthropometric parameters, energy expenditure, bone status, biochemical blood parameters including vitamins, and dietary intake by considering potential confounding variables. The investigations took place at the Institute of Nutritional Science in Giessen, Germany. Subjects were recruited through physicians, advertisements in local newspapers, notices, senior citizen meetings, and word of mouth from subjects who were already participating. Recruitment was continued until 2004, and a total of 584 participants were enrolled. Not all subjects participated in each follow-up. For enrolment, subjects had to be at least 60 years of age and physically mobile. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethical Committee of the Faculty of Medicine at the Justus-Liebig-University in Giessen. All subjects provided written informed consent. The present investigation was based on cross-sectional data collected in the follow-up in 2004, in which 350 subjects participated. Subjects with missing data on blood samples, body composition, food record, vitamin C supplement intake and lifestyle factors, including physical activity, alcohol consumption and smoking history, were excluded (n 79). Additionally, one subject was identified as an outlier regarding vitamin C status (178 μmol/l) and was therefore excluded. Hence, the following analysis included 181 women and eighty-nine men.
Anthropometric data and body composition
BMI was calculated as the measured body mass (in kg) divided by the square of the measured body height (in m)( Reference Jungert, Roth and Neuhäuser-Berthold 17 ). Subjects were classified according to their BMI into normal weight ( < 25·0 kg/m2), overweight (25·0–29·9 kg/m2) or obese ( ≥ 30·0 kg/m2). Waist circumference (WC) was determined in an upright position by a tape measure to the nearest 1·0 cm. Total body fat (TBF) mass and FFM were recorded with bioelectrical impedance analysis (Akern-RJL BIA 101/S; Data Input) according to the manufacturer's instructions and the predictive formula of Roubenoff et al. ( Reference Roubenoff, Baumgartner and Harris 18 ). The equation from Roubenoff et al. ( Reference Roubenoff, Baumgartner and Harris 18 ) was chosen because it was derived from a reference population that was comparable in age to the GISELA subjects using similar measurement conditions and because the equation has been validated against the dual-energy X-ray absorptiometry body composition measurements.
Laboratory measurements
Blood samples were collected after an overnight fast. Following immediate centrifugation, aliquots of heparin plasma, which were acidified with trichloroacetic acid for the determination of vitamin C, and aliquots of EDTA plasma for the determination of TAOS were stored at − 70°C until they were analysed after completion of the follow-up. Plasma TAOS, which is inversely related to oxidative stress, was assessed by photometer (Shimadzu UV-160A)( Reference Miller, Rice-Evans and Davies 19 ). Plasma concentrations of vitamin C were measured by photometer (Beckman Model 35 UV/VIS; Beckman Coulter, Inc.) with a between-days CV of 3·7 %( Reference Speitling, Hüppe, Kohlmeier, Kübler, Anders, Heeschen and Kohlmeier 20 ). Vitamin C deficiency was defined as plasma concentrations of < 11·4 μmol/l, and plasma concentrations of < 28 μmol/l indicated a moderate risk of developing a deficiency( Reference Schleicher, Carroll and Ford 9 ). Plasma concentrations of ≥ 50 μmol/l indicated an adequate status( 14 ).
Lifestyle factors
A validated 3 d estimated dietary record including 146 food items was used to assess the dietary intake of vitamin C and alcohol( Reference Lührmann, Herbert and Gaster 21 ). The dietary record was handed out to the participants at the date of blood sampling with a request to record their entire food consumption on three consecutive days, starting on a Sunday, directly after each food intake and a request to return the dietary record at their earliest convenience. The majority of dietary records were returned within 3 months. Using self-administered questionnaires, data on age, use of vitamin C supplements, smoking behaviour and physical activity patterns were collected. Subjects who reported using vitamin C supplements sometimes or regularly were classified as ‘supplement users’. Smoking behaviour was classified as a dichotomous variable: constant non-smokers v. current and ex-smokers. The physical activity level (PAL) of each participant was assessed as described earlier( Reference Krems, Lührmann and Neuhäuser-Berthold 22 ).
Statistical analyses
Statistical analyses were performed using SPSS® version 22.0 for Windows (IBM®). Because of non-normally distributed data, the characteristics of the study population are expressed as medians and 25th–75th percentiles. Descriptive characteristics were compared between groups via the Mann–Whitney U test or Kruskal–Wallis H test for continuous variables and via the χ2 test for categorical variables.
