Patients with severe chronic intestinal failure (CIF) due to benign aetiologies represent a group of severely handicapped people who, in addition to bowel transplantation, often require long-term or even lifelong parenteral nutrition (PN) replacement as the only life-saving solution(Reference Pironi, Boyekens and Bozzetti1–Reference Kubal, Mangus and Tector3).
A significant proportion of patients who require long-term PN are patients with Crohn’s disease or its complications, such as surgical removal of a large part of the small intestine, which results in short bowel syndrome (SBS). The term SBS is reserved for a clinical condition in which the residual length of the small intestine is less than 200 cm. In such cases, macronutrients (glucose, amino acids and fats), water, electrolytes and micronutrients must be substituted parenterally. The introduction and ongoing optimisation of home parenteral nutrition (HPN) is a successful step toward realisation of the needs of daily intravenous administration of nutrients without the need for hospitalisation(Reference Bielawska and Allard4–Reference Jckson and Buchman7).
Despite the unequivocal benefits in terms of patient survival, long-term HPN carries certain risks, such as catheter-associated infections, central venous catheter complications, electrolyte imbalance and PN-associated liver disease(Reference Jorda, Popovič and Rotovnik Kozjek8). Significant limitations in basic needs in the physical, psychological and social dimensions, such as restricted work and school attendance, decreased mobility and poor sleep significantly affect the quality of life (QoL) of these patients. The number of days a week and the number of hours during the day that a patient must be attached to the infusion plays an important role in the subjective evaluation of QoL(Reference Carlson, Bosaleus and Nordgren9–Reference Baxter, Fayers and Bozzetti11).
Therefore, the optimal weekly and daily infusion regimens need to be sensitively planned in terms of both safe and effective intravenous administration and satisfactory QoL of the patient. When a mixture of amino acids is administered intravenously, an important parameter is their utilisation, which is the transition from blood plasma to cells where they serve to restore proteins and a source of energy(Reference Iacone, Scansano and Santarpia12). Urinary loss of amino acids should also be considered in the event of a surge in plasma concentrations during infusion. Urinary losses of amino acids during their intravenous administration are mainly determined by the infusion rate (g/kg per h). If the daily requirement of amino acids is high, the infusion duration should be extended to avoid exceeding the resorption capacity in the renal tubules(Reference Macrides, Camargo and Verrey13,Reference Tietze, Sorensen and Eiskjaer14) . This issue is not being actively discussed regarding patients on long-term HPN.
The primary aim of our study was to determine the profile of essential amino acids (EAA) and non-essential amino acids (NEAA) in the serum of patients with chronic Crohn’s disease leading to CIF due to SBS who were on long-term HPN. The secondary aim was to determine the daily loss of individual amino acids in the urine during and after 12 h of infusion and compare the plasma and urinary levels of amino acids with those in a control group of healthy persons.
Materials and methods
Patients
This was a prospective observational study. A total of forty-five patients (twenty-five men and twenty women) who had been on long-term HPN for 6–75 (median 33) months due to CIF with SBS secondary to extensive small bowel resection for complications of Crohn’s disease with a residual bowel length of 80–120 cm were enrolled in this study. According to the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines (2016), they were categorised as CIF type III in the functional classification and type D3 in the clinical classification, which meant that they required intravenous supplementation of > 84 kJ/kg body weight per d and a volume of 2001–3000 ml/d(Reference Pironi, Arends and Bozzetti15). The average dose of amino acids, carbohydrates and fats administered was 1·5, 3·4 and 0·68 g/kg per d, respectively, at an infusion rate of 0·11, 0·28 and 0·06 g/kg per h, respectively. The baseline demographic, clinical and nutritional data are summarised in Table 1. The control group included thirty-five healthy volunteers (twenty men and fifteen women) of 25–58 years of age with BMI in the range of 21–25·5 kg/m2 and glomerular filtration rate mean (sd) of 84·6 (26·4) ml/min per 1·73 m2.
