Preterm infants experience significant benefits to their gastrointestinal maturation, neurodevelopmental outcome, host defence systems and nutritional status when fed their mother's own milk rather than infant formula(Reference Schanler1). The proteins present in human milk contribute to many of these beneficial effects, in particular by providing preterm infants with immunological protection and growth factors to aid in development(Reference Lonnerdal2). Furthermore, clinical studies have found that the amount of total protein, the ratio of protein:energy and the amount of individual proteins such as lactoferrin (LF) delivered to preterm infants each have an effect on growth and development(Reference de Halleux, Close and Stalport3).
Due to the importance of human milk proteins for the growth and development of preterm infants, a number of studies have investigated the protein composition of preterm milk(Reference Atkinson and Jensen4). Generally, these studies have focused on the concentration of the major proteins, recording any variation between mothers or throughout the course of lactation. Although there has been some inconsistency in the literature, higher levels of total protein and elevated concentrations of individual protective proteins in preterm milk have been reported by several authors in the early postpartum period(Reference Atkinson and Jensen4–Reference Grosse, Geller and Tomarelli6). A much higher level of compositional variation has also been found amongst preterm mothers compared with term mothers, particularly in the first 2 weeks of lactation(Reference Grosse, Geller and Tomarelli6). It has been argued that these compositional differences between term and preterm milk arise due to the immaturity or unpreparedness of the mammary gland at the time of delivery, coupled with the presence of an altered hormonal milieu(Reference Atkinson and Jensen4).
Despite a number of studies examining the concentration of proteins in preterm milk, the post-translational modification (PTM) of these proteins has largely been ignored. This is surprising given the profound effect that PTM can exert on protein functionality. For example, both protein glycosylation and phosphorylation have been found to be functionally important in human milk. The two milk proteins lactadherin and mucin are heavily glycosylated, and it is this modification that confers their ability to protect the infant against rotavirus and Escherichia coli, respectively(Reference Schroten, Hanisch and Plogmann7, Reference Yolken, Peterson and Vonderfescht8). In addition, glycosylation has been shown to be protective against proteolytic degradation(Reference van Veen, Geerts and van Berkel9) and to modulate pathogen associations of milk proteins(Reference Barboza, Pinzon and Wickramasinghe10). The phosphorylation of αs1-casein and β-casein in human milk is also of biological importance, in that this modification is critical to the formation of casein micelles and the subsequent delivery of Ca2+ and PO43 − to the infant(Reference Qi11). In addition, casein phosphopeptides, formed by the enzymatic digestion of intact casein after ingestion by the infant, have been shown to possess important biological functions, enhancing the bioavailability of Ca and other minerals(Reference Biohallab and Bougle12).
It is important that the PTM of proteins in preterm milk be further characterised. Given that post-translational protein modifications have an effect on the stability, structure and function of many bioactive milk proteins, assessing whether PTM varies between women and over the course of lactation will yield important information regarding the functionality of human milk for preterm infants. Investigating differences between term and preterm milk may also provide insights into the effect of premature delivery on protein synthesis in the mammary gland.
In the present study, we investigated the expression and PTM of proteins from preterm milk (28–32 weeks gestation) and term milk (38–41 weeks gestation) during the first 2 months of lactation. Previous studies have observed a higher degree of compositional variability, both between individuals and over the course of lactation, in preterm milk compared with term milk(Reference Atkinson and Jensen4, Reference Bauer and Gerss5). For this reason, the longitudinal profiles of preterm milk were investigated in greater detail, with more frequent sampling and a larger number of mothers analysed. The expression and glycosylation status of nine proteins were investigated: tenascin, macrophage mannose receptor (MMR), xanthine oxidoreductase (XOR), bile salt-stimulated lipase (BSSL), LF, serum albumin (SA), secretory IgA (sIgA), β-casein and α-lactalbumin (ALA). The phosphorylation of β-casein across the lactation period was also examined.
Experimental methods
Chemicals
Unless otherwise stated, all chemicals and reagents were obtained from Sigma-Aldrich.
Sample collection
Sample collection was carried out at the King Edward Memorial Hospital, Subiaco, Western Australia, and was approved by The University of Western Australia Human Research Ethics Committee and the King Edward Memorial Hospital Ethics Committee. Written informed consent was obtained from all participating donors. Preterm milk samples were obtained from healthy mothers who delivered their infants between 28 and 32 weeks of gestation. On days 7, 10, 14, 18, 21, 28 and 60 postpartum, milk was expressed using a hospital-grade breast pump between 09.00 and 12.00 hours. The total expression volume was mixed together and a 2 ml aliquot collected. Term milk samples were obtained from healthy mothers who delivered their infants between 38 and 41 weeks of gestation. Samples were collected on days 7, 14 and 30 postpartum using hand expression at the conclusion of a morning breastfeed (between 09.00 and 12.00 hours). Participant details are summarised in Table 1. All mothers gave written informed consent to participate in the research, and had experienced their milk ‘coming in’ prior to the commencement of sample collection. All milk samples were frozen within 1 h of expression at − 20°C, and transferred to − 80°C within 7 d. Upon thawing each sample for analysis, a mammalian protease inhibitor cocktail containing 4-(2-aminoethyl)benzenesulfonyl fluoride, E-64, bestatin, leupeptin, aprotinin and sodium EDTA was added.
Table 1 Details of the study population (Medians and ranges)

Casein separation
Casein was collected from skimmed milk samples, as previously described(Reference Kunz and Lonnerdal13). Briefly, milk samples (500 μl) were removed from storage at − 80°C and placed in an ice bath prior to a pH adjustment to 4·3. After 1 h, the acidified milk samples were diluted in the ratio 1:1 with 120 mm-CaCl2 and vortexed gently for 20 min. Samples were then centrifuged at 40 000 g for 1 h at 4°C, and the fat, whey and casein collected separately. The casein pellets were washed in 1 ml water, centrifuged again and resuspended in 8 m-urea and 4 % 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS) (pH 8·5).