Factors that potentially influenced vitamin C plasma concentrations were identified by the Spearman correlations. Subsequently, those factors that exhibited a significant association with vitamin C plasma concentrations were included in the backward stepwise multiple linear regression analysis to ascertain the independent predictors of vitamin C plasma concentrations. A P value of < 0·100 was set as the significance criterion for covariates to remain in the regression model. Considering the observed collinearity of body mass, BMI, WC, TBF and FFM (data not shown), we used separate models. Adjustments for multiple testing were not performed due to the exploratory character of the present study. The significance level was set at P< 0·05, and all tests were two-tailed.
Results
Characteristics of the study subjects
The characteristics of the study subjects are shown in Table 1. All except two subjects had vitamin C plasma concentrations of >28 μmol/l, but 8 % of the women (n 15) and 18 % of the men (n 16) showed plasma concentrations of < 50 μmol/l. Women had significantly higher vitamin C plasma concentrations and a lower prevalence of a vitamin C status of < 50 μmol/l (P= 0·019) than men. Median vitamin C intake was slightly under the recommendation of 100 mg/d( 23 ) in both women and men, and 56 % of the women (n 101) and 64 % of the men (n 57) had an intake of less than 100 mg/d (P= 0·196).
Table 1 Characteristics of the study population (Median values, 25th (P25) and 75th (P75) percentiles for continuous variables; absolute and relative frequencies for categorical variables)

* Mann–Whitney U test for continuous variables, χ2 test for categorical variables to investigate differences between women and men.
† To convert μmol/l to mg/dl, multiply μmol/l by 0·0176.
‡ Median dosage of vitamin C supplements was 120 mg/d in women and 55 mg/d in men, respectively, based on vitamin C supplement users only.
Overweight and obesity were noted in 47 and 22 % of the women and in 51 and 17 % of the men, respectively. Vitamin C plasma concentrations, but not vitamin C intake levels, tended to be higher in normal-weight and overweight subjects compared with obese subjects (median 74 v. 71 v. 62 μmol/l; P= 0·088). When using tertiles of absolute TBF ( ≤ 23·74 v. 23·75–31·82 v. ≥ 31·83 kg), neither vitamin C intake nor vitamin C plasma concentrations differed between tertiles (data not shown). In contrast, differences in vitamin C plasma concentrations were observed across tertiles of absolute FFM ( ≤ 39·41 v. 39·42–49·28 v. ≥ 49·29 kg), and vitamin C plasma concentrations were significantly lower in subjects in the highest FFM tertile compared with subjects in the middle or lowest FFM tertile (median 62 v. 73 v. 76 μmol/l; P< 0·001).
Past and current smokers had lower vitamin C intake (median 86 v. 93 mg/d; P= 0·049) and lower vitamin C plasma concentrations (median 66 v. 74 μmol/l; P= 0·002) than never smokers. Furthermore, in past and current smokers (median 73 v. 62 μmol/l; P= 0·005) and in never smokers (median 77 v. 62 μmol/l; P= 0·015), vitamin C plasma concentrations, but not vitamin C intake, were higher in women than in men.
Determinants of vitamin C plasma concentrations in elderly subjects
The results of the Spearman correlation are given in Table 2. Vitamin C plasma concentrations were positively associated with age, PAL and the use of vitamin C supplements, and they were negatively correlated with male sex, past or current smoking, body mass, WC and absolute FFM. BMI, absolute TBF, relative FFM, alcohol consumption, dietary intake of vitamin C and TAOS exhibited no association with vitamin C plasma concentrations and therefore were not considered in the following multiple regression analyses.
Table 2 Spearman correlations to find variables associated with vitamin C plasma concentrations
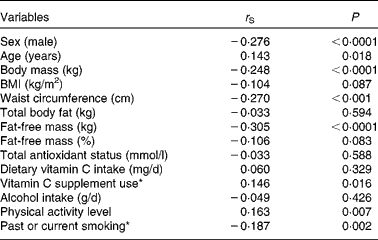
* Dummy variable (no/yes).