Table 1. Demographic, clinical and intravenous nutritional data (characteristics) of patients on home parenteral nutrition are presented as median (interquartile range) (Number and percentages)

HPN, home parenteral nutrition; GFR, glomerular filtration rate.
The following were the inclusion criteria for the study: on HPN for at least 6 months, stable clinical condition, balanced metabolic nutrition state (prealbumin > 0·2 g/l) without an acute exacerbation of the underlying disease (Crohn’s disease) or other acute illnesses (serum C-reactive protein < 5 mg/l). The exclusion criteria included uncooperative patient and acute exacerbations of the underlying disease or any other acute illnesses. Two-compartment all-in-one bags with individualised formulations of amino acids, glucose, fat emulsions and minerals that were prepared in a hospital pharmacy were used in our patients. EAA accounted for an average of 45 % of the total amino acids in the nutritional mixture.
Mode of infusion administration
Patients were administered PN at home daily in a cyclical regimen for 12 h overnight. This 12-h framework is the most commonly used in our patients with HPN to administer an average daily dose of amino acids of 1·5 g/kg perd at an infusion rate of 0·10–0·13 g/kg per h.
Central venous access via the right internal jugular vein or right subclavian vein was provided using a tunneled Broviac catheter. For intravenous administration, the portable pump Mini Rythmic PN + (B. Braun Medical) was used in 15 (30 %) patients and the administration set with flow regulator for gravity infusions Exadrop (B. Braun) was used in 30 (60 %) patients.
Sample collection
Coagulable blood was collected for serum amino acid profiling from the ulnar vein into a Sarstedt Serum-gel collection vessel 5 h after the end of the infusion of amino acids to avoid affecting the amino acid values by their current intravenous supply. The blood was then centrifuged at 5000 g for 10 min. The serum was transferred to an aliquot tube and sent to the laboratory for further processing. Due to the impossibility of determining or estimating the individually absorbed orally administered proteins or amino acids in patients with SBS, the oral support feeding was protein-free on the day of urine collection. Urine was collected over two 12 h intervals—during the infusion and after the infusion. For each urine sample, the volume was given for the possibility of calculating the waste of individual amino acids. The urinary levels of excreted amino acids during and after the infusion were compared with the 12 h amino acid output in the control group. Blood collection in the control group was performed in the morning at 8 O’clock after an overnight fast, that is, at the beginning of the 12-hour urine collection. The control group received the usual mixed diet during the day without any dietary restrictions.
Sample preparation
Blood serum samples were treated with deproteination using 10 % sulfosalicylic acid solution and an internal standard methionine sulfone. The supernatant obtained after centrifugation of the mixture at 10 000 g was acidified with lithium citrate buffer (pH 2·9), which ensures that all amino acids are in the form of a cation. Urine samples were acidified with lithium citrate buffer and methionine sulfone. The pre-prepared samples were then stored at −20°C until analysis.
Amino acid evaluation
Amino acids were analysed on an automatic amino acid analyzer Sykam S-433 (Sykam GmbH). Sykam S-433 is a computer controlled automatic liquid chromatograph that functions on the principle of ion exchange chromatography with post-column derivatisation and detection at two wavelengths. Monitoring and evaluation of the chromatogram were performed using Clarity chromatographic software (Clarity software, Data Apex).
Reagents
The following buffers and reagents used for analysis on Sykam S-433 were procured from Sykam GmbH: lithium citrate buffer 0·12 N (pH, 2·90), lithium citrate buffer 0·3 N (pH, 4·20), lithium citrate buffer 0·3 N (pH, 8·0), regeneration solution 0·45 lithium hydroxide and ninhydrin reagent solution. Sigma A-9906 amino acid standard was used to calibrate the analyzer; additionally, the internal standard (methionine sulfone) was obtained from Sigma-Aldrich. Sulfosalicylic acid solution with the addition of an internal standard and urine sample treatment solution was prepared directly in the laboratory.