Protein determination
The concentration of true protein, excluding non-protein N, was determined as previously described(Reference Mitoulas, Kent and Cox14) using a modified Bradford method and a commercial protein reagent (Bio-Rad Laboratories), with human milk standards used to calibrate the measurements. The recovery of a known amount of protein added to the milk samples was 99 (sem 1·03) % (n 12), the interassay CV was 5·0 % and the detection limit was 0·015 g/l.
Lactose determination
The lactose content of milk was determined by an enzymatic spectroscopic method(Reference Mitoulas, Kent and Cox14). The recovery of a known amount of lactose added to the milk samples was 101 (se 0·9) % (n 12). The detection limit of this assay was 0·17 g/l (n 16) and the inter-assay CV was 3·5 % (n 60).
SDS-PAGE
The skimmed milk protein analysis and analysis of casein profiles were conducted using the HOEFER gel apparatus (HOEFER Scientific Instruments) with the Laemmli gel system(Reference Laemmli15). All samples were run in duplicate on 12·5 % polyacrylamide gels using a constant current of 9 mA for 16 h at 4°C. For the analysis of skimmed milk proteins, 20 μg of protein was loaded. Gels were fixed, stained using Coomassie Blue Silver and scanned as described previously(Reference Molinari, Casadio and Arthur16).
The glycoprotein analysis was conducted using the Bio-Rad Mini-Protean tetra cell apparatus (Bio-Rad Laboratories) and the Laemmli gel system. A measure of 3 μg of skimmed milk protein samples was loaded onto precast 4–20 % gradient polyacrylamide gels (TGX precast gels; Bio-Rad Laboratories). Gels were run at a constant voltage of 200 V for 40 min. Gels were stained for glycoproteins using a Pro-Q® Emerald Glycoprotein Gel and Blot Stain Kit (Invitrogen) according to the manufacturer's instructions. Glycoproteins were imaged using a ChemiDoc XRS System (Bio-Rad Laboratories). Gels were then stained using Coomassie Blue Silver, as described earlier, in order to observe the total protein content.
Urea-PAGE
One-dimensional urea-PAGE was conducted according to Kinoshita et al. (Reference Kinoshita, Kinoshita-Kikuta and Matsubara17) using the Bio-Rad Mini-Protean tetra cell. Briefly, gels consisted of a stacking gel (4·0 % polyacrylamide (w/v), 125 mm-Tris–HCl (pH 6·8) and 4·0 m-urea) and a separating gel (6·0 % polyacrylamide (w/v), 375 mm-Tris–HCl (pH 8·8) and 4·0 m-urea). The electrophoresis running buffer (pH 8·4) was 25 mm-Tris and 192 mm-glycine. Samples were mixed with a loading buffer containing 250 mm-Tris–HCl (pH 6·8), 4·0 m-urea, 5·0 % glycerol, 2·5 % dithiothreitol (w/v) and 0·1 % bromophenol blue (w/v).
Gels were run at 20 mA/gel. A measure of 10 μg of each casein fraction was loaded into individual wells. Each sample was analysed in duplicate. Gels were run at a constant current of 20 mA for 150 min, fixed, stained using Coomassie Blue Silver and scanned in the same manner described earlier for the skimmed milk samples.
As an experimental control, dephosphorylated casein was prepared by incubating a solution containing 5 mg casein, 50 mm-Tris–HCl (pH 9·0) and 1·0 mm-MgCl2 with 2 nmol/min (2 mU) of bovine intestinal mucosa alkaline phosphatase at 37°C. After 6 h, the reaction was stopped by adding an equal volume of urea-PAGE loading buffer.
Gel analysis
Gels were analysed using the open access software package ImageJ 1.410 (National Institutes of Health; http://imagej.nih.gov/ij/). All gels were run in duplicate, and all measurements of band intensity were also conducted in duplicate. All quantitative comparisons were made using background-subtracted integrals of either Coomassie or Pro-Q Emerald electrophoretic peaks. The average CV between repeat intensity measurements of the same protein band was 2·7 % (n 200). The within-gel loading variability, given by the average CV of the total lane intensities, was 6·4 % (n 40). The between-gel variability, given by the average CV of the relative intensities of duplicate samples on different gels, was 8·4 % (n 209). Different gel analysis protocols were used for the analysis of protein expression and glycoprotein expression, as described later.
Analysis of protein expression
The percentage quantity of each protein band was calculated by measuring the band intensity and normalising it against the total intensity of each lane. By multiplying this value by the total protein concentration, the relative concentration of each protein was calculated. No protein standards were used to provide absolute quantitation from the gel analysis, and thus all measures are relative only.
Glycoprotein analysis
The intensity of each band was measured and normalised against the total amount of protein present in each lane (Coomassie Blue Silver stain). The intensity of the glycoprotein molecular weight marker on each gel was used to correct for gel-to-gel differences in staining efficiency. In order to assess whether the glycan content varied relative to the amount of protein present, the normalised intensity of each glycoprotein was divided by the percentage quantity of the corresponding protein (calculated as given earlier for the Coomassie-stained protein bands) and expressed as a unitless ratio.
MS
Bands and spots of interest were cut from the gel, destained and digested in gel with trypsin (Roche Diagnostics), as described by Shevchenko et al. (Reference Shevchenko, Tomas and Havlis18). MS analysis was conducted as described in a previous study(Reference Molinari, Casadio and Arthur16).