In the multiple regression analysis, male sex (β = − 0·214; P= 0·001) was a negative predictor of vitamin C plasma concentrations, whereas age (β = 0·181; P= 0·002), the use of vitamin C supplements (β = 0·153; P= 0·008) and PAL (β = 0·157; P= 0·007) had a positive impact on vitamin C plasma concentrations, provided that FFM was not considered in the analysis. Additionally, smoking was entered in the regression model but did not reach statistical significance (β = − 0·115; P= 0·064). When absolute FFM was included, FFM and past or current smoking were negative determinants, and age, vitamin C supplement use and PAL were positive determinants of plasma vitamin C concentrations, whereas sex was no longer an independent predictor of vitamin C plasma concentrations (Table 3). When FFM was replaced by body mass, BMI, WC or TBF, male sex remained negatively associated with vitamin C plasma concentrations (data not shown). Each regression model explained approximately 14 % of the variance in vitamin C plasma concentrations. We repeated these analyses by excluding subjects who used anti-diabetics (n 15) or thyroid hormones (n 81), because they differed in their vitamin C plasma concentrations from subjects who did not use such drugs (data not shown). As a result, absolute FFM (β = − 0·346; P< 0·0001), age (β = 0·187; P= 0·007), use of vitamin C supplements (β = 0·220; P= 0·001) and PAL (β = 0·127; P= 0·064), but not sex, were predictors of vitamin C plasma concentrations (R 2 0·189). Smoking and PAL were no longer significant independent predictors, possibly because the sample size was markedly reduced (n 178). When FFM was replaced by body mass, BMI, WC or TBF, male sex remained a negative predictor of vitamin C plasma concentrations (data not shown).
Table 3 Multiple regression analysis to identify independent determinants of vitamin C plasma concentrations in the elderly (n 270)*
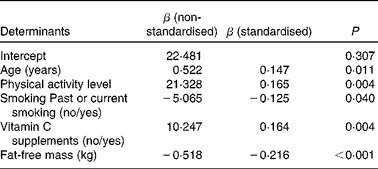
* Multiple linear regression analysis using backward stepwise procedure and vitamin C plasma concentrations in μmol/l as the dependent variable. Only variables of the final model are presented. Variables that were entered into the stepwise regression analysis but did not remain in the final model included sex (female/male). The adjusted coefficient of determination (R 2) for the final model was 0·143.
Discussion
The results of the present study indicate that lower vitamin C plasma concentrations in men compared with women are predominantly explained by higher FFM in men. A special feature of the present approach was the consideration of a variety of potential confounders, such as age, anthropometric variables, body composition, TAOS, vitamin C intake, alcohol consumption, smoking and PAL.
The present study demonstrates that male sex was only a predictor of vitamin C plasma concentrations when FFM was not considered. The inclusion of TBF or anthropometric parameters in the regression model did not influence the association between sex and vitamin C plasma concentrations.
Previous studies considered only anthropometric parameters or TBF, but not FFM, as potential determinants of vitamin C status. In several studies that were conducted in young and middle-aged subjects, a negative association between BMI and vitamin C serum/plasma concentrations was reported( Reference Galan, Viteri and Bertrais 4 , Reference Canoy, Wareham and Welch 24 – Reference García, Ronquillo and Caamaño Mdel 26 ). Furthermore, the relative body fat content( Reference Johnston, Beezhold and Mostow 25 , Reference García, Ronquillo and Caamaño Mdel 26 ) and anthropometric indices of fat distribution( Reference Canoy, Wareham and Welch 24 – Reference García, Ronquillo and Caamaño Mdel 26 ), such as WC and waist:ratio, were associated with vitamin C status in some, but not all, studies. However, these findings were often controlled for limited confounders( Reference Schleicher, Carroll and Ford 9 , Reference Johnston, Beezhold and Mostow 25 , Reference García, Ronquillo and Caamaño Mdel 26 ) or vanished after body mass was taken into account( Reference Johnston, Beezhold and Mostow 25 ). A study conducted in 545 Spanish subjects who were ≥ 65 years of age( Reference Vioque, Weinbrenner and Asensio 6 ) and a study with 979 Canadian subjects who were 20–29 years of age( Reference Cahill, Corey and El-Sohemy 8 ) could not verify an independent association between BMI categories and vitamin C serum/plasma concentrations. In the present study, TBF, BMI and WC were not predictors of vitamin C plasma concentrations, and vitamin C plasma concentrations did not differ among BMI groups or tertiles of absolute TBF. However, vitamin C plasma concentrations varied by tertiles of absolute FFM. These observations support the hypothesis that FFM, and not TBF, determines vitamin C plasma concentrations.