Validation parameters
Repeatability of retention time was ±1·5 min (monitored for alanine chromatographic peak). Reproducibility of the area of chromatographic peak was CV 3·2 % (monitored for internal standard chromatographic peak, n 50).
Statistical analysis
Data are presented as mean (standard deviation, sd) or median (interquartile range, IQR) as appropriate. Student’s t test was used for parametric data and the Mann–Whitney U test was used for non-parametric data. Significance was defined as P < 0·05. Statistical analysis was performed using MedCalc software Ltd., package version 6.0.
Statement of ethics
This study was performed in accordance with the ethical guidelines of the 1957 Declaration of Helsinki. The study was approved by the Ethical Committee of the University Hospital Brno (No. 15-270420/EK). All patients and participants provided informed consent for participation in the study.
Results
The levels of EAA and NEAA in patients on long-term HPN and their comparisons with those in healthy persons are summarised in Table 2. We did not observe a significant difference in the EAA values between the patients and controls. However, in NEAA, significantly lower levels of cystine and glutamine and significantly higher levels of glycine and ornithine were observed in patients on HPN. No significant differences were noted in the other NEAA. Table 3 summarises the amount of EAA and NEAA administered, their urinary excretion during 12 h of infusion and the remaining 12 h in comparison with the 12-h amino acid output in the controls. The first column in Table 3 shows the average amount of individual EAA and NEAA infused. The sum of these values at an average patient weight of 65 kg is 1·5 g/kg.
Table 2. Comparison of serum amino acids levels (umol/l) in patients on home parenteral nutrition (HPN) and a control group of healthy people
(Mean values and standard deviations)
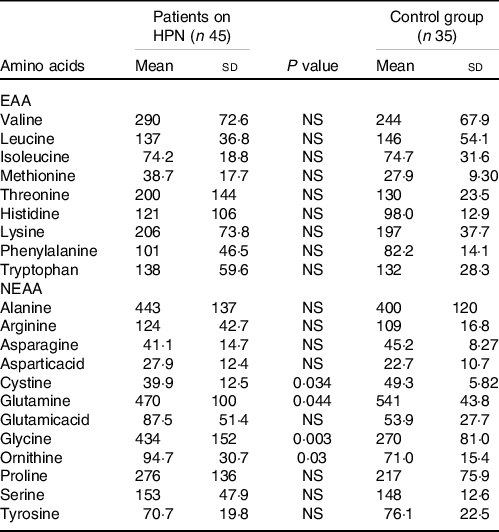
EAA, essential amino acids; NEAA, non-essential amino acids.
Table 3. Amounts of administered EAA and NEAA (g), their urinary excretion during 12 h of intravenous infusion and the remaining 12 h
(Median values and interquartile ranges)

HPN, home parenteral nutrition; AA, amino acids; EAA, essential amino acids; NEAA, non-essential amino acids; IQR, interquartile range.
* P value, significant difference in AA output during infusion v. AA output without infusion.
** P value, significant difference in AA output without infusion v. AA output in control group.
In all the administered amino acids, we recorded significantly higher values of excretion during intravenous administration. The excretion in patients when not on infusion did not differ significantly from those in the controls for most amino acids. For six amino acids (leucine, isoleucine, histidine, tryptophan, arginine and glutamine), we found significantly lower urinary levels in patients than those in the controls. Proline concentrations were below the limit of quantification, and lysine could not be identified due to ammonia peak overlap.
Summary data of the levels of amino acids excreted during and after the infusion are summarised in Table 4. During the infusion, 301 mg (0·34 %) of the administered amino acids was excreted in the urine.
Table 4. Total amino acid losses during and after the infusion in patients on home parenteral nutrition compared with the control group
(Median values and interquartile ranges)

AA, amino acids; IQR, interquartile range. Data are presented in mg as median (interquartile range).