MS/MS data were imported into the database search engine (Mascot, version 2.3.01; www.matrixscience.com). Mascot searches were conducted using the SwissProt Mammalia database (49 887 sequences) with the following settings: number of missed cleavages permitted = 1; no fixed modifications; variable modifications = methionine oxidation, cysteine carbamidomethylation; peptide tolerance = 1·2 Da, MS/MS tolerance = 0·6 Da; enzyme = trypsin; and the peptide charge = +1. A Mascot score greater than 50, with a minimum of two peptide matches, was considered to be a significant identification.
Statistical analysis
Statistical analysis was carried out using the statistics package R(19). The packages nlme(20) and multcomp(Reference Hothorn, Bretz and Westfall21) were used for linear mixed effects modelling and Tukey's contrast analysis, respectively.
Univariate analysis for variables collected at multiple time points was performed using a linear mixed models approach. This method is considered appropriate given the variation between different milk samples and the repeated measures in the data. All models included the individual milk samples as the grouping. ANOVA of the fitted model objects found that a grouping variable of the duplicate gels within the data from each milk sample did not improve the models. The mean concentrations of each milk component were compared, both within and between mothers, using general linear hypothesis tests.
Values are presented as means and standard deviations unless otherwise specified. P values < 0·05 were considered statistically significant and values smaller than 0·001 have been reported as P <0·001.
Results
Macronutrient concentration
The total protein concentration decreased across the first 2 months of lactation in both term and preterm mothers, with no difference observed between term and preterm milk on days 7, 14 and 30 (Fig. 1(a)). In term milk, the protein concentration was highest on day 7 at 17·9 (sd 2·2) g/l and decreased during lactation, with the protein concentrations on days 14 (P =0·041) and 30 (P <0·001) both significantly lower than the concentration on day 7. In preterm milk, the concentration was highest on day 7 at 19·3 (sd 5·5) g/l and remained elevated across the second week, falling only slightly to 17·5 (sd 4·5) g/l by day 14. The protein concentrations on days 14, 18, 21, 28 and 60 of lactation were all significantly lower than the concentration on day 7 (P =0·032, <0·001, <0·001, <0·001 and<0·001, respectively).

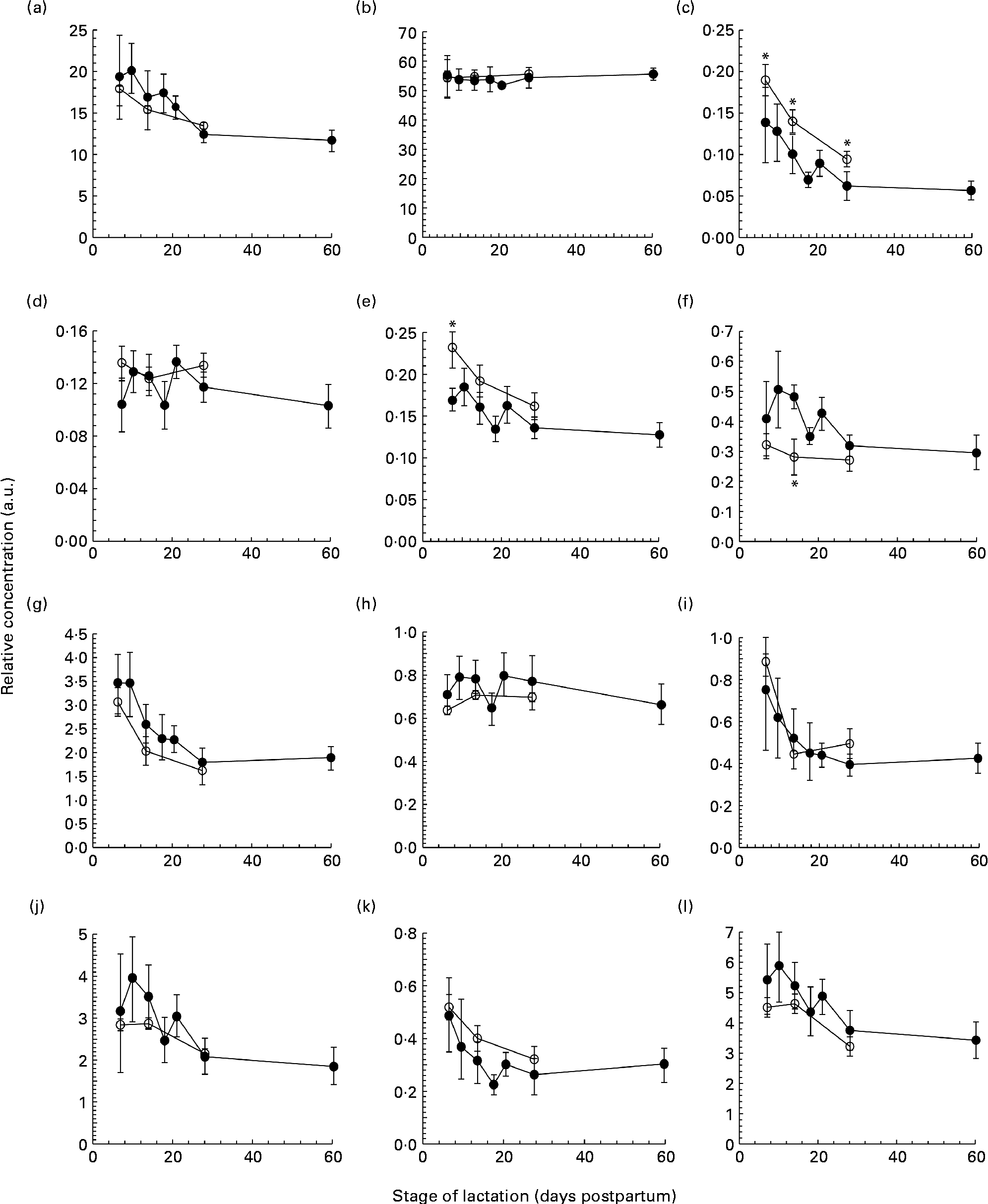
Fig. 1 The concentration of (a) protein (g/l), (b) lactose (g/l), (c) tenascin (arbitrary units (a.u.)), (d) macrophage mannose receptor (a.u.), (e) xanthine oxidoreductase (a.u.), (f) bile salt-stimulated lipase (a.u.), (g) lactoferrin (a.u.), (h) serum albumin (a.u.), (i) secretory Ig A (a.u.), (j) 28 kDa β-casein, (k) 16 kDa β-casein and (l) α-lactalbumin (a.u.) in preterm milk (●) and term milk (○) during 60 d of lactation, expressed in a.u. Values are means and standard deviations represented by vertical bars. * Time points with a significantly different concentration (P <0·05) in term and preterm milk.