In addition to FFM playing a role in determining vitamin C plasma concentrations, we initially assumed that the frequently observed lower vitamin C status in men as compared to women might be attributed to a lower intake of vitamin C, lower TAOS, higher consumption of alcohol or higher proportion of smokers in male subjects. However, sex remained a predictor of vitamin C plasma concentrations after controlling for these factors. Consequently, these factors seem unlikely to be the major contributing factors for the observed impact of sex on vitamin C status in independently living elderly subjects. Nonetheless, in addition to absolute FFM, current or past smoking was negatively associated with vitamin C plasma concentrations, whereas age, PAL and the use of vitamin C supplements were positively associated with vitamin C plasma concentrations in the present study population.
In previous studies, smoking was associated with a lower vitamin C status in middle-aged( Reference Hampl, Taylor and Johnston 3 – Reference Faure, Preziosi and Roussel 5 , Reference Schleicher, Carroll and Ford 9 , Reference Dietrich, Block and Norkus 12 ) and elderly( Reference Hampl, Taylor and Johnston 3 , Reference Vioque, Weinbrenner and Asensio 6 , Reference Schleicher, Carroll and Ford 9 , Reference Ravindran, Vashist and Gupta 10 ) subjects, which is in accordance with the present results. The lower status in smokers may be a consequence of greater metabolic turnover of ascorbic acid due to an increased production of free radicals as a result of cigarette smoke rather than lower vitamin C intake( Reference Galan, Viteri and Bertrais 4 , Reference Alberg 11 ).
In agreement with the present results, in the Third National Health and Nutrition Examination Survey (NHANES III), Hampl et al. ( Reference Hampl, Taylor and Johnston 3 ) found a lower prevalence of vitamin C deficiency in subjects aged ≥ 65 years than in younger subjects. By contrast, in populations with a high prevalence of vitamin C deficiency, a negative relationship between age and vitamin C status has been reported( Reference Ravindran, Vashist and Gupta 10 ), whereas some studies of middle-aged( Reference Galan, Viteri and Bertrais 4 , Reference Faure, Preziosi and Roussel 5 ) and elderly subjects( Reference Vioque, Weinbrenner and Asensio 6 ) show no association. In the NHANES 2003–4, the mean vitamin C serum concentration declined during adolescence, then reached a plateau and increased with advancing age( Reference Schleicher, Carroll and Ford 9 ). Therefore, the authors suggested a non-linear relationship( Reference Schleicher, Carroll and Ford 9 ). However, previous studies frequently did not control for relevant confounders, such as FFM or vitamin C intake. One reason for our observation may be the age-related decline in FFM (r S − 0·116; P= 0·056) and concurrent increase in vitamin C intake (r S 0·214; P< 0·001) that was noticed in the GISELA subjects. However, age remained a positive predictor of vitamin C plasma concentrations after controlling for FFM and vitamin C intake in the present study. In addition to a decline in FFM, old age has also been associated with a decrease in FFM hydration( Reference Virgili, D'Amicis and Ferro-Luzzi 27 ). Thus, a decrease in fluid volume might further explain the increase in vitamin C plasma concentration that occurs with advancing age.
To the best of our knowledge, until now, the impact of PAL on vitamin C plasma concentrations in elderly subjects has not been investigated. In the present study, regular physical activity was associated with higher vitamin C plasma concentrations, even after applying multiple adjustments, including FFM. The reason for this finding is unclear. One may speculate that subjects who perform regular physical activity might have better health, better nutritional conditions and concomitant antioxidant status. The fact that TAOS showed no direct association with vitamin C plasma concentrations in the present study population could be ascribed to a limitation of the method, i.e. the exclusive measurement of the scavenging of the non-physiologic 2,2′-azino-bis-3-ethylbensthiazoline-6-sulfonic acid radical; thus, other reactive oxygen species are not considered by this approach( Reference Strube, Haenen and Van Den Berg 28 ).