Discussion
The aim of our study was to provide insight into the issue of amino acid administration within HPN in its current form. Historical works on amino acid profile in the serum and urine of patients on PN using protein hydrolysates reported urinary losses during infusion of 1–10 % (1–10 g)(Reference Eckhard and Davidson16–Reference Jacobson19). To our knowledge, there are no recent studies on this topic related to HPN in CIF. Further, the character and composition of amino acid mixtures; their content in all-in-one bags with glucose, fat emulsions, minerals, trace elements and vitamins; and the method of their intravenous administration using infusion pumps, often in a mobile version, have fundamentally changed.
With the increasing number of patients with HPN and the duration of its application, considerable attention has also been recently paid to its impact on the QoL of these patients. Important evaluation criteria of QoL include, in addition to the occurrence of septic complications and necessary rehospitalisations, the necessary number of infusion days per week and the number of hours of attachment to the infusion. The length of the HPN infusion is currently based on the maximum infusion rate requirement for glucose (0·42 g/kg per h) and the infused volume (2·9 ml/kg/h). The infusion rate for amino acids could be 0·12 g/kg per h(Reference Pironi, Boyekens and Bozzetti1,Reference Pironi, Arends and Bozzetti15,Reference Dreesen, Foulon and Vanhaecht20) .
We did not observe significant differences in the serum EAA concentrations in our patients in comparison with the controls. In the case of NEAA, levels of glutamine and cysteine were significantly reduced and those of glycine and ornithine were significantly increased in our patients. The lower levels of glutamine could be explained by its lack in our nutritional amino acid mixture as well as the low cystine content relative to other amino acids. Commercial mixed amino acid formulas do not contain appreciable cysteine levels because of its instability in solutions.
The urinary levels of EAA and NEAA in the 12 h during the infusion were significantly higher in the patients than those during 12 h without infusion. It should be noted, however, that the excretion of virtually all amino acids was in the order of milligrams, while they were administered in grams in the infusion. Only the excretion levels of histidine, glycine and serine reached values of 60·3, 63·8 and 22·4 mg (median), respectively. Urinary amino acid levels correspond to their fractional excretion in the renal tubules. While this value is < 1 % for most amino acids, it is 6·5 %, 2·4 % and 2·2 % for histidine, glycine and serine, respectively(Reference Macrides, Camargo and Verrey13,Reference Tietze, Sorensen and Eiskjaer14) . The lower urinary content of some amino acids during the infusion-free interval could be due to reduced glomerular filtration and increased tubular resorption in the absence of infused water volume.
The urinary excretion of amino acids was 301 mg during the infusion and 104 mg without infusion (P < 0·0001). The total waste of amino acids in the urine during the 12-h infusion accounted for only 0·34 % (0·23–0·46) of the administered dose. We found that urinary loss of only milligrams of amino acids is present at an infusion rate of 0·11 g/kg/h. Due to the required daily dose of amino acids, the duration of the possible infusion rate varies. In future, we would like to focus on finding a cut-off value for the infusion rate with respect to acceptable urinary amino acid loss and its minimum duration. We hope that our study will inspire further research in this topic.
The duration of the daily infusion in HPN, which is a significant factor influencing the quality of life of patients with HPN, is affected by the rate of infusion, that is, g/kg per h, in the case of amino acids as well as glucose. Until the critical value of the amino acid infusion rate, and the corresponding acceptable value of their utilisation and excretion in the urine, is experimentally verified, and the required amount of amino acids should be administered at a rate of 0·10–0·13 g/kg per h.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
This work is supported by Ministry of Health of the Czech Republic – DRO (FNBr, 65269705).
M. D. jr and M. Š. participated in the conceptualisation, methodology, investigation, data curation, reviewing and editing; AM participated in the methodology, investigation, data curation and statistic; MD participated in the conceptualisation, methodology, investigation, data curation, writing of the original draft, reviewing and editing.
The authors have no conflict of interest to declare.