The lactose concentration changed minimally across the first 2 months of lactation in the preterm milk samples (Fig. 1(b)). The lactose concentration on day 7 was 55·3 (sd 8·8) g/l. The concentration remained steady at this level, with concentrations of 54·5 (sd 2·9) g/l and 55·7 (sd 3·8) g/l at days 28 and 60, respectively. In term milk, the lactose concentration was constant at 55 (sd 7·0) g/l on day 7 and remained constant thereafter. No difference was found between the concentration of lactose in term and preterm milk.
Protein profiles
Each milk sample was analysed using SDS-PAGE in order to identify changes in the protein profile over the course of lactation in term and preterm milk. The identity of nine major proteins was determined using matrix-assisted laser desorption/ionisation time-of-flight-time-of-flight MS (MALDI-TOF-TOF MS) (Table 2). In term milk, the concentration of tenascin (Fig. 1(c)), XOR (Fig. 1(e)), LF (Fig. 1(g)), sIgA (Fig. 1(i)), β-casein (Fig. 1(j), (k)) and ALA (Fig. 1(l)) decreased during lactation, with the concentration on day 30 being significantly lower than that on day 7 (P <0·05 for all comparisons). No change in concentration over time was observed for MMR, SA and BSSL in term milk.
Table 2 Protein identifications

Similar longitudinal patterns were observed in preterm milk. The concentrations of tenascin (Fig. 1(c)), LF (Fig. 1(g)) and sIgA (Fig. 1(i)) were highest on day 7 and decreased over time, with the concentration on days 14, 18, 21, 28 and 60 all being significantly lower to that on both days 7 and 10 (all pairwise comparisons P <0·05). Similarly, the concentration of ALA also decreased significantly after day 7 in preterm milk (Fig. 1(l)), with the concentration of ALA on days 18, 21, 28 and 60 all being lower than that on day 7 (all pairwise comparisons P <0·05). The two β-casein bands (28 and 16 kDa) displayed slightly different profiles to each other, although both also decreased in concentration over time. For the 28 kDa β-casein band (Fig. 1(j)), the concentration on days 28 and 60 were both lower to the concentration on each of the days 7, 10, 14, 18 and 21. This was the same pattern of difference observed for BSSL (Fig. 1(f)). For the 16 kDa β-casein band (Fig. 1(k)), the concentration decreased more noticeably over time, with the concentration on each subsequent time point being lower compared with that on day 7 (all pairwise comparisons P <0·05). No significant difference was observed during the 60-d period in preterm milk for SA (Fig. 1(h)). Both MMR (Fig. 1(d)) and XOR (Fig. 1(e)) showed only a slight decrease in concentration over time, with the concentration on day 60 being lower than that on days 10, 14 and 21 (all pairwise comparisons P <0·05).
Comparing term and preterm milk, no difference was found for the concentration of MMR, LF, SA, sIgA, β-casein or ALA. However, differences were found between term and preterm milk for the concentration of tenascin, XOR and BSSL. The concentration of tenascin in term milk was higher on days 7, 14 and 30 (P <0·05) compared with preterm milk (Fig. 1(c)). XOR was present at a higher concentration in term milk on day 7 (P =0·02), but was similar on days 14 and 30 (Fig. 1(e)). BSSL had a similar concentration in term and preterm milk on days 7 and 30 of lactation, but was lower in term milk on day 14 (P <0·001) (Fig. 1(f)).
Glycosylation status of individual proteins
ProQ Emerald Stain 300 was used to detect glycoproteins on SDS-PAGE gels. A number of proteins, including tenascin, MMR, BSSL, LF, SA, sIgA and ALA, were identified as glycoproteins, exhibiting differing levels of glycosylation. The concentration of each of these glycoproteins varied over the first 60 d of lactation. However, when expressed relative to the concentration of the respective proteins, the glycan content of MMR (Fig. 2(b)), BSSL (Fig. 2(c)), LF (Fig. 2(d)), sIgA (Fig. 2(f)) and ALA (Fig. 2(g)) was found to be constant during the 60 d period in both term and preterm mothers. For tenascin (Fig. 2(a)) and SA (Fig. 2(e)), the extent of protein glycosylation was found to differ throughout lactation in term and preterm mothers.