In contrast to previous investigations( Reference Hampl, Taylor and Johnston 3 , Reference Galan, Viteri and Bertrais 4 , Reference Vioque, Weinbrenner and Asensio 6 , Reference Schleicher, Carroll and Ford 9 ), dietary vitamin C intake was not associated with vitamin C plasma concentrations in the present cohort. Vitamin C intake was also only moderately associated with vitamin C plasma concentrations in a meta-analysis of twenty-six epidemiological studies with subjects who were < 65 years of age( Reference Dehghan, Akhtar-Danesh and McMillan 29 ). Studies that have shown an association between vitamin C intake and serum/plasma concentrations were often performed with middle-aged subjects( Reference Galan, Viteri and Bertrais 4 ), subjects with lower vitamin C status( Reference Hampl, Taylor and Johnston 3 , Reference Vioque, Weinbrenner and Asensio 6 , Reference Schleicher, Carroll and Ford 9 ) and/or higher vitamin C intake levels( Reference Hampl, Taylor and Johnston 3 , Reference Vioque, Weinbrenner and Asensio 6 ) as compared to the GISELA subjects, or they analysed dichotomous variables( Reference Schleicher, Carroll and Ford 9 ). The association of vitamin C intake with vitamin C plasma and tissue concentrations follows a sigmoidal relationship( Reference Padayatty, Katz and Wang 30 ), i.e. the association appears to approximate an asymptote at plasma concentrations of >50 μmol/l( 14 ). In the present study, because the median vitamin C plasma concentrations were 76 and 62 μmol/l in women and men, respectively, the concentrations might have reached a plateau; thus, further increase may rely on a higher intake dosage. This could explain why the use of vitamin C supplements positively affected vitamin C plasma concentrations in the present subjects despite the relatively low proportion of supplement users as compared with other studies, in which 37–47 % of the subjects consumed supplements( Reference Schleicher, Carroll and Ford 9 ).
For the first time, the present study shows that sex differences in vitamin C plasma concentrations are related to differences in FFM. However, some limitations have to be considered. As with any cross-sectional study, causal inferences cannot be derived. Further limitations include the sample size, the imbalanced sex ratio and the use of self-reported data on dietary intake, smoking behaviour and physical activity. Likewise, dietary vitamin C intake was recorded after blood sampling was performed, which may have biased the association between vitamin C intake and vitamin C plasma concentrations. Furthermore, the number of current smokers was small in the present study (n 12), which may have attenuated the association between smoking and vitamin C plasma concentrations.
Even though we considered a variety of potentially confounding factors, we cannot exclude the possibility that other confounding factors may have influenced the results. The observation in the present study that only 14 % of the variability in vitamin C plasma concentrations were explained by FFM, age, the use of vitamin C supplements and PAL may be ascribed to the rather homogeneous study population with regard to these variables. It should also be mentioned that the GISELA subjects were volunteers, exhibited a higher level of education, consumed lower quantities of alcohol and were less often smokers than their peers in the general German population, and the generalisability of the present findings should therefore be confirmed in other populations.
Future studies should elucidate whether the relationship between FFM and vitamin C plasma concentrations depends only on distribution volume or possibly also on metabolic aspects related to FFM. Moreover, the question arises of whether the differences in plasma concentrations that are caused by differences in body composition are also observed in other indicators of vitamin C status, such as vitamin C concentrations in leucocytes or functional markers.
In conclusion, the present investigation provides an explanation for the frequently noticed sex differences in vitamin C status. The higher absolute FFM and the consequential higher distribution volume in men contribute to lower vitamin C plasma concentrations in men as compared with women. Therefore, absolute FFM should be considered in the interpretation of plasma vitamin C concentrations when vitamin C status and the impact of vitamin C on health status are analysed. Whether lower vitamin C plasma concentrations in men are associated with health consequences warrants further investigation.
Acknowledgements
The authors thank all participants in the GISELA study as well as the staff of the Department of Human Nutrition who helped in the data acquisition.
The present investigation received no specific grant from any funding agency in the public, commercial or non-profit sectors.
The authors' responsibilities are as follows: A. J. performed the statistical analysis, interpreted the data and wrote the manuscript. M. N.-B. formulated the research question, designed the study, conducted the research, proofread the manuscript and had primary responsibility for final content. All authors read and approved the final manuscript.
None of the authors had a conflict of interest.