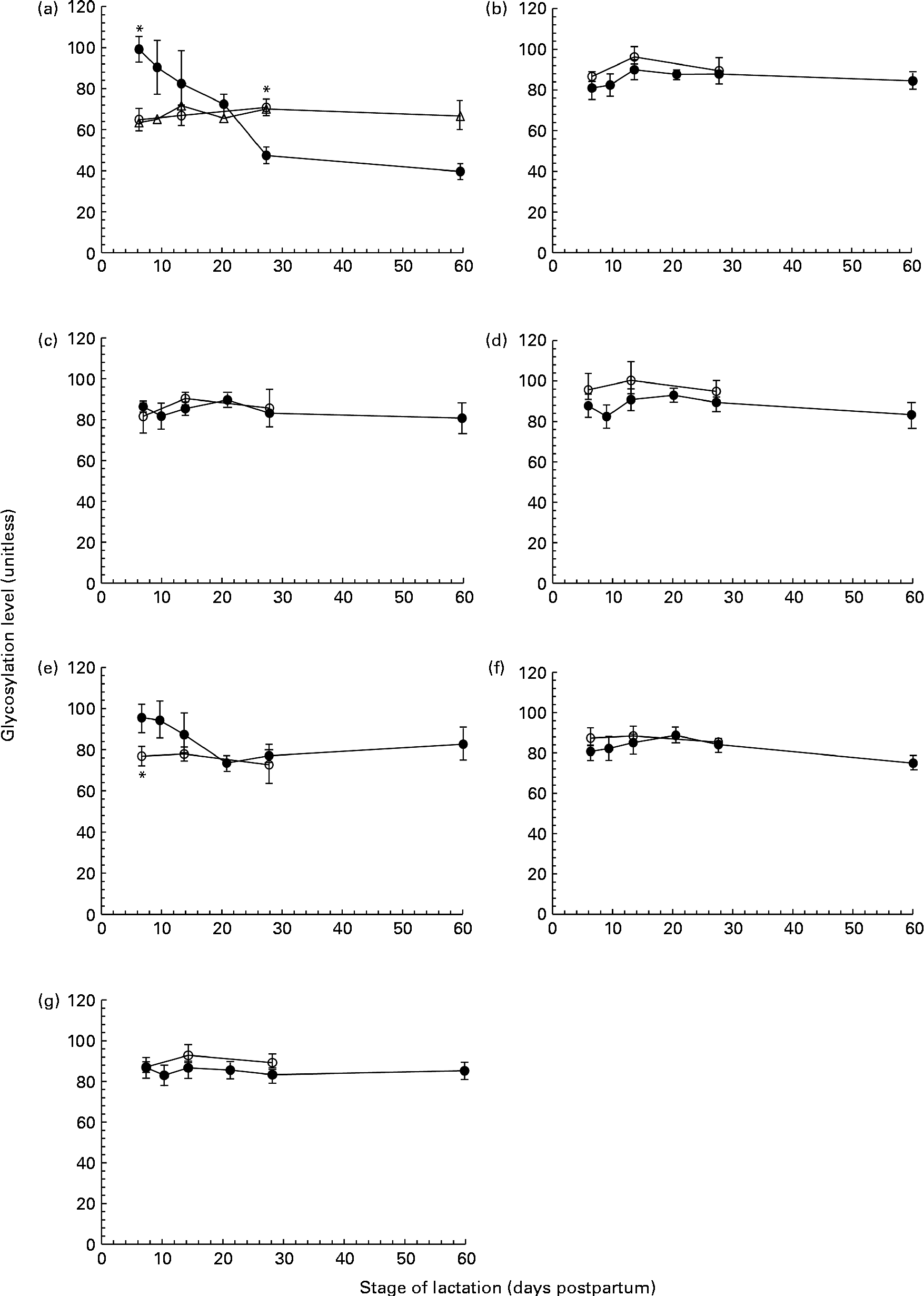
Fig. 2 The level of protein glycosylation during the first 60 d of lactation in milk from term (T, n 8) and preterm (PT, n 17) mothers. (a) Tenascin (○, n 8T; ●, n 7PT; Δ, n 10 PT), (b) macrophage mannose receptor (○, n 8 T; ●, n 17 PT), (c) bile-salt stimulated lipase (○, n 8 T; ●, n 17 PT), (d) lactoferrin (○, n 8 T; ●, n 17 PT), (e) serum albumin (○, n 8 T; ●, n 17 PT), (f) secretory Ig A (○, n 8 T; ●, n 17 PT) and (g) α-lactalbumin (○, n 8 T; ●, n 17 PT). Values (unitless) are expressed as the ratio of ProQ Emerald stain (glycosylation) to Coomassie Blue Silver stain (protein expression) for individual protein bands. Two distinct patterns were observed for the glycosylation of tenascin amongst the preterm mother cohort, and are thus shown separately in (a). For all other protein bands, all term and preterm mothers are grouped together. Values are means and standard deviations represented by vertical bars. * Significant differences (P <0·05) between term and preterm milk.
No change in the extent of tenascin glycosylation was observed during lactation in the term population, whereas in the preterm population, two distinct patterns were observed, with the level of glycosylation either remaining constant or decreasing over lactation for individual mothers. For the analysis of tenascin glycosylation, mothers were grouped according to these patterns (Fig. 2(a)). The level of glycosylation was constant during lactation for ten of the preterm mothers. For the remaining seven mothers, the level of glycosylation decreased markedly across the 60 d of lactation, with the ratio of carbohydrate:protein being higher at day 7 than at days 21, 28 and 60 (P =0·041, 0·004 and 0·002, respectively). For these seven mothers, the level of glycosylation was significantly different compared with the term population on both days 7 (P <0·001) and 28 (P =0·02). There was no significant difference between the two groups of preterm mothers for any of the other variables measured.
The glycosylation of SA (Fig. 2(e)) in preterm milk also displayed changes across the first 60 d of lactation. The ratios of carbohydrate:protein on days 21 and 28 were both lower than those on days 7 (P =0·006, 0·011) and 10 (P =0·007, 0·014), respectively. In term milk, no change in the level of glycosylation was observed during lactation. A significant difference was found between the glycan content of SA in term and preterm milk on day 7 (P =0·03), with a higher degree of glycosylation observed in the preterm milk.
Casein phosphorylation
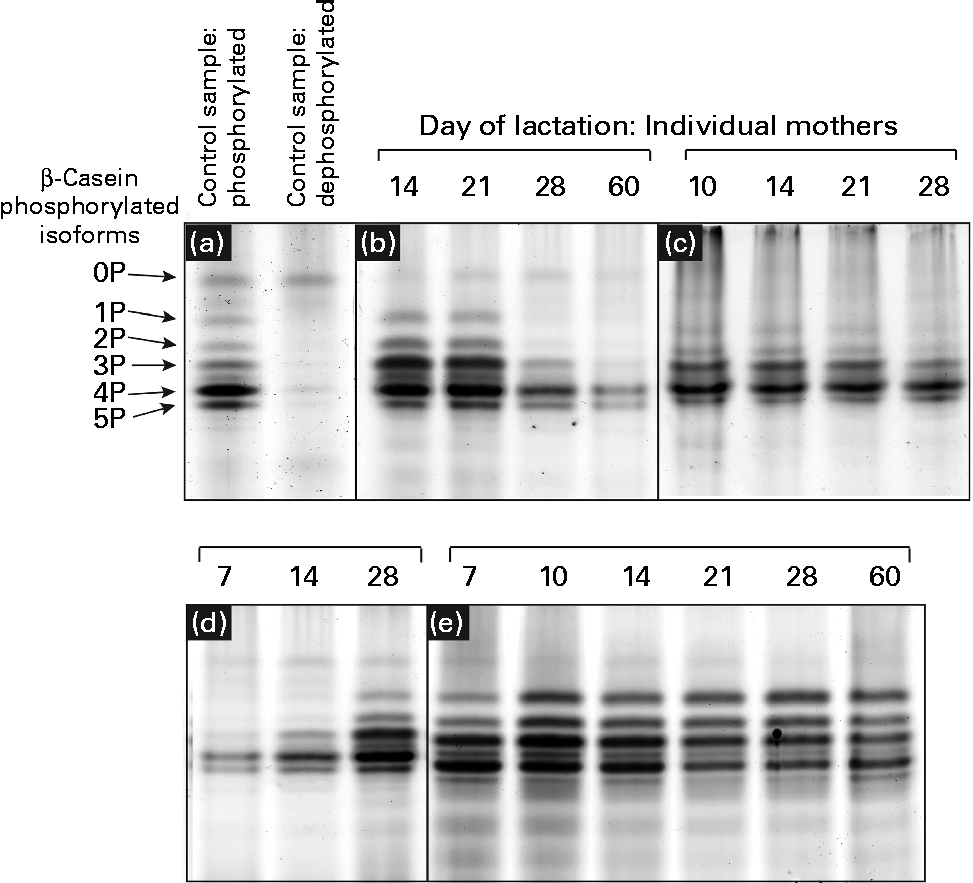
Urea-PAGE was used to separate the differentially phosphorylated forms of β-casein in term and preterm milk. Six distinct bands were visible in the electrophoretograms, corresponding to β-casein with 0, 1, 2, 3, 4 and 5 attached phosphate groups, respectively (Fig. 3(a)). These assignments were made based on a range of evidence. Using MALDI-TOF-TOF MS, each band was identified as β-casein, with the same two peptides identified in each band: m/z 1468·8 (residues 120–132, SPTIPFFDPQIPK) and m/z 1633·9 (residues 176–189, VLPIPQQVVPYPQR). No phosphorylated peptides were detected, so it was not possible to assign bands to particular forms of casein. The dephosphorylated form of β-casein was identified using alkaline phosphatase to create a sample of dephosphorylated casein (Fig. 3(a)). The corresponding forms of the multiple-phosphorylated proteins were then assigned to the protein bands based on the description given by Kinoshita et al. (Reference Kinoshita, Kinoshita-Kikuta and Matsubara17).

Fig. 3 Patterns of casein phosphorylation in human milk over the first 2 months of lactation in term (n 8) and preterm mothers (n 16). A pooled control sample of casein was dephosphorylated by incubation with alkaline phosphatase. (a) The casein samples after and prior to dephosphorylation are depicted. There was considerable variation between the longitudinal patterns of casein phosphorylation between individual mothers, with four basic patterns observed: (b) increase in phosphorylation, (c) no change, highly phosphorylated, (d) decrease in phosphorylation and (e) no change, less phosphorylation.
The casein phosphorylation patterns present in preterm skimmed milk varied both between and within mothers (Fig. 3), with marked differences between term and preterm milk. Four different longitudinal patterns were observed. In term milk, the level of phosphorylation decreased over time in milk from seven of the eight mothers analysed (Fig. 3(d)). In preterm milk, the level of phosphorylation increased over the course of lactation in six of the sixteen samples analysed (Fig. 3(b)). In the remaining ten mothers, no change in phosphorylation pattern was observed over time. For six of these mothers, β-casein was present in five phosphorylated casein isoforms of relatively even intensity (Fig. 3(e)). This pattern was also observed in one of the term mothers. For the remaining four preterm mothers, only three phosphorylated casein isoforms were visible, with the band representing casein with four attached phosphate groups being significantly more intensely stained compared with the others (Fig. 3(c)).
Discussion
In the present study, the protein composition of term (38–41 weeks gestation) and preterm (28–32 weeks gestation) milk over the first 2 months of lactation was investigated. One of the strengths of the present study is that the relative concentration and PTM of a large number of proteins were measured simultaneously in the same set of milk samples, enabling relationships between them to be examined. The concentration, glycosylation and phosphorylation of the major proteins in human milk were found to vary over the course of lactation. Differences between term and preterm milk were found for the glycosylation of tenascin and SA and for the phosphorylation of β-casein. This is the first time that longitudinal changes in these protein modifications have been reported in human milk samples.
We used a gel-based assay to measure the relative concentration of nine of the most abundant proteins in human milk, namely tenascin, MMR, XOR, BSSL, LF, SA, sIgA, β-casein (28 and 16 kDa isoforms) and ALA, together with the concentration of total protein and lactose. With the exception of MMR, each of the individual proteins and the concentration of total protein decreased throughout the first 60 d of lactation in both term and preterm mothers (Fig. 1). Protein expression was similar in term and preterm milk, except for the proteins tenascin and XOR, which had a higher concentration in term milk on days 7 and 14 of lactation. Whilst many other studies have found higher concentrations of total protein, LF and sIgA in preterm milk compared with term milk, these differences tend to be highest in the first few days postpartum(Reference Atkinson and Jensen4, Reference Montagne, Cuilliere and Mole22). In the present study, samples were not collected until 7 d postpartum, which may account for why differences between term and preterm milk were not observed.
It is to be noted that between days 7 and 60 postpartum in milk from preterm mothers, the concentrations of total protein, the immunological proteins LF and sIgA and the nutritional protein ALA all fell by over 40 %. Given the significance of these proteins to preterm infant growth and development(Reference Lonnerdal23), it is important that this change in protein concentration be taken into account when feeding human milk to preterm infants. In a hospital setting, preterm infants can receive human milk either from their own mother (often expressed on an earlier occasion and frozen for storage) or milk from a donor mother at a later stage of lactation. This milk is then routinely fortified with additional protein, energy and minerals in order to meet the high nutritional demands of preterm infants(Reference Ziegler24). Using this standard protocol, it is possible that widely different quantities of total protein, as well as of individual bioactive proteins, will be delivered to preterm infants. Indeed, studies that have implemented adjustable fortification regimes to ensure that infants receive the appropriate level of protein and energy have observed improved infant outcomes(Reference de Halleux, Close and Stalport3, Reference Arslanoglu, Moro and Ziegler25). The results of the present study support the rationale of these adjustable approaches.
In addition to mapping the longitudinal changes in protein expression in preterm mothers, we investigated the PTM of these proteins and found the majority of the abundant proteins in preterm milk to be glycosylated (Fig. 2(b)). Glycosylation is an important protein modification in human milk, and has been shown to have profound effects on both the protection of infants from pathogens and infant development(Reference Brines and Brock26, Reference Newburg27). Whereas both tenascin and SA showed changes in the extent of glycosylation during lactation and between-term and preterm mothers, the degree of glycosylation of LF, MMR, BSSL, sIgA and ALA was constant during the first 60 d of lactation (Fig. 2). These results conflict with previous studies conducted in term milk, which reported BSSL, XOR, κ-casein and LF to be differentially glycosylated over the same time period(Reference Froehlich, Dodds and Barboza28–Reference Kunz and Lonnerdal30). It is possible that this disparity is due to differences in the number of milk samples analysed in each study. The previous studies in term milk observed the differential glycosylation during lactation of XOR and LF in only one mother(Reference Froehlich, Dodds and Barboza28) and of BSSL in four mothers(Reference Landberg, Huang and Stromqvist29), whereas the present study is based on a cohort of seventeen preterm mothers and eight term mothers. It also needs to be acknowledged that, although we did not find any changes in the extent of glycosylation of MMR, BSSL, sIgA, LF or ALA during lactation, this does not preclude the possibility of there being significant changes in the nature of the carbohydrates attached to the protein. Certainly, changes in the composition of protein glycans and milk oligosaccharides have been reported, both between women and throughout lactation(Reference Barboza, Pinzon and Wickramasinghe10, Reference Landberg, Huang and Stromqvist29, Reference Chaturvedi, Warren and Altaye31), and it is likely that this was also the case in the present study.
We did observe a difference in protein glycosylation between term and preterm mothers for the protein tenascin. In term milk, no change in the level of glycosylation was observed during lactation. This was similar to the pattern observed in ten of the preterm mothers. However, for the remaining seven preterm mothers, the amount of bound carbohydrate per unit protein fell significantly over the first 60 d of lactation (Fig. 2(a)). Whilst its precise role in the mammary gland and human milk is unclear, there are indications that the heavily glycosylated extracellular protein tenascin is involved in the extensive tissue remodelling associated with both the periods of pregnancy and involution, acting to suppress the expression of milk-specific proteins during these times(Reference Hsia and Schwarzbauer32–Reference Cella, Chiquet-Ehrismann and Hynes34). The functionality of tenascin is based on its adhesion to other components of the extracellular matrix and to cell surface receptors, and thus glycosylation, which alters its surface structure and adhesive properties, is critical to its function. The differential glycosylation of tenascin observed in the subset of preterm mothers during lactation, and compared with the term mothers, certainly warrants further investigation. Given the difference between term and preterm milk, and the probable roles of tenascin in the remodelling of the mammary gland and the suppression of milk proteins, it is possible that the different patterns of tenascin glycosylation observed are related to the developmental state of the mammary gland or to patterns of protein expression. Further investigation with a much larger sample set is needed to assess these possibilities.
Serum albumin was also found to be differentially glycosylated during lactation in preterm milk, although unlike tenascin, a consistent pattern was observed amongst each of the mothers (Fig. 2(e)). The level of glycosylation decreased during lactation and was higher compared with term milk at day 7 (Fig. 2(e)). Serum albumin is an abundant protein in human milk that enters milk from the bloodstream rather than being synthesised in the mammary gland(Reference Monks and Neville35). It is known to bind to a number of different ligands in milk, potentially acting as a passive carrier(Reference Lonnerdal, Atkinson, Robert and Jensen36). It is not normally considered to be a major glycoprotein in milk, and indeed was not found to be glycosylated at all in a select group of term mothers(Reference Froehlich, Dodds and Barboza28). However, serum albumin has been found in plasma to be non-enzymatically glycosylated (glycated) on a number of different sites(Reference van Boekel, van den Bergh and Hoenders37). Interestingly, the level of serum albumin glycation has been used as a marker of oxidative stress, with higher levels of glycation observed during disease states such as diabetes(Reference Oettl and Stauber38). As serum albumin enters the milk from the bloodstream, it may be possible to view its glycation status as a reflection of the glycation experienced by the circulating serum albumin. Alternatively, the albumin may become glycated after entering the milk space, instead reflecting the oxidative status of milk. Indeed, preterm delivery has been associated with both higher levels of systemic oxidative stress(Reference Joshi, Mehendale and Dangat39) and with lower levels of antioxidants present in human milk(Reference Quiles, Ochoa and Ramirez-Tortosa40), potentially accounting for the higher level of serum albumin glycation observed in preterm milk. The observed fall in the glycation of serum albumin throughout lactation in preterm mothers (Fig. 2(e)) may, therefore, reflect either decreasing levels of maternal oxidative stress as lactation progresses or a change in the oxidative balance in milk itself. Whilst the glycation of serum albumin has been shown to have an effect on protein function(Reference Nakajou, Watanabe and Kragh-Hansen41), it is unclear whether it has any effect on its role in human milk.
In addition to investigating the glycosylation of human milk proteins in term and preterm milk, we also examined the longitudinal patterns of β-casein phosphorylation. A longitudinal trend was observed amongst the term population, with the phosphorylation state of β-casein decreasing during lactation in seven of the eight mothers analysed (Fig. 3). In the preterm population, no change in the distribution of phosphorylated isoforms was observed over time in ten of the sixteen mothers, whereas in the other six mothers, the level of phosphorylation increased during lactation (Fig. 3). This is the most comprehensive report of longitudinal changes in β-casein phosphorylation in either term or preterm mothers, with previous studies reporting differences between samples collected from two to four mothers only(Reference Poth, Deeth and Alewood42, Reference Kunz and Lonnerdal43). The factors regulating the differences in β-casein phosphorylation between mothers and over the course of lactation are unclear. Genetic factors may account for some of the inter-individual variation, with many different polymorphisms of both β-casein and casein kinase genes reported(Reference Greenberg, Groves and Dower44). It is also possible that hormonal differences between term and preterm mothers and within mothers over the course of lactation may account for the observed differences in casein phosphorylation. Casein kinase, for example, has been found to respond to a number of different hormonal signals(Reference Allende and Allende45). The changing hormonal milieu throughout lactation may have an effect on casein kinase activity and, consequently, on the extent of β-casein phosphorylation.
The biological significance of this variation in casein phosphorylation patterns is unknown. Certainly, the formation of casein micelles, structures enabling the transmission of otherwise insoluble concentrations of Ca and phosphate to the infant, is dependent on the phosphorylation of both β-casein and αs1-casein(Reference Qi11). In addition to the transport of Ca and phosphate, another important function of the casein micelle is the curding or coagulation it undergoes in the infant stomach when acted upon by the enzyme chymosin. Curding results in a delay of the entry of milk constituents into the small intestine, thereby improving digestibility and facilitating the spacing of feeding intervals(Reference Miller, Witherly and Clark46). It is not clear whether the differing casein phosphorylation patterns affect either the structure of the casein micelle and its ability to transport Ca and phosphate or the behaviour of casein micelles in the infant stomach. However, the differing phosphorylation pattern of β-casein would certainly have an effect on the nature of the casein peptides produced during digestion. Casein phosphopeptides perform a large range of bioactive functions in the infant, including increasing mineral absorption, immunomodulation and opioid roles(Reference Meisel and Bockelmann47, Reference Ferraretto, Signorile and Gravaghi48). It is possible that the differing patterns of β-casein phosphorylation, in both between term and preterm mothers, and over the course of lactation, exert a biological effect within breastfed infants through the production of different sets of casein peptides.
A limitation of the present study is that data regarding the volume of milk produced by each mother were unavailable. This is unfortunate from a nutritional perspective, as it prevents any analysis of the total amount of each protein received by the infant. Furthermore, it precludes an analysis of the relationship between protein concentration and milk production. There are conflicting reports in the literature regarding this relationship, particularly with regard to differences between term and preterm milk(Reference Atkinson and Jensen4, Reference Bauer and Gerss5), and further studies are required. In addition, it would be interesting to investigate whether the PTM differences that we observed in the preterm mothers are related to levels of milk production, as this may provide insight into the physiological mechanisms responsible for the observed variation.
Conclusions
The concentration of the major proteins in human milk was found to decrease over the first month of lactation and to be similar in both term and preterm milk. With the exception of the glycoproteins, tenascin and serum albumin, the carbohydrate content of the proteins did not change over this time period and was also similar in term and preterm milk. The phosphorylation of β-casein was found to differ between term and preterm milk and to vary widely amongst preterm mothers. Further investigation is required to determine whether these modifications have an effect on protein function and are of clinical importance to preterm infants.
Acknowledgements
The present study was funded by an unrestricted grant from Medela AG (Switzerland) to the University of Western Australia. C. E. M. was supported by a scholarship from the Western Australian Women's Service Guild (2009–11) and an Australian Postgraduate Award (2009–11). B. T. H. contributed to the sample collection and reviewed the manuscript. Y. S. C., P. G. A. and P. E. H. critically reviewed the manuscript. C. E. M. was involved in the study design, performed the laboratory work, data analysis and wrote the initial manuscript. None of the authors had a financial or personal conflict of interest regarding the content of this manuscript.